
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Tilman Borggrefe | + 2529 word(s) | 2529 | 2021-05-12 08:54:27 | | | |
| 2 | Peter Tang | Meta information modification | 2529 | 2021-05-19 04:48:46 | | |
Video Upload Options
Enzymes, such as histone methyltransferases and demethylases, histone acetyltransferases and deacetylases, and DNA methyltransferases are known as epigenetic modifiers that are often implicated in tumorigenesis and disease. One of the best-studied chromatin-based mechanism is X chromosome inactivation (XCI), a process that establishes facultative heterochromatin on only one X chromosome in females and establishes the right dosage of gene expression. The specificity factor for this process is the long non-coding RNA Xinactivespecifictranscript (Xist), which is upregulated from one X chromosome in female cells. Subsequently, Xist is bound by the corepressor SHARP/SPEN, recruiting and/or activating histone deacetylases (HDACs), leading to the loss of active chromatin marks such as H3K27ac. In addition, polycomb complexes PRC1 and PRC2 establish wide-spread accumulation of H3K27me3 and H2AK119ub1 chromatin marks. The lack of active marks and establishment of repressive marks set the stage for DNA methyltransferases (DNMTs) to stably silence the X chromosome.
1. Long Non-Coding RNAs and Cancer
Less than 2% of the genome is transcribed in protein-encoding mRNAs; however, most of it is actively transcribed, which suggests that a fraction produces non-coding RNAs (ncRNAs). ncRNAs are classified based on their size in small ncRNAs (<200 bp) and long ncRNAs (>200 bp, also referred to as lncRNAs) [1][2]. In this review, we focus on lncRNAs.
lncRNAs can be classified based on their genomic localization [3] as well as on their cellular distribution [4]. It is proposed that lncRNAs are organized in secondary and tertiary structures [5] that may offer binding surfaces for proteins containing RNA-recognition motives (RRMs). lncRNAs are capable of interacting with coactivators or corepressors of transcription, recruiting them to specific genes or genomic regions [6][7][8][9]. In addition, lncRNAs are also able to regulate alternative splicing events by interacting with splicing factors [6][10].
Several lncRNAs have been associated with variety of diseases. Metastasis-associated lung adenocarcinoma transcript 1 (MALAT1) was found to be upregulated in renal cell carcinoma (RCC), gastric cancer (GC), gallbladder cancer (GBC), colorectal cancer (CRC), multiple myeloma, clear cell renal cell carcinoma (ccRCC), and glioma, as well as in osteosarcoma [11][12][13][14][15][16][17][18], and it has been proposed as a molecular marker therein [14][15][16][19]. The lncRNA imprinted H19 gene is maternally expressed and strongly downregulated directly after birth [20][21][22]. It was shown that H19 is strongly upregulated in gastric cancer [23][24][25], similarly to several other lncRNAs, such as PVT1 oncogene (PVT1), gastric carcinoma high expressed transcript 1 (GHET1), antisense ncRNA in the INK4 locus (ANRIL), SPRY4 intronic transcript 1 (SPRY4-IT1), and the already mentioned MALAT1 [18][26][27][28][29][30]. H19 is also upregulated in other cancer types, such as esophageal cancer, CRC and lung cancer [25]. Another example is represented by homeobox (HOX) transcript antisense RNA (HOTAIR), which is upregulated in hepatocellular carcinoma [31], in colorectal cancer [32], in gastric cancer [33], and pancreatic cancer [34].
In this review, we will focus our attention on X inactive specific transcript (Xist; XIST in human), a lncRNA whose main function is to inactivate one X chromosome in female cells to achieve dosage compensation between males (XY) and females (XX) (see below). Recent studies highlighted its frequent deregulation in cancer. XIST is responsible for silencing several genes, and the observation that the X-linked oncogenes ARAF-1 and ETS-like 1 (ELK-1) are overexpressed in tumors with multiple active X chromosomes [35] suggests that the deregulation of XIST may be associated with cancer. Several studies observed defective X chromosome inactivation (XCI) in breast and basal-like cancer and linked the deregulation of the X chromosome to breast cancer (BC) [36][37][38][39][40][41][42], to ovarian cancer [43], as well as to cancers in patients affected by Klinefelter syndrome [44]. This deregulation is usually given by a loss of XIST as result of disappearance of the inactive X chromosome (Xi) and amplification of the active one (Xa) [37][38][40][43][44].
The gathered knowledge of these studies suggest that lncRNAs are important mediators of pathological conditions and they may, in the future, serve as potential therapeutic targets.
XCI serves as a powerful paradigm to study chromatin dynamics at a chromosomal scale. XCI co-evolved with the mammalian sex chromosomes as a mechanism to equalize the dosage of X-encoded genes between male XY and female XX cells. The central player in this process is Xist, which was discovered as the first functional lncRNA in mammals, being upregulated from the future Xi, coating the Xi in cis, thereby recruiting chromatin remodelers directly and indirectly rendering the X chromosome inactive. Xist is located on the X chromosome and it is surrounded by several other lncRNA-encoding genes, including Tsix, just proximal to Xist (Jpx), and five prime to XistT (Ftx), which, in mouse, have been shown to be involved in Xist regulation through different mechanisms, including transcriptional interference, RNA-mediated recruitment of chromatin remodelers, and through transcription co-activation [45][46][47][48]. Xist encodes a 17 kb lncRNA (19 kb in human) that contains six repeat structures that play a crucial, sometimes redundant, role in Xist-mediated silencing as well as localization [49]. So far, most of the functional studies have been performed in mouse where deletions of the most 5′ located repeat A led to a silencing phenotype despite the fact that Xist spreading was unaffected. Several studies indicated that SHARP [SMRT (silencing mediator for retinoid or thyroid hormone receptors) and HDACs (histone deacetylases)-associated repressor protein], encoded by the SPEN (split ends) gene [also called SHARP or Mint (Msx2-interacting nuclear target protein)], is a crucial factor in the X inactivation process through interacting with the A repeat sequence and recruitment of several repressor complex members, such as nuclear receptor corepressor (NCoR), SMRT, and nucleosome remodeling deacetylase (NuRD) complexes [50][51][52][53][54][55] (see Table 1).
Table 1. Proteins and complexes involved in the regulation of X chromosome inactivation (XCI). The “Disease(s)” column indicates diseases caused by mutations in the XCI related genes/proteins described in this table. The functional link between these mutations and XCI remains to be investigated.
