
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Yahya Choonara | + 3887 word(s) | 3887 | 2021-05-07 10:13:59 | | | |
| 2 | Vivi Li | Meta information modification | 3887 | 2021-05-19 03:23:51 | | |
Video Upload Options
Nanotechnology has aided in the advancement of drug delivery for the treatment of several neurological disorders including depression. Depression is a relatively common mental disorder which is characterized by a severe imbalance of neurotransmitters. Several current therapeutic regimens against depression display drawbacks which include low bioavailability, delayed therapeutic outcome, undesirable side effects and drug toxicity due to high doses.
1. Introduction
Depression is a common mental disorder that is characterized by a persistent feeling of sadness, low self-esteem, disturbed appetite, suicidal thoughts, insomnia and loss of interest [1]. Depression is caused by several aspects which include pathological effects, social activities such as drug and alcohol abuse and biological factors [2]. According to research done by the World Health Organization (W.H.O) in 2017, more than 300 million people (approximately 4.4% of the world’s population) suffer from depression [1] making it one of the top two causes of disability-adjusted life years currently [2]. Pathological causes of depression include a chemical imbalance in the brain, energy metabolic decline and alteration in body hormones [3]. According to the serotonin hypothesis, depression is a result of dysfunctional serotonergic activities [4] which results in reduced serotonin levels in the brain. Several classes of antidepressant therapy that are currently on the market include selective serotonin reuptake inhibitors (SSRI), tricyclic antidepressants, serotonin-norepinephrine reuptake inhibitors, and monoamine oxidase inhibitors. SSRI such as paroxetine, vilazodone, and fluvoxamine are first-line treatment options in adults with depression, albeit with several contraindications [5]. The side effects of current medication include delayed therapeutic onset, low bioavailability, erectile dysfunction, weight gain, dry mouth, nervousness, and insomnia. Some currently approved antidepressant drugs pass through extensive first-pass metabolism which results in reduced oral bioavailability [5]. The time taken by the drug to reach the saturation point is usually prolonged, resulting in delayed therapeutic onset and reduced therapeutic efficacy. Furthermore, as the bioavailability is low, higher doses are required, leading to an increased prevalence of side effects. The therapeutic effect is also limited because of the presence of the blood-brain barrier (BBB) and the blood–cerebrospinal fluid barrier (BCSFB). Traditional medicines have a limited capacity of crossing the BBB and BCSFB [6]. According to current research, nanotechnology-based delivery platforms can be employed to ameliorate the above-mentioned limitations [7][8]. The uses of nanomedicine, biopolymers and nanocarriers have gained significant attention on overcoming these gaps [7].
Nano-based drug delivery strategies offer various advantages in the treatment of chronic diseases by site-specific and targeted delivery, thereby improving the efficacy of approved formulations [7]. Additionally, nanoparticles can improve plasma bioavailability profiles, further enhancing a sustained delivery of antidepressants, resulting in reduced side effects on account of lowered dosing frequencies. Nanomedicine has been used to overcome the limitations of the BBB, as they penetrate through it due to their small size ≤100 nm [9]. Furthermore, nanoparticles can target specific receptors enabled by complexation to ligands such as transferrin and glutathione for improving therapeutic efficacy [9][10][11]. In this review, we discuss different drug carriers, ligands and biopolymers that can improve the bioavailability and therapeutic efficacy of antidepressants by reducing undesirable side effects and dosing frequencies, to achieve safe, desired clinical outcomes.
2. Nanocarriers Employed as Therapeutic Delivery Platforms of Antidepressants
Nanocarriers possess attractive properties which include a high surface-area-to-volume ratio, controlled drug release, targeted delivery, multi-functionality, and a great potential for surface modification [12][13]. Moreover, their nano size has conferred on them the ability to penetrate the BBB and target the brain, rendering them desirable for neurotherapy and diagnosis. Nanocarriers can be employed to enhance drug solubility, circulating time, stability, and the biocompatibility of antidepressants targeted to the brain [12][14]. Moreover, nanocarriers minimize hepatic first-pass metabolism and protect bioactive agents from hydrolytic and enzymatic degradation [15]. They show great potential in improving antidepressant drug delivery due to their characteristics [12]. The use of nanocarriers to improve the efficacy of delivery systems of antidepressants has gained increased attention among researchers [12].
