
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Fumi Miyagawa | + 988 word(s) | 988 | 2021-04-23 11:32:20 | | | |
| 2 | Catherine Yang | -29 word(s) | 959 | 2021-05-18 11:22:46 | | |
Video Upload Options
Drug-induced hypersensitivity syndrome/drug reaction with eosinophilia and systemic symptoms (DIHS/DRESS) is a severe type of adverse drug eruption associated with multiorgan involvement and the reactivation of human herpesvirus 6, which arises after prolonged exposure to a limited number of drugs. Severe complications, some of which are related to cytomegalovirus reactivation, can be fatal. DIHS/DRESS is distinct from other drug reactions, as it involves herpes virus reactivation and can lead to the subsequent development of autoimmune diseases. The current consensus on the pathogenesis of DIHS/DRESS is that it occurs as a result of complex interactions between several herpesviruses and comprehensive immune responses, including drug-specific immune responses and antiviral immune responses. Although our understanding of the pathophysiology of DIHS/DRESS has evolved considerably over the last decade, the precise pathomechanisms of this complex disease remain largely unknown. This entry describes the clinical features of DIHS/DRESS, including the associated complications and sequelae.
1. Clinical Symptoms
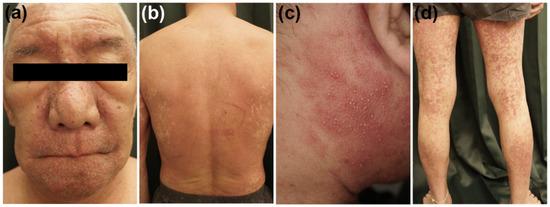
DIHS/DRESS is characterized by a delayed onset, i.e., it usually occurs 3 weeks to 3 months after the initiation of treatment with the causative drug, and the deterioration of clinical symptoms after the cessation of such treatment [1][2][3]. These features are peculiar to DIHS/DRESS and are not seen in other types of drug eruptions. A limited number of drugs, including carbamazepine, phenytoin, phenobarbital, zonisamide, lamotrigine, mexiletine, dapsone, sulfasalazine, minocycline, allopurinol, and vancomycin, are implicated cause in most cases of DIHS/DRESS [4][3][5]. HHV-6 reactivation has also been demonstrated to be involved in DIHS/DRESS, and it generally occurs 2–3 weeks after the onset of rashes in DIHS/DRESS [6][7][8]. The symptoms of DIHS/DRESS include rash development associated with facial and periorbital edema (Figure 1a), lymphadenopathy, and fever [3]. DIHS/DRESS typically begins with a fever and maculopapular eruptions, which often generalize into severe exfoliative dermatitis or erythroderma (Figure 1b). Pinhead-sized pustules (Figure 1c), blisters, and purpura (Figure 1d) are occasionally present. There is usually no mucocutaneous involvement. The laboratory findings of DIHS/DRESS include leukocytosis, eosinophilia, atypical lymphocytosis, and liver abnormalities, which can vary in severity. Systemic involvement includes hepatitis and/or interstitial pneumonia. Interstitial nephritis or carditis may also be found. The typical clinical course of DIHS/DRESS involves a bimodal distribution of clinical symptoms, i.e., they are most severe at onset and 2–3 weeks after onset (at the time of HHV-6 reactivation). However, in severe cases symptoms can continue to deteriorate or several flare-ups can be seen, even weeks after the discontinuation of the causative drug. Some patients develop autoimmune diseases after the resolution of DIHS/DRESS (Figure 1). The mortality rate of DIHS/DRESS ranges from approximately 10–20% [3][5], and the risk of death is correlated with the degree of hepatic or renal involvement [3]. The complications and sequelae observed in DIHS/DRESS are detailed below.
2. Complications
3. Sequelae (Autoimmune Disease)
The development of autoimmune diseases, such as autoimmune thyroiditis, type I diabetes mellitus, and autoimmune hemolytic anemia, can occur as a late complication of DIHS/DRESS, i.e., several months to years after the resolution of DIHS/DRESS [13][14]. The overall cumulative incidence of long-term sequelae in DIHS/DRESS has been reported to be 11.5% [13]. A study of the sequelae of 145 DIHS/DRESS patients conducted by the Asian Research Committee on Severe Cutaneous Adverse Reactions (ASCAR) revealed that the following autoimmune diseases can newly develop after the resolution of DIHS/DRESS: thyroid diseases, diabetes mellitus, systemic lupus erythematosus, arthritis, alopecia, and vitiligo [14]. The development of other autoimmune diseases, including sclerodermoid graft-versus-host disease-like lesions [15] and chronic inflammatory demyelinating polyneuropathy [16], has also been reported in sporadic case reports. The subsequent development of type III polyglandular autoimmune syndrome has also been reported in a 6-year-old boy who developed multiple autoimmune diseases, including thyroiditis, type I diabetes, alopecia, vitiligo, and uveitis due to Vogt-Koyanagi-Harada disease after the resolution of DIHS/DRESS [17]. The immunological mechanism underlying the development of autoimmune diseases in DIHS/DRESS currently remains unknown. A recent report suggested that higher plasma interferon (IFN)-γ-induced protein (IP)-10 levels are associated with the development of long-term sequelae in patients with DIHS/DRESS [18]. IP-10 has already been known to be associated with idiopathic autoimmune diseases, such as type 1 diabetes, thyroiditis, vitiligo, and alopecia areata [18].
References
- Shiohara, T.; Iijima, M.; Ikezawa, Z.; Hashimoto, K. The diagnosis of a DRESS syndrome has been sufficiently established on the basis of typical clinical features and viral reactivations. Br. J. Dermatol. 2007, 156, 1083–1084.