|
Protein/Complex |
Subunits |
Function(s) in XCI |
Disease(s) |
References |
|---|---|---|---|---|
|
DNMT3B |
- |
DNA methyltransferase |
AML, FSHD, HD, ICF, PR |
|
|
hnRNPK |
- |
Bridging protein between Xist and ncPRC1 |
AKS, AML, KLS, KS, MF, OS |
|
|
ncPRC1 |
PCGF3/5 RING1A/B RYBP/YAF |
E3 ubiquitin ligase |
MDS |
|
|
NCoR1/2 * |
GPS2 HDAC3 NCoR1/2 * TBL1 TBLR1 |
Deacetylase |
ASDs, BC, CC, HCC, ID, MB, NDDs, OMZL, PS, SCZ |
[79][80][81][82][83][84][85][86][87][88][89][90][91][92][93][94][95] |
|
PRC1 |
CBX2/4/6/7/8 PCGF1-6 PHC1-3 RING1A/B SCMH1/L2 |
E3 ubiquitin ligase/Recognition of histone methylation |
BC, DD, DSD, ESCC, GC, MCL, MDS, OSS, PM |
|
|
PRC2 |
AEBP2 EZH2 ** EED JARID2 RBBP4/7 SUZ12 |
Methyltransferase |
AML, DS-AMKL, DLBCL, ETP-ALL, FL, HCC, MDS, MPN, T-ALL, T-PLL |
[76][106][107][108][109][110][111][112][113][114][115][116][117][118][119][120][121][122][123][124][125][126][127] |
|
SHARP |
- |
Adaptor protein that recruits the HDAC3-containing NCoR1/2 complexes |
ACC, BC, DLBCL, MCL, NDDs, PASC, SMZL |
[51][52][53][54][55][128][129][130][131][132][133][134][135][136][137][138][139] |
ACC: Adenoid cystic carcinoma; AEBP2: Adipocyte enhancer-binding protein 2; AKS: Au–Kline syndrome; AML: Acute myeloid leukemia; ASDs: Autism spectrum disorders; BC: Breast cancer; CC: Colon cancer; CBX2/4/6/7/8: Chromobox homolog 2/4/6/7/8; DD: Developmental disorder; DLBCL: Diffuse large B-cell lymphoma; DNMT3B: DNA methyltransferase 3B; DS-AMKL: Acute megakaryoblastic leukemia associated with Down syndrome; DSD: Disorders of sex development; EED: Embryonic ectoderm development; ESCC: Esophageal squamous cell carcinoma; ETP-ALL: Early T-cell precursor acute lymphoblastic leukaemia Early T-cell precursor acute lymphoblastic leukaemia; EZH2: Enhancer of zeste 2; FL: Follicular lymphoma; FSHD: Facioscapulohumeral dystrophy; GC: Gastric cancer; GPS2: G-protein pathway suppressor 2; HCC: Hepatocellular carcinoma; HD: Hirschsprung disease; HDAC3: Histone deacetylase 3; hnRNPK: Heterogeneous nuclear ribonucleoprotein K; ICF: Immunodeficiency, centromeric instability and facial anomalies; ID: Intellectual disability; JARID2: Jumanji and AT-rich interaction domain-containing 2; KLS: Kabuki-like syndrome; KS: Kabuki syndrome; MB: Medulloblastoma; MCL: Mantle cell lymphoma; MDS: Myelodysplastic syndromes; MF: Mycosis fungoides; MPN: myeloproliferative neoplasm; NCoR1/2: Nuclear receptor corepressor; ncPRC1: non-canonical PRC1 complex; NDDs: Neurodevelopmental disorders; OMZL: Ocular marginal zone lymphoma; OS: Okamoto syndrome; OSS: Osteosarcoma; PASC: Pancreatic adenosquamous carcinoma; PCGF1-6: PcG ring finger 1-6; PCGF3/5: PcG ring finger 3/5; PHC1-3: Polyhomeotic homolog 1-3; PM: Primary microcephaly; PR: Prostate cancer; PRC1: Polycomb repressive complex 1; PRC2: Polycomb repressive complex 2; PS: Pierpont syndrome; RBBP4/7: Retinoblastoma binding protein 4/7; RING1A/B: Really interesting new gene 1A/B; RYBP/YAF: RING1 And YY1 Binding Protein/YY1-associated factor; SCMH1/L2: Sex comb on midleg homolog 1/L2; SCZ: Schizophrenia; SHARP: SMRT (silencing mediator for retinoid or thyroid hormone receptors) and HDACs (histone deacetylases)-associated repressor protein; SMZL: Splenic marginal zone lymphoma; SUZ12: Suppressor of zeste 12; T-ALL: T-cell acute lymphoblastic leukemia; T-PLL: T-cell prolymphocytic leukemia; TBL1: Transducin β-like protein 1; TBLR1: Transducing β-like 1 (TBL1)-related protein; XCI: X chromosome inactivation; Xist: X inactive specific transcript; * NCoR2 is also known as SMRT (silencing mediator for retinoid or thyroid hormone receptors); ** EZH2 is also known as KMT6A (lysine (K) methyltransferase 6A).
SHARP is transiently enriched at the promoters and enhancers of genes that are subject to XCI and it recruits NCoR/SMRT complexes that contain HDACs, leading to histone deacetylation [55]. SHARP localization also shows overlap with NuRD complex members predominantly at promoters, and its action is only required during the initiation phase of XCI, as removal of SHARP after Xi is established has no effect [55][140]). As a consequence of the action of SHARP and its associated protein complexes, promoters and enhancers are deacetylated in a stepwise manner, paving the way for the action of the polycomb group (PcG) protein repressive complexes PRC1 and PRC2 that play a crucial role in the establishment and maintenance of the silent state of the Xi. PRC1 is a large multi-protein complex that is recruited to Xist through heterogeneous nuclear ribonucleoprotein K (hnRNPK) that acts as a bridge between PRC1 and Xist Repeat B and, to a lesser extent, Repeat C [65][66][77]. PRC1-directed deposition of monoubiquitination of K119 of histone H2A (H2A119ub1) is mediated by the core PRC1 complex member really interesting new gene 1 isoform A or B (RING1A/B) and, in turn, is recognized by PRC2 subunit jumanji and AT-rich interaction domain-containing 2 (JARID2) facilitating trimethylation on K27 of histone H3 (H3K27me3) by the enhancer of zeste 2/lysine (K) methyltransferase 6A (EZH2/KMT6A) [141][142][143]. Subsequently, PRC1 and PRC2 recruitment is re-enforced through the recruitment of PRC1 that recognizes the trimethylation of K27 of histone H3 (H3K27me3) through chromobox-containing protein (CBX), which further promotes H2AK119ub1 deposition, facilitating the spreading of silencing [144][145][146]. At a later stage of the XCI process, de novo DNA methyltransferases (DNMTs) are recruited to lock in the silent state through the deposition of DNA methylation at promoters and CpG islands (CGI). These studies highlight the concerted action of chromatin readers and writers directing the right order of epigenetic events required to establish the Xi that is propagated through a near infinite number of cell divisions.
2. The Inactive X Chromosome Status in Cancer
The complete loss or alteration of the Xi is frequently observed in breast and ovarian cancers, amongst other types of cancer [147][148]. Initial studies showed that Xist/XIST RNA is essential for the initiation and establishment of XCI during development, but dispensable to maintain the Xi in female somatic cells [149][150]. Even so, more recent studies making use of more sensitive techniques detect the reactivation of X-linked genes upon nearly complete or partial Xist/XIST depletion. The human X chromosome codes for more than 900 coding genes [151], including several tumor suppressor genes and oncogenes [152][153]. Thus, gene dosage changes that are caused by potential reactivation or silencing of X-linked genes could be detrimental. So far, only one well documented study in mice revealed a clear causal relationship between Xist deletion in the hematopoietic lineage and high penetrance hematopoietic cancer [154].
In human, the absence of the Xi (Barr body) in female cancer cells and presence of multiple Xa’s have been frequently associated with different forms of cancer, such as breast cancer [38][40][44]. However, these events are primarily attributed to the loss of the Xi and duplication of the Xa due to chromosome segregation errors (see Figure 1) [38][40][44].
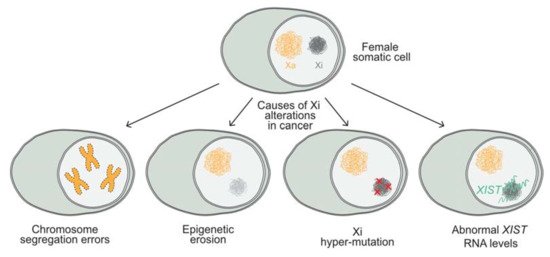
Epigenetic alterations that are caused by epigenetic erosion of the Xi have also been described. These erosion events affect histone modification, deposition, and DNA methylation, leading to the reactivation of X-linked genes in breast cancer cell lines and primary tumors [155]. Moreover, the Xi in female cancer genomes has been shown to accumulate more mutations than the autosomes in various cancer types, including medulloblastoma, breast cancer, glioblastoma, and acute myeloid leukemia (AML) [156]. Interestingly, recent studies suggest that high XIST expression levels correlate with a poor survival in various types of cancer [157]. Some of these studies propose that XIST acts as a competing endogenous RNA (ceRNA) [158][159], by depleting microRNAs. As a consequence, specific RNA targets cannot be degraded, which may lead to the dysregulation of downstream genes [160][161]. So far, both epigenetic and genetic changes have been observed in relation to the Xi of cancer cells, but whether these alterations are driving events that give a selective advantage to cancer cells is under debate. Nevertheless, evidence suggests that the Xi epigenetic status and XIST expression levels are potential cancer biomarkers as a readout for genomic instability or epigenomic changes. Therefore, understanding the factors and mechanisms that render and maintain the X chromosome inactive, both during embryonic development and in somatic cells during the maintenance phase of XCI, is of crucial importance.
3. Chromatin Modifiers That Act in XCI
The regulation of the X chromosome is controlled by chromatin modifiers that build up heterochromatin formation by deacetylating and methylating histone tails, finally leading to the DNA methylation of regulatory CpG islands (see Figure 2).
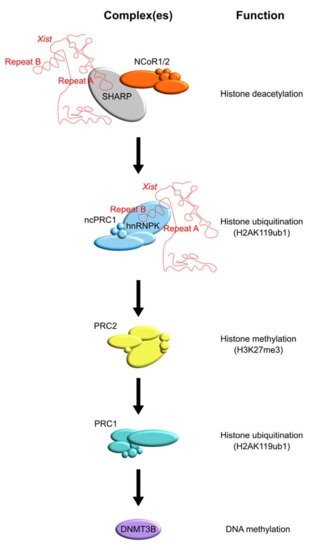
Specific enzymes that play a central role in XCI are HDACs, the PRC1 and PRC2 complexes, and DNMTs (see Table 1). Recently, the SHARP protein has been identified as a direct Xist interactor. This protein bridges Xist to HDACs allowing for histone deacetylation at the X chromosome.