2.1. Dendrimers
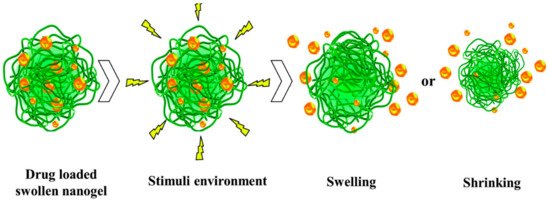
2.2. Nanogels
2.3. Polymeric Micelles
2.4. Nanoliposomes
2.5. Carbon Nanotubes (CNT)
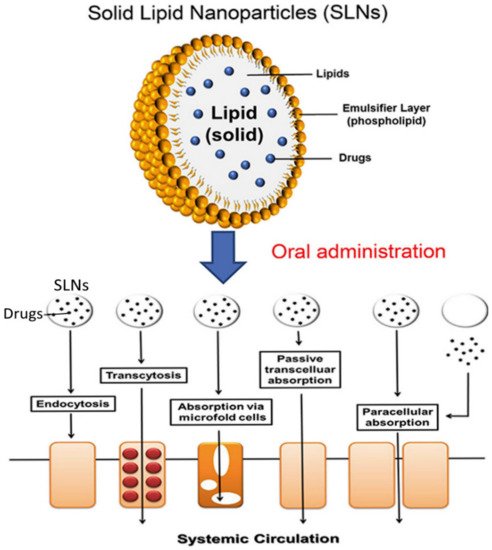
2.6. Solid-Lipid Nanoparticles (SLN)
2.7. Polymeric Nanoparticles
2.8. Magnetic Nanoparticles
Magnetic nanoparticles (MNPs) are generally spherical and crystalline nanoparticles that are composed of elements with unpaired electrons such as iron (Fe), nickel (Ni) and chromium (Cr) which confer magnetic properties on them. Their magnetic properties are harnessed for drug delivery through the application of an external magnetic field. Iron oxide is the most employed core because it exhibits high physiological stability and is easily removed through the endogenous iron metabolic pathway [60]. On account of their small size, MNPs can easily penetrate the brain matrix by temporarily creating pores in the BBB endothelium. The size and magnetic properties of synthesized MNPs are dependent on the physiological characteristics of the targeted organ [61]. Including their magnetic properties, the attractive characteristics of MNPs which include biocompatibility, low toxicity, easily modifiable surfaces have sprouted interest in drug delivery research [62]. Furthermore, since they can bind to several compounds such as drugs, antibodies and proteins, they can be directed to different receptors using an external magnetic field [62]. Despite mounting in vitro and in vivo data that indicate the potential applications of MNPs and other nano formulations, only a very few clinical trials have assessed their efficacy and safety on CNS conditions such as depression [62]. A study that was done using iron oxide nanoparticles proved that they are biocompatible and highly biodegradable under in vivo conditions. Interestingly, after metabolism, the iron can easily be incorporated into erythrocytes to form a part of hemoglobin, making it an added advantage [60]. In vivo studies that were done using rats to investigate the effects of iron oxide nanoparticles on depression treatment indicated that iron oxide nanoparticles are beneficial in reducing the symptoms of depression [63].
In another study, paroxetine and duloxetine-loaded nanogels were formulated to investigate the effect of MNPs on the efficacy of the antidepressants. The study showed that MNPs enhanced the release of the antidepressant. Magnetic fields induced stress on the nanoparticles, and this resulted in improved swelling properties of the nanogel. The group concluded that the use of magnetic nanoparticles could enhance the drug loading capacity and the sustained release profile of the formulation [25]. A previous study displayed that MNPs might be cleared by macrophages before reaching the targeted receptor or organ and the nanoparticles tend to aggregate due to strong magnetic interactions [62] which might result in increased toxicity and tissue damage. Moreover, in the absence of surface coating, the MNPs are prone to oxidation which may lead to the loss of magnetic field properties. However, aggregation can be prevented by coating the MNP with biopolymers, such as PEG and chitosan, which stabilize the nanoparticles. This might result in a reduction of antiparticle surface interaction [64]. Summary of nanocarriers discussed is presented in Table 1.