- Kardaun, S.; Sidoroff, A.; Valeyrie-Allanore, L.; Halevy, S.; Davidovici, B.; Mockenhaupt, M.; Roujeau, J. Variability in the clinical pattern of cutaneous side-effects of drugs with systemic symptoms: Does a DRESS syndrome really exist? Br. J. Dermatol. 2007, 156, 609–611.
- Shiohara, T.; Inaoka, M.; Kano, Y. Drug-induced Hypersensitivity Syndrome(DIHS): A Reaction Induced by a Complex Interplay among Herpesviruses and Antiviral and Antidrug Immune Responses. Allergol. Int. 2006, 55, 1–8.
- Shiohara, T.; Mizukawa, Y. Drug-induced hypersensitivity syndrome (DiHS)/drug reaction with eosinophilia and systemic symptoms (DRESS): An update in 2019. Allergol. Int. 2019, 68, 301–308.
- Kardaun, S.H.; Sekula, P.; Valeyrie-Allanore, L.; Liss, Y.; Chu, C.Y.; Creamer, D.; Sidoroff, A.; Naldi, L.; Mockenhaupt, M.; Roujeau, J.C.; et al. Drug reaction with eosinophilia and systemic symptoms (DRESS): An original multisystem adverse drug reaction. Results from the prospective RegiSCAR study. Br. J. Dermatol. 2013, 169, 1071–1080.
- Descamps, V.; Bouscarat, F.; Laglenne, S.; Aslangul, E.; Veber, B.; Saraux, J.-L.; Grange, M.; Grossin, M.; Navratil, E.; Crickx, B.; et al. Human herpesvirus 6 infection associated with anticonvulsant hypersensitivity syndrome and reactive haemophagocytic syndrome. Br. J. Dermatol. 1997, 137, 605–608.
- Suzuki, Y.; Inagi, R.; Aono, T.; Yamanishi, K.; Shiohara, T. Human Herpesvirus 6 Infection as a Risk Factor for the Development of Severe Drug-Induced Hypersensitivity Syndrome. Arch. Dermatol. 1998, 134, 1108–1112.
- Tohyama, M.; Yahata, Y.; Yasukawa, M.; Inagi, R.; Urano, Y.; Yamanishi, K.; Hashimoto, K. Severe hypersensitivity syndrome due to sulfasala-zine associated with reactivation of human herpesvirus 6. Arch. Dermatol. 1998, 134, 1113–1117.
- Bourgeois, G.P.; Cafardi, J.A.; Groysman, V.; Hughey, L.C. A review of DRESS-associated myocarditis. J. Am. Acad. Dermatol. 2012, 66, e229–e236.
- Asano, Y.; Kagawa, H.; Kano, Y.; Shiohara, T. Cytomegalovirus disease during severe drug eruptions: Report of 2 cases and ret-rospective study of 18 patients with drug-induced hypersensitivity syndrome. Arch. Dermatol. 2009, 145, 1030–1036.
- Mizukawa, Y.; Hirahara, K.; Kano, Y.; Shiohara, T. Drug-induced hypersensitivity syndrome/drug reaction with eosinophilia and systemic symptoms severity score: A useful tool for assessing disease severity and predicting fatal cytomegalovirus disease. J. Am. Acad. Dermatol. 2019, 80, 670–678.e2.
- Chen, Y.C.; Chiang, H.H.; Cho, Y.T.; Chang, C.Y.; Chen, K.L.; Yang, C.W.; Lee, Y.H.; Chu, C.Y. Human herpes virus reactivations and dynamic cytokine profiles in patients with cutaneous adverse drug reactions—A prospective comparative study. Allergy 2015, 70, 568–575.
- Chen, Y.C.; Chang, C.Y.; Cho, Y.T.; Chiu, H.C.; Chu, C.Y. Long-term sequelae of drug reaction with eosinophilia and systemic symptoms: A retrospective cohort study from Taiwan. J. Am. Acad. Dermatol. 2013, 68, 459–465.
- Kano, Y.; Tohyama, M.; Aihara, M.; Matsukura, S.; Watanabe, H.; Sueki, H.; Iijima, M.; Morita, E.; Niihara, H.; Asada, H.; et al. Sequelae in 145 patients with drug-induced hyper-sensitivity syndrome/drug reaction with eosinophilia and systemic symptoms: Survey conducted by the Asian Research Committee on Severe Cutaneous Adverse Reactions (ASCAR). J. Dermatol. 2015, 42, 276–282.
- Kano, Y.; Sakuma, K.; Shiohara, T. Sclerodermoid graft-versus-host disease-like lesions occurring after drug-induced hypersensi-tivity syndrome. Br. J. Dermatol. 2007, 156, 1061–1063.
- Iinuma, S.; Kanno, K.; Honma, M.; Kinouchi, M.; Ishida-Yamamoto, A. Drug-induced hypersensitivity syndrome followed by chronic inflammatory demyelinating polyneuropathy. J. Dermatol. 2018, 45, e310–e311.
- Morita, C.; Yanase, T.; Shiohara, T.; Aoyama, Y. Aggressive treatment in paediatric or young patients with drug-induced hyper-sensitivity syndrome (DiHS)/drug reaction with eosinophilia and systemic symptoms (DRESS) is associated with future de-velopment of type III polyglandular autoimmune syndrome. BMJ Case Rep. 2018, 2018, bcr2018225528.
- Yang, C.W.; Cho, Y.T.; Hsieh, Y.C.; Hsu, S.H.; Chen, K.L.; Chu, C.Y. The interferon-gamma-induced protein 10/CXCR3 axis is associated with human herpesvirus-6 reactivation and the development of sequelae in drug reaction with eosinophilia and systemic symptoms. Br. J. Dermatol. 2020, 183, 909–919.