References
- Gao, N.; Li, Y.; Li, J.; Gao, Z.; Yang, Z.; Li, Y.; Liu, H.; Fan, T. Long Non-Coding RNAs: The Regulatory Mechanisms, Research Strategies, and Future Directions in Cancers. Front. Oncol. 2020, 10, 598817.
- Statello, L.; Guo, C.J.; Chen, L.L.; Huarte, M. Gene regulation by long non-coding RNAs and its biological functions. Nat. Rev. Mol. Cell Biol. 2020.
- Thum, T.; Condorelli, G. Long noncoding RNAs and microRNAs in cardiovascular pathophysiology. Circ. Res. 2015, 116, 751–762.
- Chen, L.L. Linking Long Noncoding RNA Localization and Function. Trends Biochem. Sci. 2016, 41, 761–772.
- Liu, F.; Somarowthu, S.; Pyle, A.M. Visualizing the secondary and tertiary architectural domains of lncRNA RepA. Nat. Chem. Biol. 2017, 13, 282–289.
- Mercer, T.R.; Dinger, M.E.; Mattick, J.S. Long non-coding RNAs: Insights into functions. Nat. Rev. Genet. 2009, 10, 155–159.
- Kotake, Y.; Nakagawa, T.; Kitagawa, K.; Suzuki, S.; Liu, N.; Kitagawa, M.; Xiong, Y. Long non-coding RNA ANRIL is required for the PRC2 recruitment to and silencing of p15(INK4B) tumor suppressor gene. Oncogene 2011, 30, 1956–1962.
- Wang, K.C.; Chang, H.Y. Molecular mechanisms of long noncoding RNAs. Mol. Cell 2011, 43, 904–914.
- Li, Y.; Wang, Z.; Shi, H.; Li, H.; Li, L.; Fang, R.; Cai, X.; Liu, B.; Zhang, X.; Ye, L. HBXIP and LSD1 Scaffolded by lncRNA Hotair Mediate Transcriptional Activation by c-Myc. Cancer Res. 2016, 76, 293–304.
- Tripathi, V.; Ellis, J.D.; Shen, Z.; Song, D.Y.; Pan, Q.; Watt, A.T.; Freier, S.M.; Bennett, C.F.; Sharma, A.; Bubulya, P.A.; et al. The nuclear-retained noncoding RNA MALAT1 regulates alternative splicing by modulating SR splicing factor phosphorylation. Mol. Cell 2010, 39, 925–938.
- Zhang, H.M.; Yang, F.Q.; Chen, S.J.; Che, J.; Zheng, J.H. Upregulation of long non-coding RNA MALAT1 correlates with tumor progression and poor prognosis in clear cell renal cell carcinoma. Tumour Biol. 2015, 36, 2947–2955.
- Ma, K.X.; Wang, H.J.; Li, X.R.; Li, T.; Su, G.; Yang, P.; Wu, J.W. Long noncoding RNA MALAT1 associates with the malignant status and poor prognosis in glioma. Tumour Biol. 2015, 36, 3355–3359.
- Dong, Y.; Liang, G.; Yuan, B.; Yang, C.; Gao, R.; Zhou, X. MALAT1 promotes the proliferation and metastasis of osteosarcoma cells by activating the PI3K/Akt pathway. Tumour Biol. 2015, 36, 1477–1486.
- Cho, S.F.; Chang, Y.C.; Chang, C.S.; Lin, S.F.; Liu, Y.C.; Hsiao, H.H.; Chang, J.G.; Liu, T.C. MALAT1 long non-coding RNA is overexpressed in multiple myeloma and may serve as a marker to predict disease progression. BMC Cancer 2014, 14, 809.
- Zheng, H.T.; Shi, D.B.; Wang, Y.W.; Li, X.X.; Xu, Y.; Tripathi, P.; Gu, W.L.; Cai, G.X.; Cai, S.J. High expression of lncRNA MALAT1 suggests a biomarker of poor prognosis in colorectal cancer. Int. J. Clin. Exp. Pathol. 2014, 7, 3174–3181.
- Hirata, H.; Hinoda, Y.; Shahryari, V.; Deng, G.; Nakajima, K.; Tabatabai, Z.L.; Ishii, N.; Dahiya, R. Long Noncoding RNA MALAT1 Promotes Aggressive Renal Cell Carcinoma through Ezh2 and Interacts with miR-205. Cancer Res. 2015, 75, 1322–1331.
- Wu, X.S.; Wang, X.A.; Wu, W.G.; Hu, Y.P.; Li, M.L.; Ding, Q.; Weng, H.; Shu, Y.J.; Liu, T.Y.; Jiang, L.; et al. MALAT1 promotes the proliferation and metastasis of gallbladder cancer cells by activating the ERK/MAPK pathway. Cancer Biol. Ther. 2014, 15, 806–814.
- Wang, J.; Su, L.; Chen, X.; Li, P.; Cai, Q.; Yu, B.; Liu, B.; Wu, W.; Zhu, Z. MALAT1 promotes cell proliferation in gastric cancer by recruiting SF2/ASF. Biomed. Pharmacother. 2014, 68, 557–564.
- Wang, Y.; Xue, D.; Li, Y.; Pan, X.; Zhang, X.; Kuang, B.; Zhou, M.; Li, X.; Xiong, W.; Li, G.; et al. The Long Noncoding RNA MALAT-1 is A Novel Biomarker in Various Cancers: A Meta-analysis Based on the GEO Database and Literature. J. Cancer 2016, 7, 991–1001.
- Poirier, F.; Chan, C.T.; Timmons, P.M.; Robertson, E.J.; Evans, M.J.; Rigby, P.W. The murine H19 gene is activated during embryonic stem cell differentiation in vitro and at the time of implantation in the developing embryo. Development 1991, 113, 1105–1114.
- Gabory, A.; Jammes, H.; Dandolo, L. The H19 locus: Role of an imprinted non-coding RNA in growth and development. Bioessays 2010, 32, 473–480.
- Gabory, A.; Ripoche, M.A.; Yoshimizu, T.; Dandolo, L. The H19 gene: Regulation and function of a non-coding RNA. Cytogenet. Genome Res. 2006, 113, 188–193.
- Li, H.; Yu, B.; Li, J.; Su, L.; Yan, M.; Zhu, Z.; Liu, B. Overexpression of lncRNA H19 enhances carcinogenesis and metastasis of gastric cancer. Oncotarget 2014, 5, 2318–2329.
- Yang, F.; Bi, J.; Xue, X.; Zheng, L.; Zhi, K.; Hua, J.; Fang, G. Up-regulated long non-coding RNA H19 contributes to proliferation of gastric cancer cells. FEBS J. 2012, 279, 3159–3165.
- Yoshimura, H.; Matsuda, Y.; Yamamoto, M.; Kamiya, S.; Ishiwata, T. Expression and role of long non-coding RNA H19 in carcinogenesis. Front. Biosci. (Landmark Ed.) 2018, 23, 614–625.
- Xie, S.S.; Jin, J.; Xu, X.; Zhuo, W.; Zhou, T.H. Emerging roles of non-coding RNAs in gastric cancer: Pathogenesis and clinical implications. World J. Gastroenterol. 2016, 22, 1213–1223.
- Zhang, E.B.; Kong, R.; Yin, D.D.; You, L.H.; Sun, M.; Han, L.; Xu, T.P.; Xia, R.; Yang, J.S.; De, W.; et al. Long noncoding RNA ANRIL indicates a poor prognosis of gastric cancer and promotes tumor growth by epigenetically silencing of miR-99a/miR-449a. Oncotarget 2014, 5, 2276–2292.
- Yang, F.; Xue, X.; Zheng, L.; Bi, J.; Zhou, Y.; Zhi, K.; Gu, Y.; Fang, G. Long non-coding RNA GHET1 promotes gastric carcinoma cell proliferation by increasing c-Myc mRNA stability. FEBS J. 2014, 281, 802–813.
- Kong, R.; Zhang, E.B.; Yin, D.D.; You, L.H.; Xu, T.P.; Chen, W.M.; Xia, R.; Wan, L.; Sun, M.; Wang, Z.X.; et al. Long noncoding RNA PVT1 indicates a poor prognosis of gastric cancer and promotes cell proliferation through epigenetically regulating p15 and p16. Mol. Cancer 2015, 14, 82.
- Peng, W.; Wu, G.; Fan, H.; Wu, J.; Feng, J. Long noncoding RNA SPRY4-IT1 predicts poor patient prognosis and promotes tumorigenesis in gastric cancer. Tumour. Biol. 2015, 36, 6751–6758.