Table 1. Summary of nanocarriers.
|
Type of Nanocarrier |
Drug Delivery Characteristics |
Structure |
Drawbacks |
References |
|---|---|---|---|---|
|
Dendrimers |
Rapid cellular entry, high drug loading capacity, improved half-life, biocompatibility |
Highly branched, Monodisperse structure, |
Non-degradable in physiological environment, Large particle size |
|
|
Nanogels |
Large surface area, high entrapping rate, biocompatible, high loading capacity, |
Hydrogels, cross-linked hydrophilic polymer networks, |
Physically cross-linked nanogels are less stable |
|
|
Polymeric micelles |
Increased half-life, solubility and stability, biodegradable, biocompatible |
Amphiphilic Block copolymers, |
Low drug loading capacity, Premature leaking, |
|
|
Nanoliposomes |
Enhanced encapsulating rate, biocompatible, biodegradable, improved intracellular uptake |
Lipid vesicles, amphiphilic phospholipids |
poor stability in aqueous |
|
|
Carbon nanotubes |
Improved cell-penetrating ability, biocompatibility, high drug entrapping rate, |
Tubular morphology, two or more layers, allotropes of carbon |
Mechanism is not known, too small, low solubility, permeability can be affected with temperature |
|
|
Solid Lipid Nanoparticles |
Excellent drug release profile, stable, biodegradable, large surface area |
Spherical structure, |
Poor incorporation rate, prone to gelation, loading capacity depends on length of the hydrocarbon chain, |
|
|
Polymeric nanoparticles |
High cell-penetrating rate, prolong duration, biodegradable, enhanced stability, |
Spherical shape, |
Easily eliminated in the bloodstream |
|
|
Magnetic nanoparticles |
High stability, biocompatible, improve drug targeting |
Spherical structure, crystals. |
Easily eliminated from the body, prone to aggregation |
References
- World Health Organization. Depression and Other Common Mental Disorders: Global Health Estimates; World Health Organization: Geneva, Switzerland, 2017.
- Atnafie, S.A.; Muluneh, N.Y.; Getahun, K.A.; Woredekal, A.T.; Kahaliw, W. Depression, anxiety, stress, and associated factors among khat chewers in Amhara region, Northwest Ethiopia. Depression Res. Treat. 2020, 2020, 7934892.
- Hasler, G. Pathophysiology of depression: Do we have any solid evidence of interest to clinicians? World Psychiatry 2010, 9, 155–161.
- Maes, M.; Meltzer, Y.H. Psychopharmacology; The Fourth Generation of Progress. In The Serotonin Hypothesis of Major Depression; Raven Press: New York, NY, USA, 1994; pp. 933–944.
- Strawn, J.R.; Geracioti, L.; Rajdev, N.; Clemenza, K.; Levine, A. Pharmacotherapy for generalized anxiety disorder in adult and pediatric patients: An evidence-based treatment review. Expert Opin. Pharmacother. 2018, 19, 1057–1070.
- Soni, S.; Ruhela, R.K.; Medhi, B. Nanomedicine in Central Nervous System (CNS) disorders: A present and future prospective. Adv. Pharm. Bull. 2016, 6, 319–335.
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; del Pilar Rodriguez-Torres, M.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71.
- Kondiah, P.P.; Choonara, Y.E.; Marimuthu, T.; Kondiah, P.J.; Du Toit, L.C.; Kumar, P.; Pillay, V. Nanotechnological paradigms for neurodegenerative disease interventions. In Advanced 3D-Printed Systems and Nanosystems for Drug Delivery and Tissue Engineering; Elsevier BV: Cambridge, MA, USA, 2020; pp. 277–292.
- Saeedi, M.; Eslamifar, M.; Khezri, K.; Dizaj, S.M. Applications of nanotechnology in drug delivery to the central nervous system. Biomed. Pharmacother. 2019, 111, 666–675.
- Jain, A.; Jain, S.K. Ligand-appended BBB-targeted nanocarriers (LABTNs). Crit. Rev. Ther. Drug Carr. Syst. 2015, 32, 149–180.
- Kondiah, P.P.; Choonara, Y.E.; Kondiah, P.J.; Marimuthu, T.; Kumar, P.; Du Toit, L.C.; Modi, G.; Pillay, V. Nanocomposites for therapeutic application in multiple sclerosis. In Applications of Nanocomposite Materials in Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2018; pp. 391–408.
- Din, F.U.; Aman, W.; Ullah, I.; Qureshi, O.S.; Mustapha, O.; Shafique, S.; Zeb, A. Effective use of nanocarriers as drug delivery systems for the treatment of selected tumors. Int. J. Nanomed. 2017, 12, 7291–7309.