- Geng, Y.J.; Xie, S.L.; Li, Q.; Ma, J.; Wang, G.Y. Large intervening non-coding RNA HOTAIR is associated with hepatocellular carcinoma progression. J. Int. Med. Res. 2011, 39, 2119–2128.
- Kogo, R.; Shimamura, T.; Mimori, K.; Kawahara, K.; Imoto, S.; Sudo, T.; Tanaka, F.; Shibata, K.; Suzuki, A.; Komune, S.; et al. Long noncoding RNA HOTAIR regulates polycomb-dependent chromatin modification and is associated with poor prognosis in colorectal cancers. Cancer Res. 2011, 71, 6320–6326.
- Xu, Z.Y.; Yu, Q.M.; Du, Y.A.; Yang, L.T.; Dong, R.Z.; Huang, L.; Yu, P.F.; Cheng, X.D. Knockdown of long non-coding RNA HOTAIR suppresses tumor invasion and reverses epithelial-mesenchymal transition in gastric cancer. Int. J. Biol. Sci. 2013, 9, 587–597.
- Kim, K.; Jutooru, I.; Chadalapaka, G.; Johnson, G.; Frank, J.; Burghardt, R.; Kim, S.; Safe, S. HOTAIR is a negative prognostic factor and exhibits pro-oncogenic activity in pancreatic cancer. Oncogene 2013, 32, 1616–1625.
- Kawakami, T.; Okamoto, K.; Sugihara, H.; Hattori, T.; Reeve, A.E.; Ogawa, O.; Okada, Y. The roles of supernumerical X chromosomes and XIST expression in testicular germ cell tumors. J. Urol 2003, 169, 1546–1552.
- Ganesan, S.; Silver, D.P.; Greenberg, R.A.; Avni, D.; Drapkin, R.; Miron, A.; Mok, S.C.; Randrianarison, V.; Brodie, S.; Salstrom, J.; et al. BRCA1 supports XIST RNA concentration on the inactive X chromosome. Cell 2002, 111, 393–405.
- Pageau, G.J.; Hall, L.L.; Lawrence, J.B. BRCA1 does not paint the inactive X to localize XIST RNA but may contribute to broad changes in cancer that impact XIST and Xi heterochromatin. J. Cell Biochem. 2007, 100, 835–850.
- Richardson, A.L.; Wang, Z.C.; de Nicolo, A.; Lu, X.; Brown, M.; Miron, A.; Liao, X.; Iglehart, J.D.; Livingston, D.M.; Ganesan, S. X chromosomal abnormalities in basal-like human breast cancer. Cancer Cell 2006, 9, 121–132.
- Silver, D.P.; Dimitrov, S.D.; Feunteun, J.; Gelman, R.; Drapkin, R.; Lu, S.D.; Shestakova, E.; Velmurugan, S.; Denunzio, N.; Dragomir, S.; et al. Further evidence for BRCA1 communication with the inactive X chromosome. Cell 2007, 128, 991–1002.
- Sirchia, S.M.; Ramoscelli, L.; Grati, F.R.; Barbera, F.; Coradini, D.; Rossella, F.; Porta, G.; Lesma, E.; Ruggeri, A.; Radice, P.; et al. Loss of the inactive X chromosome and replication of the active X in BRCA1-defective and wild-type breast cancer cells. Cancer Res. 2005, 65, 2139–2146.
- Sirchia, S.M.; Tabano, S.; Monti, L.; Recalcati, M.P.; Gariboldi, M.; Grati, F.R.; Porta, G.; Finelli, P.; Radice, P.; Miozzo, M. Misbehaviour of XIST RNA in breast cancer cells. PLoS ONE 2009, 4, e5559.
- Vincent-Salomon, A.; Ganem-Elbaz, C.; Manie, E.; Raynal, V.; Sastre-Garau, X.; Stoppa-Lyonnet, D.; Stern, M.H.; Heard, E. X inactive-specific transcript RNA coating and genetic instability of the X chromosome in BRCA1 breast tumors. Cancer Res. 2007, 67, 5134–5140.
- Benoit, M.H.; Hudson, T.J.; Maire, G.; Squire, J.A.; Arcand, S.L.; Provencher, D.; Mes-Masson, A.M.; Tonin, P.N. Global analysis of chromosome X gene expression in primary cultuRes. of normal ovarian surface epithelial cells and epithelial ovarian cancer cell lines. Int. J. Oncol. 2007, 30, 5–17.
- Kawakami, T.; Zhang, C.; Taniguchi, T.; Kim, C.J.; Okada, Y.; Sugihara, H.; Hattori, T.; Reeve, A.E.; Ogawa, O.; Okamoto, K. Characterization of loss-of-inactive X in Klinefelter syndrome and female-derived cancer cells. Oncogene 2004, 23, 6163–6169.
- Sun, S.; del Rosario, B.C.; Szanto, A.; Ogawa, Y.; Jeon, Y.; Lee, J.T. Jpx RNA activates Xist by evicting CTCF. Cell 2013, 153, 1537–1551.
- Barakat, T.S.; Loos, F.; van Staveren, S.; Myronova, E.; Ghazvini, M.; Grootegoed, J.A.; Gribnau, J. The trans-activator RNF12 and cis-acting elements effectuate X chromosome inactivation independent of X-pairing. Mol. Cell 2014, 53, 965–978.
- Furlan, G.; Gutierrez-Hernandez, N.; Huret, C.; Galupa, R.; van Bemmel, J.G.; Romito, A.; Heard, E.; Morey, C.; Rougeulle, C. The Ftx Noncoding Locus Controls X Chromosome Inactivation Independently of Its RNA Products. Mol. Cell 2018, 70, 462–472.e468.
- Mutzel, V.; Okamoto, I.; Dunkel, I.; Saitou, M.; Giorgetti, L.; Heard, E.; Schulz, E.G. A symmetric toggle switch explains the onset of random X inactivation in different mammals. Nat. Struct. Mol. Biol. 2019, 26, 350–360.
- Wutz, A.; Rasmussen, T.P.; Jaenisch, R. Chromosomal silencing and localization are mediated by different domains of Xist RNA. Nat. Genet. 2002, 30, 167–174.
- Shi, Y.; Downes, M.; Xie, W.; Kao, H.Y.; Ordentlich, P.; Tsai, C.C.; Hon, M.; Evans, R.M. Sharp, an inducible cofactor that integrates nuclear receptor repression and activation. Genes Dev. 2001, 15, 1140–1151.
- Chu, C.; Zhang, Q.C.; da Rocha, S.T.; Flynn, R.A.; Bharadwaj, M.; Calabrese, J.M.; Magnuson, T.; Heard, E.; Chang, H.Y. Systematic discovery of Xist RNA binding proteins. Cell 2015, 161, 404–416.
- Moindrot, B.; Cerase, A.; Coker, H.; Masui, O.; Grijzenhout, A.; Pintacuda, G.; Schermelleh, L.; Nesterova, T.B.; Brockdorff, N. A Pooled shRNA Screen Identifies Rbm15, Spen, and Wtap as Factors Required for Xist RNA-Mediated Silencing. Cell Rep. 2015, 12, 562–572.
- Monfort, A.; di Minin, G.; Postlmayr, A.; Freimann, R.; Arieti, F.; Thore, S.; Wutz, A. Identification of Spen as a Crucial Factor for Xist Function through Forward Genetic Screening in Haploid Embryonic Stem Cells. Cell Rep. 2015, 12, 554–561.
- McHugh, C.A.; Chen, C.K.; Chow, A.; Surka, C.F.; Tran, C.; McDonel, P.; Pandya-Jones, A.; Blanco, M.; Burghard, C.; Moradian, A.; et al. The Xist lncRNA interacts directly with SHARP to silence transcription through HDAC3. Nature 2015, 521, 232–236.
- Dossin, F.; Pinheiro, I.; Zylicz, J.J.; Roensch, J.; Collombet, S.; le Saux, A.; Chelmicki, T.; Attia, M.; Kapoor, V.; Zhan, Y.; et al. SPEN integrates transcriptional and epigenetic control of X-inactivation. Nature 2020, 578, 455–460.
- Gendrel, A.V.; Apedaile, A.; Coker, H.; Termanis, A.; Zvetkova, I.; Godwin, J.; Tang, Y.A.; Huntley, D.; Montana, G.; Taylor, S.; et al. Smchd1-dependent and -independent pathways determine developmental dynamics of CpG island methylation on the inactive X chromosome. Dev. Cell 2012, 23, 265–279.
- Hansen, R.S.; Wijmenga, C.; Luo, P.; Stanek, A.M.; Canfield, T.K.; Weemaes, C.M.; Gartler, S.M. The DNMT3B DNA methyltransferase gene is mutated in the ICF immunodeficiency syndrome. Proc. Natl. Acad. Sci. USA 1999, 96, 14412–14417.
- Jiang, Y.L.; Rigolet, M.; Bourc’his, D.; Nigon, F.; Bokesoy, I.; Fryns, J.P.; Hulten, M.; Jonveaux, P.; Maraschio, P.; Megarbane, A.; et al. DNMT3B mutations and DNA methylation defect define two types of ICF syndrome. Hum. Mutat. 2005, 25, 56–63.
- Rechavi, E.; Lev, A.; Eyal, E.; Barel, O.; Kol, N.; Barhom, S.F.; Pode-Shakked, B.; Anikster, Y.; Somech, R.; Simon, A.J. A Novel Mutation in a Critical Region for the Methyl Donor Binding in DNMT3B Causes Immunodeficiency, Centromeric Instability, and Facial Anomalies Syndrome (ICF). J. Clin. Immunol. 2016, 36, 801–809.
- Shirohzu, H.; Kubota, T.; Kumazawa, A.; Sado, T.; Chijiwa, T.; Inagaki, K.; Suetake, I.; Tajima, S.; Wakui, K.; Miki, Y.; et al. Three novel DNMT3B mutations in Japanese patients with ICF syndrome. Am. J. Med. Genet. 2002, 112, 31–37.
- Xu, G.L.; Bestor, T.H.; Bourc’his, D.; Hsieh, C.L.; Tommerup, N.; Bugge, M.; Hulten, M.; Qu, X.; Russo, J.J.; Viegas-Pequignot, E. Chromosome instability and immunodeficiency syndrome caused by mutations in a DNA methyltransferase gene. Nature 1999, 402, 187–191.
- Zhao, S.G.; Chen, W.S.; Li, H.; Foye, A.; Zhang, M.; Sjostrom, M.; Aggarwal, R.; Playdle, D.; Liao, A.; Alumkal, J.J.; et al. The DNA methylation landscape of advanced prostate cancer. Nat. Genet. 2020, 52, 778–789.
- Herold, T.; Metzeler, K.H.; Vosberg, S.; Hartmann, L.; Jurinovic, V.; Opatz, S.; Konstandin, N.P.; Schneider, S.; Zellmeier, E.; Ksienzyk, B.; et al. Acute myeloid leukemia with del(9q) is characterized by frequent mutations of NPM1, DNMT3A, WT1 and low expression of TLE4. Genes Chromosomes Cancer 2017, 56, 75–86.
- van den Boogaard, M.L.; Lemmers, R.; Balog, J.; Wohlgemuth, M.; Auranen, M.; Mitsuhashi, S.; van der Vliet, P.J.; Straasheijm, K.R.; van den Akker, R.F.P.; Kriek, M.; et al. Mutations in DNMT3B Modify Epigenetic Repression of the D4Z4 Repeat and the Penetrance of Facioscapulohumeral Dystrophy. Am. J. Hum. Genet. 2016, 98, 1020–1029.
- Pintacuda, G.; Wei, G.; Roustan, C.; Kirmizitas, B.A.; Solcan, N.; Cerase, A.; Castello, A.; Mohammed, S.; Moindrot, B.; Nesterova, T.B.; et al. hnRNPK Recruits PCGF3/5-PRC1 to the Xist RNA B-Repeat to Establish Polycomb-Mediated Chromosomal Silencing. Mol. Cell 2017, 68, 955–969.e910.
- Nakamoto, M.Y.; Lammer, N.C.; Batey, R.T.; Wuttke, D.S. hnRNPK recognition of the B motif of Xist and other biological RNAs. Nucleic Acids Res. 2020, 48, 9320–9335.
- Gallardo, M.; Lee, H.J.; Zhang, X.; Bueso-Ramos, C.; Pageon, L.R.; McArthur, M.; Multani, A.; Nazha, A.; Manshouri, T.; Parker-Thornburg, J.; et al. hnRNP K Is a Haploinsufficient Tumor Suppressor that Regulates Proliferation and Differentiation Programs in Hematologic Malignancies. Cancer Cell 2015, 28, 486–499.
- Au, P.Y.B.; Goedhart, C.; Ferguson, M.; Breckpot, J.; Devriendt, K.; Wierenga, K.; Fanning, E.; Grange, D.K.; Graham, G.E.; Galarreta, C.; et al. Phenotypic spectrum of Au-Kline syndrome: A report of six new cases and review of the literature. Eur. J. Hum. Genet. 2018, 26, 1272–1281.
- Bastidas-Torres, A.N.; Cats, D.; Mei, H.; Szuhai, K.; Willemze, R.; Vermeer, M.H.; Tensen, C.P. Genomic analysis reveals recurrent deletion of JAK-STAT signaling inhibitors HNRNPK and SOCS1 in mycosis fungoides. Genes Chromosomes Cancer 2018, 57, 653–664.
- Lange, L.; Pagnamenta, A.T.; Lise, S.; Clasper, S.; Stewart, H.; Akha, E.S.; Quaghebeur, G.; Knight, S.J.; Keays, D.A.; Taylor, J.C.; et al. A de novo frameshift in HNRNPK causing a Kabuki-like syndrome with nodular heterotopia. Clin. Genet. 2016, 90, 258–262.
- Dentici, M.L.; Barresi, S.; Niceta, M.; Pantaleoni, F.; Pizzi, S.; Dallapiccola, B.; Tartaglia, M.; Digilio, M.C. Clinical spectrum of Kabuki-like syndrome caused by HNRNPK haploinsufficiency. Clin. Genet. 2018, 93, 401–407.
- Miyake, N.; Inaba, M.; Mizuno, S.; Shiina, M.; Imagawa, E.; Miyatake, S.; Nakashima, M.; Mizuguchi, T.; Takata, A.; Ogata, K.; et al. A case of atypical Kabuki syndrome arising from a novel missense variant in HNRNPK. Clin. Genet. 2017, 92, 554–555.
- Naarmann-de-Vries, I.S.; Sackmann, Y.; Klein, F.; Ostareck-Lederer, A.; Ostareck, D.H.; Jost, E.; Ehninger, G.; Brummendorf, T.H.; Marx, G.; Rollig, C.; et al. Characterization of acute myeloid leukemia with del(9q)—Impact of the genes in the minimally deleted region. Leuk Res. 2019, 76, 15–23.
- Maystadt, I.; Deprez, M.; Moortgat, S.; Benoit, V.; Karadurmus, D. A second case of Okamoto syndrome caused by HNRNPK mutation. Am. J. Med. Genet. A 2020, 182, 1537–1539.
- Okamoto, N. Okamoto syndrome has featuRes. overlapping with Au-Kline syndrome and is caused by HNRNPK mutation. Am. J. Med. Genet. A 2019, 179, 822–826.
- Di Carlo, V.; Mocavini, I.; di Croce, L. Polycomb complexes in normal and malignant hematopoiesis. J. Cell Biol. 2019, 218, 55–69.
- Almeida, M.; Pintacuda, G.; Masui, O.; Koseki, Y.; Gdula, M.; Cerase, A.; Brown, D.; Mould, A.; Innocent, C.; Nakayama, M.; et al. PCGF3/5-PRC1 initiates Polycomb recruitment in X chromosome inactivation. Science 2017, 356, 1081–1084.
- Palau, A.; Garz, A.K.; Diesch, J.; Zwick, A.; Malinverni, R.; Valero, V.; Lappin, K.; Casquero, R.; Lennartsson, A.; Zuber, J.; et al. Polycomb protein RING1A limits hematopoietic differentiation in myelodysplastic syndromes. Oncotarget 2017, 8, 115002–115017.
- Zhang, J.; Kalkum, M.; Chait, B.T.; Roeder, R.G. The N-CoR-HDAC3 nuclear receptor corepressor complex inhibits the JNK pathway through the integral subunit GPS2. Mol. Cell 2002, 9, 611–623.
- Underhill, C.; Qutob, M.S.; Yee, S.P.; Torchia, J. A novel nuclear receptor corepressor complex, N-CoR, contains components of the mammalian SWI/SNF complex and the corepressor KAP-1. J. Biol. Chem. 2000, 275, 40463–40470.
- Pareja, F.; Ferrando, L.; Lee, S.S.K.; Beca, F.; Selenica, P.; Brown, D.N.; Farmanbar, A.; da Cruz-Paula, A.; Vahdatinia, M.; Zhang, H.; et al. The genomic landscape of metastatic histologic special types of invasive breast cancer. NPJ Breast Cancer 2020, 6, 53.
- Nishi, A.; Numata, S.; Tajima, A.; Zhu, X.; Ito, K.; Saito, A.; Kato, Y.; Kinoshita, M.; Shimodera, S.; Ono, S.; et al. De novo non-synonymous TBL1XR1 mutation alters Wnt signaling activity. Sci. Rep. 2017, 7, 2887.