- Laffleur, F.; Keckeis, V. Advances in drug delivery systems: Work in progress still needed? Int. J. Pharm. 2020, 590, 119912.
- Fuller, E.G.; Scheutz, G.M.; Jimenez, A.; Lewis, P.; Savliwala, S.; Liu, S.; Sumerlin, B.S.; Rinaldi, C. Theranostic nanocarriers combining high drug loading and magnetic particle imaging. Int. J. Pharm. 2019, 572, 118796.
- Chowdhury, A.; Kunjiappan, S.; Panneerselvam, T.; Somasundaram, B.; Bhattacharjee, C. Nanotechnology and nanocarrier-based approaches on treatment of degenerative diseases. Int. Nano Lett. 2017, 7, 91–122.
- Sherje, A.P.; Jadhav, M.; Dravyakar, B.R.; Kadam, D. Dendrimers: A versatile nanocarrier for drug delivery and targeting. Int. J. Pharm. 2018, 548, 707–720.
- Parajapati, S.K.; Maurya, S.D.; Das, M.K.; Tilak, V.K.; Verma, K.K.; Dhakar, R.C. Potential application of dendrimers in drug delivery: A concise review and update. J. Drug Deliv. Ther. 2016, 6, 71–85.
- Xu, L.; Zhang, H.; Wu, Y. Dendrimer Advances for the Central Nervous System Delivery of Therapeutics. ACS Chem. Neurosci. 2013, 5, 2–13.
- Florendo, M.; Figacz, A.; Srinageshwar, B.; Sharma, A.; Swanson, D.; Dunbar, G.L.; Rossignol, J. Use of Polyamidoamine Dendrimers in Brain Diseases. Molecules 2018, 23, 2238.
- Soni, K.S.; Desale, S.S.; Bronich, T.K. Nanogels: An overview of properties, biomedical applications and obstacles to clinical translation. J. Control. Release 2016, 240, 109–126.
- Danish, A.; Sheikh, T.; Abrar, M.; Chaos, S.; Bagwan, R.; Kulkarni, A. Nanogel: A versatile nano-scopic platform for oral drug delivery. World J. Pharm. Sci. 2018, 7, 685–693.
- Neamtu, I.; Rusu, A.G.; Diaconu, A.; Nita, L.E.; Chiriac, A.P. Basic concepts and recent advances in nanogels as carriers for medical applications. Drug Deliv. 2017, 24, 539–557.
- Vashist, A.; Kaushik, A.; Vashist, A.; Bala, J.; Nikkhah-Moshaie, R.; Sagar, V.; Nair, M. Nanogels as potential drug nanocarriers for CNS drug delivery. Drug Discov. Today 2018, 23, 1436–1443.
- Dange, S.; Kamble, M.; Bhalerao, K.; Chaudhari, P.; Bhosale, A.; Nanjwade, B.; Shinde, S. Formulation and evaluation of venlafaxine nanostructured lipid carriers. J. Bionanosci. 2014, 8, 81–89.
- Casolaro, M.; Casolaro, I. Polyelectrolyte hydrogel platforms for the delivery of antidepressant drugs. Gels 2016, 2, 24.
- Amin, M.C.I.M.; Butt, A.M.; Amjad, M.W.; Kesharwani, P. Polymeric micelles for drug targeting and delivery. In Nanotechnology-Based Approaches for Targeting and Delivery of Drugs and Genes; Elsevier BV: Amsterdam, The Netherlands, 2017; pp. 167–202.
- Zhang, Y.; Huang, Y.; Li, S. Polymeric micelles: Nanocarriers for cancer-targeted drug delivery. AAPS PharmSciTech 2014, 15, 862–871.
- Subramani, K.; Ahmed, W. Emerging Nanotechnologies in Dentistry. In Nanoparticulate Drug Delivery Systems for Oral Cancer Treatment; William Andrew Publishing: Boston, MA, USA, 2012; pp. 333–345.
- Mohapatra, S.S.; Ranjan, S.; Dasgupta, N.; Mishra, R.K.; Thomas, S. Nanocarriers for Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2019.
- Abourehab, M.A.; Ahmed, O.A.; Balata, G.F.; Almalki, W.H. Self-assembled biodegradable polymeric micelles to improve dapoxetine delivery across the blood–brain barrier. Int. J. Nanomed. 2018, ume 13, 3679–3687.