- Jung, H.; Yoo, H.Y.; Lee, S.H.; Shin, S.; Kim, S.C.; Lee, S.; Joung, J.G.; Nam, J.Y.; Ryu, D.; Yun, J.W.; et al. The mutational landscape of ocular marginal zone lymphoma identifies frequent alterations in TNFAIP3 followed by mutations in TBL1XR1 and CREBBP. Oncotarget 2017, 8, 17038–17049.
- Heinen, C.A.; Jongejan, A.; Watson, P.J.; Redeker, B.; Boelen, A.; Boudzovitch-Surovtseva, O.; Forzano, F.; Hordijk, R.; Kelley, R.; Olney, A.H.; et al. A specific mutation in TBL1XR1 causes Pierpont syndrome. J. Med. Genet. 2016, 53, 330–337.
- Pugh, T.J.; Weeraratne, S.D.; Archer, T.C.; Pomeranz-Krummel, D.A.; Auclair, D.; Bochicchio, J.; Carneiro, M.O.; Carter, S.L.; Cibulskis, K.; Erlich, R.L.; et al. Medulloblastoma exome sequencing uncovers subtype-specific somatic mutations. Nature 2012, 488, 106–110.
- Pons, L.; Cordier, M.P.; Labalme, A.; Till, M.; Louvrier, C.; Schluth-Bolard, C.; Lesca, G.; Edery, P.; Sanlaville, D. A new syndrome of intellectual disability with dysmorphism due to TBL1XR1 deletion. Am. J. Med. Genet. A 2015, 167A, 164–168.
- Stessman, H.A.; Xiong, B.; Coe, B.P.; Wang, T.; Hoekzema, K.; Fenckova, M.; Kvarnung, M.; Gerdts, J.; Trinh, S.; Cosemans, N.; et al. Targeted sequencing identifies 91 neurodevelopmental-disorder risk genes with autism and developmental-disability biases. Nat. Genet. 2017, 49, 515–526.
- O’Roak, B.J.; Vives, L.; Fu, W.; Egertson, J.D.; Stanaway, I.B.; Phelps, I.G.; Carvill, G.; Kumar, A.; Lee, C.; Ankenman, K.; et al. Multiplex targeted sequencing identifies recurrently mutated genes in autism spectrum disorders. Science 2012, 338, 1619–1622.
- Saitsu, H.; Tohyama, J.; Walsh, T.; Kato, M.; Kobayashi, Y.; Lee, M.; Tsurusaki, Y.; Miyake, N.; Goto, Y.; Nishino, I.; et al. A girl with West syndrome and autistic featuRes. harboring a de novo TBL1XR1 mutation. J. Hum. Genet. 2014, 59, 581–583.
- Riehmer, V.; Erger, F.; Herkenrath, P.; Seland, S.; Jackels, M.; Wiater, A.; Heller, R.; Beck, B.B.; Netzer, C. A heritable microduplication encompassing TBL1XR1 causes a genomic sister-disorder for the 3q26.32 microdeletion syndrome. Am. J. Med. Genet. A 2017, 173, 2132–2138.
- Ivanov, I.; Lo, K.C.; Hawthorn, L.; Cowell, J.K.; Ionov, Y. Identifying candidate colon cancer tumor suppressor genes using inhibition of nonsense-mediated mRNA decay in colon cancer cells. Oncogene 2007, 26, 2873–2884.
- Ciriello, G.; Sinha, R.; Hoadley, K.A.; Jacobsen, A.S.; Reva, B.; Perou, C.M.; Sander, C.; Schultz, N. The molecular diversity of Luminal A breast tumors. Breast Cancer Res. Treat 2013, 141, 409–420.
- Stephens, P.J.; Tarpey, P.S.; Davies, H.; van Loo, P.; Greenman, C.; Wedge, D.C.; Nik-Zainal, S.; Martin, S.; Varela, I.; Bignell, G.R.; et al. The landscape of cancer genes and mutational processes in breast cancer. Nature 2012, 486, 400–404.
- Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature 2012, 490, 61–70.
- Xu, X.R.; Huang, J.; Xu, Z.G.; Qian, B.Z.; Zhu, Z.D.; Yan, Q.; Cai, T.; Zhang, X.; Xiao, H.S.; Qu, J.; et al. Insight into hepatocellular carcinogenesis at transcriptome level by comparing gene expression profiles of hepatocellular carcinoma with those of corresponding noncancerous liver. Proc. Natl. Acad. Sci. USA 2001, 98, 15089–15094.
- Awad, S.; Al-Dosari, M.S.; Al-Yacoub, N.; Colak, D.; Salih, M.A.; Alkuraya, F.S.; Poizat, C. Mutation in PHC1 implicates chromatin remodeling in primary microcephaly pathogenesis. Hum. Mol. Genet. 2013, 22, 2200–2213.
- Deshpande, A.M.; Akunowicz, J.D.; Reveles, X.T.; Patel, B.B.; Saria, E.A.; Gorlick, R.G.; Naylor, S.L.; Leach, R.J.; Hansen, M.F. PHC3, a component of the hPRC-H complex, associates with E2F6 during G0 and is lost in osteosarcoma tumors. Oncogene 2007, 26, 1714–1722.
- Chen, Z.Y.; Sun, S.X.; Zhu, S.X.; Bu, J. Identification of the Roles of Chromobox Family Members in Gastric Cancer: A Study Based on Multiple Datasets. Biomed. Res. Int. 2020, 2020, 5306509.
- Deciphering Developmental Disorders, S. Large-scale discovery of novel genetic causes of developmental disorders. Nature 2015, 519, 223–228.
- Lee, J.H.; Zhao, X.M.; Yoon, I.; Lee, J.Y.; Kwon, N.H.; Wang, Y.Y.; Lee, K.M.; Lee, M.J.; Kim, J.; Moon, H.G.; et al. Integrative analysis of mutational and transcriptional profiles reveals driver mutations of metastatic breast cancers. Cell Discov. 2016, 2, 16025.
- Schulte, I.; Batty, E.M.; Pole, J.C.; Blood, K.A.; Mo, S.; Cooke, S.L.; Ng, C.; Howe, K.L.; Chin, S.F.; Brenton, J.D.; et al. Structural analysis of the genome of breast cancer cell line ZR-75-30 identifies twelve expressed fusion genes. BMC Genom. 2012, 13, 719.
- Turnpenny, P.D.; Wright, M.J.; Sloman, M.; Caswell, R.; van Essen, A.J.; Gerkes, E.; Pfundt, R.; White, S.M.; Shaul-Lotan, N.; Carpenter, L.; et al. Missense Mutations of the Pro65 Residue of PCGF2 Cause a Recognizable Syndrome Associated with Craniofacial, Neurological, Cardiovascular, and Skeletal Features. Am. J. Hum. Genet. 2018, 103, 786–793.
- Zhang, L.; Zhou, Y.; Cheng, C.; Cui, H.; Cheng, L.; Kong, P.; Wang, J.; Li, Y.; Chen, W.; Song, B.; et al. Genomic analyses reveal mutational signatuRes. and frequently altered genes in esophageal squamous cell carcinoma. Am. J. Hum. Genet. 2015, 96, 597–611.
- Biason-Lauber, A.; Konrad, D.; Meyer, M.; DeBeaufort, C.; Schoenle, E.J. Ovaries and female phenotype in a girl with 46,XY karyotype and mutations in the CBX2 gene. Am. J. Hum. Genet. 2009, 84, 658–663.
- Ferreira, B.I.; Garcia, J.F.; Suela, J.; Mollejo, M.; Camacho, F.I.; Carro, A.; Montes, S.; Piris, M.A.; Cigudosa, J.C. Comparative genome profiling across subtypes of low-grade B-cell lymphoma identifies type-specific and common aberrations that target genes with a role in B-cell neoplasia. Haematologica 2008, 93, 670–679.
- da Rocha, S.T.; Boeva, V.; Escamilla-Del-Arenal, M.; Ancelin, K.; Granier, C.; Matias, N.R.; Sanulli, S.; Chow, J.; Schulz, E.; Picard, C.; et al. Jarid2 Is Implicated in the Initial Xist-Induced Targeting of PRC2 to the Inactive X Chromosome. Mol. Cell 2014, 53, 301–316.
- Cancer Genome Atlas Research Network; Ley, T.J.; Miller, C.; Ding, L.; Raphael, B.J.; Mungall, A.J.; Robertson, A.; Hoadley, K.; Triche, T.J., Jr.; Laird, P.W.; et al. Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N. Engl. J. Med. 2013, 368, 2059–2074.
- Yoshida, K.; Toki, T.; Okuno, Y.; Kanezaki, R.; Shiraishi, Y.; Sato-Otsubo, A.; Sanada, M.; Park, M.J.; Terui, K.; Suzuki, H.; et al. The landscape of somatic mutations in Down syndrome-related myeloid disorders. Nat. Genet. 2013, 45, 1293–1299.
- Haferlach, T.; Nagata, Y.; Grossmann, V.; Okuno, Y.; Bacher, U.; Nagae, G.; Schnittger, S.; Sanada, M.; Kon, A.; Alpermann, T.; et al. Landscape of genetic lesions in 944 patients with myelodysplastic syndromes. Leukemia 2014, 28, 241–247.
- Bejar, R.; Stevenson, K.; Abdel-Wahab, O.; Galili, N.; Nilsson, B.; Garcia-Manero, G.; Kantarjian, H.; Raza, A.; Levine, R.L.; Neuberg, D.; et al. Clinical effect of point mutations in myelodysplastic syndromes. N. Engl. J. Med. 2011, 364, 2496–2506.
- Lindsley, R.C.; Mar, B.G.; Mazzola, E.; Grauman, P.V.; Shareef, S.; Allen, S.L.; Pigneux, A.; Wetzler, M.; Stuart, R.K.; Erba, H.P.; et al. Acute myeloid leukemia ontogeny is defined by distinct somatic mutations. Blood 2015, 125, 1367–1376.
- Papaemmanuil, E.; Gerstung, M.; Malcovati, L.; Tauro, S.; Gundem, G.; Van Loo, P.; Yoon, C.J.; Ellis, P.; Wedge, D.C.; Pellagatti, A.; et al. Clinical and biological implications of driver mutations in myelodysplastic syndromes. Blood 2013, 122, 3616–3627.
- Ernst, T.; Chase, A.J.; Score, J.; Hidalgo-Curtis, C.E.; Bryant, C.; Jones, A.V.; Waghorn, K.; Zoi, K.; Ross, F.M.; Reiter, A.; et al. Inactivating mutations of the histone methyltransferase gene EZH2 in myeloid disorders. Nat. Genet. 2010, 42, 722–726.
- Nikoloski, G.; Langemeijer, S.M.; Kuiper, R.P.; Knops, R.; Massop, M.; Tonnissen, E.R.; van der Heijden, A.; Scheele, T.N.; Vandenberghe, P.; de Witte, T.; et al. Somatic mutations of the histone methyltransferase gene EZH2 in myelodysplastic syndromes. Nat. Genet. 2010, 42, 665–667.
- Lohr, J.G.; Stojanov, P.; Lawrence, M.S.; Auclair, D.; Chapuy, B.; Sougnez, C.; Cruz-Gordillo, P.; Knoechel, B.; Asmann, Y.W.; Slager, S.L.; et al. Discovery and prioritization of somatic mutations in diffuse large B-cell lymphoma (DLBCL) by whole-exome sequencing. Proc. Natl. Acad. Sci. USA 2012, 109, 3879–3884.
- Morin, R.D.; Mendez-Lago, M.; Mungall, A.J.; Goya, R.; Mungall, K.L.; Corbett, R.D.; Johnson, N.A.; Severson, T.M.; Chiu, R.; Field, M.; et al. Frequent mutation of histone-modifying genes in non-Hodgkin lymphoma. Nature 2011, 476, 298–303.
- Bodor, C.; Grossmann, V.; Popov, N.; Okosun, J.; O’Riain, C.; Tan, K.; Marzec, J.; Araf, S.; Wang, J.; Lee, A.M.; et al. EZH2 mutations are frequent and represent an early event in follicular lymphoma. Blood 2013, 122, 3165–3168.
- Kiel, M.J.; Velusamy, T.; Rolland, D.; Sahasrabuddhe, A.A.; Chung, F.; Bailey, N.G.; Schrader, A.; Li, B.; Li, J.Z.; Ozel, A.B.; et al. Integrated genomic sequencing reveals mutational landscape of T-cell prolymphocytic leukemia. Blood 2014, 124, 1460–1472.
- Morin, R.D.; Johnson, N.A.; Severson, T.M.; Mungall, A.J.; An, J.; Goya, R.; Paul, J.E.; Boyle, M.; Woolcock, B.W.; Kuchenbauer, F.; et al. Somatic mutations altering EZH2 (Tyr641) in follicular and diffuse large B-cell lymphomas of germinal-center origin. Nat. Genet. 2010, 42, 181–185.
- Zhang, J.; Ding, L.; Holmfeldt, L.; Wu, G.; Heatley, S.L.; Payne-Turner, D.; Easton, J.; Chen, X.; Wang, J.; Rusch, M.; et al. The genetic basis of early T-cell precursor acute lymphoblastic leukaemia. Nature 2012, 481, 157–163.
- Ma, X.; Liu, Y.; Liu, Y.; Alexandrov, L.B.; Edmonson, M.N.; Gawad, C.; Zhou, X.; Li, Y.; Rusch, M.C.; Easton, J.; et al. Pan-cancer genome and transcriptome analyses of 1,699 paediatric leukaemias and solid tumours. Nature 2018, 555, 371–376.
- Seki, M.; Kimura, S.; Isobe, T.; Yoshida, K.; Ueno, H.; Nakajima-Takagi, Y.; Wang, C.; Lin, L.; Kon, A.; Suzuki, H.; et al. Recurrent SPI1 (PU.1) fusions in high-risk pediatric T cell acute lymphoblastic leukemia. Nat. Genet. 2017, 49, 1274–1281.
- Ntziachristos, P.; Tsirigos, A.; van Vlierberghe, P.; Nedjic, J.; Trimarchi, T.; Flaherty, M.S.; Ferres-Marco, D.; da Ros, V.; Tang, Z.; Siegle, J.; et al. Genetic inactivation of the polycomb repressive complex 2 in T cell acute lymphoblastic leukemia. Nat. Med. 2012, 18, 298–301.
- Score, J.; Hidalgo-Curtis, C.; Jones, A.V.; Winkelmann, N.; Skinner, A.; Ward, D.; Zoi, K.; Ernst, T.; Stegelmann, F.; Dohner, K.; et al. Inactivation of polycomb repressive complex 2 components in myeloproliferative and myelodysplastic/myeloproliferative neoplasms. Blood 2012, 119, 1208–1213.
- Iwata, S.; Takenobu, H.; Kageyama, H.; Koseki, H.; Ishii, T.; Nakazawa, A.; Tatezaki, S.; Nakagawara, A.; Kamijo, T. Polycomb group molecule PHC3 regulates polycomb complex composition and prognosis of osteosarcoma. Cancer Sci. 2010, 101, 1646–1652.
- Brecqueville, M.; Cervera, N.; Adelaide, J.; Rey, J.; Carbuccia, N.; Chaffanet, M.; Mozziconacci, M.J.; Vey, N.; Birnbaum, D.; Gelsi-Boyer, V.; et al. Mutations and deletions of the SUZ12 polycomb gene in myeloproliferative neoplasms. Blood Cancer J. 2011, 1, e33.
- Gao, S.B.; Sun, S.L.; Zheng, Q.L.; Zhang, L.; Zhu, Y.; Jin, G.H.; Xue, L.X. Genetic alteration and misexpression of Polycomb group genes in hepatocellular carcinoma. Am. J. Cancer Res. 2015, 5, 2969–2979.
- Carter, A.C.; Xu, J.; Nakamoto, M.Y.; Wei, Y.; Zarnegar, B.J.; Shi, Q.; Broughton, J.P.; Ransom, R.C.; Salhotra, A.; Nagaraja, S.D.; et al. Spen links RNA-mediated endogenous retrovirus silencing and X chromosome inactivation. Elife 2020, 9.
- Minajigi, A.; Froberg, J.; Wei, C.; Sunwoo, H.; Kesner, B.; Colognori, D.; Lessing, D.; Payer, B.; Boukhali, M.; Haas, W.; et al. Chromosomes. A comprehensive Xist interactome reveals cohesin repulsion and an RNA-directed chromosome conformation. Science 2015, 349.
- Trotman, J.B.; Lee, D.M.; Cherney, R.E.; Kim, S.O.; Inoue, K.; Schertzer, M.D.; Bischoff, S.R.; Cowley, D.O.; Calabrese, J.M. Elements at the 5’ end of Xist harbor SPEN-independent transcriptional antiterminator activity. Nucleic Acids Res. 2020, 48, 10500–10517.
- Stephens, P.J.; Davies, H.R.; Mitani, Y.; van Loo, P.; Shlien, A.; Tarpey, P.S.; Papaemmanuil, E.; Cheverton, A.; Bignell, G.R.; Butler, A.P.; et al. Whole exome sequencing of adenoid cystic carcinoma. J. Clin. Investig. 2013, 123, 2965–2968.