- Movassaghian, S.; Merkel, O.M.; Torchilin, V.P. Applications of polymer micelles for imaging and drug delivery. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2015, 7, 691–707.
- Vieira, D.B.; Gamarra, L.F. Getting into the brain: Liposome-based strategies for effective drug delivery across the blood–brain barrier. Int. J. Nanomed. Dove Med. Press 2016, 1–34.
- Nomani, M.S.; Samy, J.G. Nanoliposome: An alternative approach for drug delivery system. Int. J. Adv. Pharm. Med. Bioallied. Sci. 2016, 2016, 1–10.
- Mura, P. Advantages of the combined use of cyclodextrins and nanocarriers in drug delivery: A review. Int. J. Pharm. 2020, 579, 119181.
- Piazzini, V.; Landucci, E.; Graverini, G.; Pellegrini-Giampietro, D.E.; Bilia, A.R.; Bergonzi, M.C. Stealth and cationic nanoliposomes as drug delivery systems to increase andrographolide BBB permeability. Pharmaceutics 2018, 10, 128.
- Daeihamed, M.; Dadashzadeh, S.; Haeri, A.; Akhlaghi, M.F. Potential of liposomes for enhancement of oral drug absorption. Curr. Drug Deliv. 2016, 13, 1.
- Ramalho, M.J.; Andrade, S.; Loureiro, J.A.; Pereira, M.D.C. Nanotechnology to improve the Alzheimer’s disease therapy with natural compounds. Drug Deliv. Transl. Res. 2019, 10, 380–402.
- Mutlu, N.B.; DeĞim, Z.; Yılmaz, Ş.; Eşsiz, D.; Nacar, A. New perspective for the treatment of Alzheimer diseases: Liposomal rivastigmine formulations. Drug Dev. Ind. Pharm. 2011, 37, 775–789.
- Rotman, M.; Welling, M.M.; Bunschoten, A.; de Backer, M.E.; Rip, J.; Nabuurs, R.J.; Gaillard, P.J.; van Buchem, M.A.; van der Maarel, S.M.; van der Weerd, L. Enhanced glutathione PEGylated liposomal brain delivery of an anti-amyloid single domain antibody fragment in a mouse model for Alzheimer’s disease. J. Control. Release 2015, 203, 40–50.
- Medyantseva, E.P.; Brusnitsyn, D.V.; Varlamova, R.M.; Maksimov, A.A.; Konovalova, O.A.; Budnikov, H.C. Surface modification of electrodes by carbon nanotubes and gold and silver nanoparticles in monoaminoxidase biosensors for the determination of some antidepressants. J. Anal. Chem. 2017, 72, 362–370.
- Komane, P.P.; Choonara, Y.E.; du Toit, L.C.; Kumar, P.; Kondiah, P.P.D.; Modi, G.; Pillay, V.; Komane, P.P. Diagnosis and treatment of neurological and ischemic disorders employing carbon nanotube technology. J. Nanomater. 2016, 2016, 1–19.
- Sengel, C.T.; Alpturk, O. Nanoconjugate Nanocarriers for Drug Delivery. In Carbon Nanotubes for Drug Delivery; Apple Academic Press: New York, NY, USA, 2018; pp. 348–376.
- Pitroda, J.; Jethwa, B.; Dave, S.K. A critical review on carbon nanotubes. Int. J. Constr. Res. Civ. Eng. 2016, 2, 36–42.
- Guo, Q.; You, H.; Yang, X.; Lin, B.; Zhu, Z.; Lu, Z.; Li, X.; Zhao, Y.; Mao, L.; Shen, S.; et al. Functional single-walled carbon nanotubes ‘CAR’ for targeting dopamine delivery into the brain of parkinsonian mice. Nanoscale 2017, 9, 10832–10845.
- Naseri, N.; Valizadeh, H.; Zakeri-Milani, P. Solid Lipid nanoparticles and nanostructured lipid carriers: Structure, preparation and application. Adv. Pharm. Bull. 2015, 5, 305–313.
- Lin, C.-H.; Chen, C.-H.; Lin, Z.-C.; Fang, J.-Y. Recent advances in oral delivery of drugs and bioactive natural products using solid lipid nanoparticles as the carriers. J. Food Drug Anal. 2017, 25, 219–234.