- Hansen, M.H.; Cedile, O.; Blum, M.K.; Hansen, S.V.; Ebbesen, L.H.; Bentzen, H.H.N.; Thomassen, M.; Kruse, T.A.; Kavan, S.; Kjeldsen, E.; et al. Molecular characterization of sorted malignant B cells from patients clinically identified with mantle cell lymphoma. Exp. Hematol. 2020, 84, 7–18.e12.
- Jain, P.; Zhang, S.; Kanagal-Shamanna, R.; Ok, C.Y.; Nomie, K.; Gonzalez, G.N.; Gonzalez-Pagan, O.; Hill, H.A.; Lee, H.J.; Fayad, L.; et al. Genomic profiles and clinical outcomes of de novo blastoid/pleomorphic MCL are distinct from those of transformed MCL. Blood Adv. 2020, 4, 1038–1050.
- Hill, H.A.; Qi, X.; Jain, P.; Nomie, K.; Wang, Y.; Zhou, S.; Wang, M.L. Genetic mutations and featuRes. of mantle cell lymphoma: A systematic review and meta-analysis. Blood Adv. 2020, 4, 2927–2938.
- Hartert, K.T.; Wenzl, K.; Krull, J.E.; Manske, M.; Sarangi, V.; Asmann, Y.; Larson, M.C.; Maurer, M.J.; Slager, S.; Macon, W.R.; et al. Targeting of inflammatory pathways with R2CHOP in high-risk DLBCL. Leukemia 2020.
- Parry, M.; Rose-Zerilli, M.J.; Gibson, J.; Ennis, S.; Walewska, R.; Forster, J.; Parker, H.; Davis, Z.; Gardiner, A.; Collins, A.; et al. Whole exome sequencing identifies novel recurrently mutated genes in patients with splenic marginal zone lymphoma. PLoS ONE 2013, 8, e83244.
- Rossi, D.; Trifonov, V.; Fangazio, M.; Bruscaggin, A.; Rasi, S.; Spina, V.; Monti, S.; Vaisitti, T.; Arruga, F.; Fama, R.; et al. The coding genome of splenic marginal zone lymphoma: Activation of NOTCH2 and other pathways regulating marginal zone development. J. Exp. Med. 2012, 209, 1537–1551.
- Ma, H.; Song, B.; Guo, S.; Li, G.; Jin, G. Identification of germline and somatic mutations in pancreatic adenosquamous carcinoma using whole exome sequencing. Cancer Biomark 2020, 27, 389–397.
- Wang, T.; Hoekzema, K.; Vecchio, D.; Wu, H.; Sulovari, A.; Coe, B.P.; Gillentine, M.A.; Wilfert, A.B.; Perez-Jurado, L.A.; Kvarnung, M.; et al. Large-scale targeted sequencing identifies risk genes for neurodevelopmental disorders. Nat. Commun. 2020, 11, 4932.
- Zylicz, J.J.; Bousard, A.; Zumer, K.; Dossin, F.; Mohammad, E.; da Rocha, S.T.; Schwalb, B.; Syx, L.; Dingli, F.; Loew, D.; et al. The Implication of Early Chromatin Changes in X Chromosome Inactivation. Cell 2019, 176, 182–197.e123.
- Blackledge, N.P.; Farcas, A.M.; Kondo, T.; King, H.W.; McGouran, J.F.; Hanssen, L.L.P.; Ito, S.; Cooper, S.; Kondo, K.; Koseki, Y.; et al. Variant PRC1 complex-dependent H2A ubiquitylation drives PRC2 recruitment and polycomb domain formation. Cell 2014, 157, 1445–1459.
- Kalb, R.; Latwiel, S.; Baymaz, H.I.; Jansen, P.W.; Muller, C.W.; Vermeulen, M.; Muller, J. Histone H2A monoubiquitination promotes histone H3 methylation in Polycomb repression. Nat. Struct. Mol. Biol. 2014, 21, 569–571.
- Cooper, S.; Grijzenhout, A.; Underwood, E.; Ancelin, K.; Zhang, T.; Nesterova, T.B.; Anil-Kirmizitas, B.; Bassett, A.; Kooistra, S.M.; Agger, K.; et al. Jarid2 binds mono-ubiquitylated H2A lysine 119 to mediate crosstalk between Polycomb complexes PRC1 and PRC2. Nat. Commun. 2016, 7, 13661.
- Min, J.; Zhang, Y.; Xu, R.M. Structural basis for specific binding of Polycomb chromodomain to histone H3 methylated at Lys 27. Genes Dev. 2003, 17, 1823–1828.
- Gao, Z.; Zhang, J.; Bonasio, R.; Strino, F.; Sawai, A.; Parisi, F.; Kluger, Y.; Reinberg, D. PCGF homologs, CBX proteins, and RYBP define functionally distinct PRC1 family complexes. Mol. Cell 2012, 45, 344–356.
- Wang, L.; Brown, J.L.; Cao, R.; Zhang, Y.; Kassis, J.A.; Jones, R.S. Hierarchical recruitment of polycomb group silencing complexes. Mol. Cell 2004, 14, 637–646.
- Chaligne, R.; Heard, E. X-chromosome inactivation in development and cancer. FEBS Lett. 2014, 588, 2514–2522.
- Pageau, G.J.; Hall, L.L.; Ganesan, S.; Livingston, D.M.; Lawrence, J.B. The disappearing Barr body in breast and ovarian cancers. Nat. Rev. Cancer 2007, 7, 628–633.
- Brown, C.J.; Willard, H.F. The human X-inactivation centre is not required for maintenance of X-chromosome inactivation. Nature 1994, 368, 154–156.
- Csankovszki, G.; Panning, B.; Bates, B.; Pehrson, J.R.; Jaenisch, R. Conditional deletion of Xist disrupts histone macroH2A localization but not maintenance of X inactivation. Nat. Genet. 1999, 22, 323–324.
- Ross, M.T.; Grafham, D.V.; Coffey, A.J.; Scherer, S.; McLay, K.; Muzny, D.; Platzer, M.; Howell, G.R.; Burrows, C.; Bird, C.P.; et al. The DNA sequence of the human X chromosome. Nature 2005, 434, 325–337.
- Liu, R.; Kain, M.; Wang, L. Inactivation of X-linked tumor suppressor genes in human cancer. Future Oncol. 2012, 8, 463–481.
- Spatz, A.; Borg, C.; Feunteun, J. X-chromosome genetics and human cancer. Nat. Rev. Cancer 2004, 4, 617–629.
- Yildirim, E.; Kirby, J.E.; Brown, D.E.; Mercier, F.E.; Sadreyev, R.I.; Scadden, D.T.; Lee, J.T. Xist RNA is a potent suppressor of hematologic cancer in mice. Cell 2013, 152, 727–742.
- Chaligne, R.; Popova, T.; Mendoza-Parra, M.A.; Saleem, M.A.; Gentien, D.; Ban, K.; Piolot, T.; Leroy, O.; Mariani, O.; Gronemeyer, H.; et al. The inactive X chromosome is epigenetically unstable and transcriptionally labile in breast cancer. Genome Res. 2015, 25, 488–503.
- Jager, N.; Schlesner, M.; Jones, D.T.; Raffel, S.; Mallm, J.P.; Junge, K.M.; Weichenhan, D.; Bauer, T.; Ishaque, N.; Kool, M.; et al. Hypermutation of the inactive X chromosome is a frequent event in cancer. Cell 2013, 155, 567–581.
- Yin, S.; Dou, J.; Yang, G.; Chen, F. Long non-coding RNA XIST expression as a prognostic factor in human cancers: A meta-analysis. Int. J. Biol. Markers 2019, 34, 327–333.
- Salmena, L.; Poliseno, L.; Tay, Y.; Kats, L.; Pandolfi, P.P. A ceRNA hypothesis: The Rosetta Stone of a hidden RNA language? Cell 2011, 146, 353–358.
- Thomson, D.W.; Dinger, M.E. Endogenous microRNA sponges: Evidence and controversy. Nat. Rev. Genet. 2016, 17, 272–283.
- Zhang, Y.; Xu, Y.; Feng, L.; Li, F.; Sun, Z.; Wu, T.; Shi, X.; Li, J.; Li, X. Comprehensive characterization of lncRNA-mRNA related ceRNA network across 12 major cancers. Oncotarget 2016, 7, 64148–64167.
- Madhi, H.; Kim, M.H. Beyond X-Chromosome Inactivation: The Oncogenic Facet of XIST in Human Cancers. Biomed. Sci. Lett. 2019, 25, 113–122.