- Fonseca-Santos, B.; Chorilli, M.; Gremião, M.P.D. Nanotechnology-based drug delivery systems for the treatment of Alzheimer’s disease. Int. J. Nanomed. 2015, 10, 4981–5003.
- Noack, A.; Hause, G.; Mäder, K. Physicochemical characterization of curcuminoid-loaded solid lipid nanoparticles. Int. J. Pharm. 2012, 423, 440–451.
- Vijayanand, P.; Jyothi, V.; Aditya, N.; Mounika, A. Development and characterization of solid lipid nanoparticles containing herbal extract: In vivo antidepressant activity. J. Drug Deliv. 2018, 2018, 1–7.
- Lloyd-Parry, O.; Downing, C.; Aleisaei, E.; Jones, C.; Coward, K. Nanomedicine applications in women’s health: State of the art. Int. J. Nanomed. 2018, 13, 1963–1983.
- Wang, S.; Chen, T.; Chen, R.; Hu, Y.; Chen, M.; Wang, Y. Emodin loaded solid lipid nanoparticles: Preparation, characterization and antitumor activity studies. Int. J. Pharm. 2012, 430, 238–246.
- Ganesan, P.; Ramalingam, P.; Karthivashan, G.; Ko, Y.T.; Choi, D.-K. Recent developments in solid lipid nanoparticle and surface-modified solid lipid nanoparticle delivery systems for oral delivery of phyto-bioactive compounds in various chronic diseases. Int. J. Nanomed. 2018, 13, 1569–1583.
- Kulthe, S.S.; Choudhari, Y.M.; Inamdar, N.N.; Mourya, V. Polymeric micelles: Authoritative aspects for drug delivery. Des. Monomers Polym. 2012, 15, 465–521.
- Neha, B.; Ganesh, B.; Preeti, K.; Guru, S. Drug delivery to the brain using polymeric nanoparticles: A review. Int. J. Pharm. Life Sci. 2013, 2, 107–121.
- Beiranvand, S.; Sorori, M.M. Pain management using nanotechnology approaches. Artif. Cells Nanomed. Biotechnol. 2019, 47, 462–468.
- Kasinathan, N.; Jagani, H.V.; Alex, A.T.; Volety, S.M.; Rao, J.V. Strategies for drug delivery to the central nervous system by systemic route. Drug Deliv. 2015, 22, 243–257.
- AlAbsi, A.; Khoudary, A.C.; Abdelwahed, W. The antidepressant effect of L-tyrosine-loaded nanoparticles: Behavioral aspects. Ann. Neurosci. 2016, 23, 89–99.
- Tong, G.-F.; Qin, N.; Sun, L.-W. Development and evaluation of Desvenlafaxine loaded PLGA-chitosan nanoparticles for brain delivery. Saudi Pharm. J. 2017, 25, 844–851.
- Rajput, R.; Kumar, S.; Nag, P.; Singh, M. Fabrication and characterization of chitosan based polymeric Escitalopram nanoparticles. J. Appl. Pharm. Sci. 2016, 6, 171–177.
- Arora, G.; Midha, K.; Kaur, S.; Marwah, S. An overview of the nanoparticles in CNS targeted drug delivery: An emerging trend. Curr. Trends Pharm. Clin. Trials 2019, 2, 2–5.
- D’Agata, F.; Ruffinatti, F.A.; Boschi, S.; Stura, I.; Rainero, I.; Abollino, O.; Cavalli, R.; Guiot, C. Magnetic nanoparticles in the central nervous system: Targeting principles, applications and safety issues. Molecules 2017, 23, 9.
- Denkbaş, E.B.; Çelik, E.; Erdal, E.; Kavaz, D.; Akbal, Ö.; Kara, G.; Bayram, C. Magnetically based nanocarriers in drug delivery. Nanobiomater. Drug Deliv. 2016, 285–331.
- Saeidienik, F.; Shahraki, M.R.; Fanaei, H.; Badini, F. The effects of iron oxide nanoparticles administration on depression symptoms induced by LPS in male wistar rats. Basic Clin. Neurosci. J. 2018, 9, 209–216.
- Gutiérrez, L.; De La Cueva, L.; Moros, M.; Mazarío, E.; De Bernardo, S.; De La Fuente, J.M.; Morales, M.D.P.; Salas, G. Aggregation effects on the magnetic properties of iron oxide colloids. Nanotechnology 2019, 30, 112001.





