
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Caroline Pellet-Many | + 1497 word(s) | 1497 | 2021-05-07 09:50:28 |
Video Upload Options
In zebrafish, the spatiotemporal development of the vascular system is well described due to its stereotypical nature. However, the cellular and molecular mechanisms orchestrating post-embryonic vascular development, the maintenance of vascular homeostasis, or how coronary vessels integrate into the growing heart are less well studied. In the context of cardiac regeneration, the central cellular mechanism by which the heart regenerates a fully functional myocardium relies on the proliferation of pre-existing cardiomyocytes; however the epicardium and the endocardium are also known to play key roles in the regenerative process. Remarkably, revascularisation of the injured
tissue occurs within a few hours after cardiac damage, thus generating a vascular network acting as a scaffold for the regenerating myocardium. The activation of the endocardium leads to the secretion of cytokines, further supporting the proliferation of the cardiomyocytes. In this review, we focus on recent advances in the understanding of the development of the endocardium and the coronary vasculature in zebrafish as well as their pivotal roles in the heart regeneration process.
1. The Endothelium in Heart Development
In the past 25 years, the zebrafish model has facilitated a wealth of discoveries about angiogenesis and cardiac morphogenesis. It presents several advantages, such as the generation of large sample sets, with a single breeding pair producing ≥200 embryos per mating, their ease of genetic manipulation, and rapid early ex utero development. Within 48 h post fertilisation (hpf), the major organs and body plan are completely formed, and the first circulatory loop is established by 24 hpf [1]. Zebrafish embryos are also transparent for the first 24 h of development, after which pigmentation can be suppressed by adding the chemical 1-phenyl 2-thiourea (PTU) to the water or by the breeding of casper, TraNac, or crystal mutant lines, which lack pigmentation throughout adulthood [2][3][4].
Because zebrafish embryos are small in size and readily fit within conventional microscopy set ups, they are much easier to investigate than mammalian models. A seminal study by Isogai and colleagues, who used confocal microangiography to observe lumenised vessels, provided a comprehensive description of the first seven days of vascular development, publicly available online: (https://zfish.nichd.nih.gov/Intro%20Page/intro1.html (accessed on 13 January 2021)) [5]. Apart from providing a key resource to the research community, their study also identified that the zebrafish embryonic vasculature is largely stereotypical, i.e., almost all embryos form any given blood vessels with the same pattern at the same timepoint.
The generation of new transgenic fluorescent reporter lines, advances in microscopy techniques, and the development of morpholino technology, which transiently suppresses mRNA translation, allowed the initial characterisation of molecules central to the development of the vascular system. The use of live in vivo imaging of cardiovascular processes at single-cell resolution enabled the identification of the molecular mechanisms of vessel development in unprecedented detail.
In vertebrates, the cardiovascular system consists of the heart and all blood and lymphatic vessels in the body. It is the first organ system to become functional in the zebrafish embryo and is crucial to survival past 7 days post fertilisation (dpf). Heart tissue consists of three layers: the myocardium, epicardium, and endocardium. The myocardium is a functional syncytium made up of cardiomyocytes (CM) that contract in response to action potentials induced by pacemaker cells. The endocardium is the inner lining of the heart. It arises from vascular endothelial cells [6] and contributes to the formation of the cardiac valves and part of the coronary vasculature [7][8]. The epicardium is the visceral layer of the pericardium, it is made up of mesothelial cells that protect the heart and stabilise it in its anatomic position. It also supports the coronary vessel network that supplies the heart itself with blood flow. The coronary vasculature arises later during development, from E11 in mice and much later in zebrafish [9].
The zebrafish heart begins forming at 12 hpf when myocardial progenitors migrate from the lateral plate mesoderm to the midline and, by 19 hpf, fuse with endocardial progenitors. Together, they create the cardiac cone that involutes to form the linear heart tube circa 24 hpf. Looping occurs from 36 hpf onwards and, by 48 hpf, the distinct curvatures of the atrium and ventricle can be identified. At 72 hpf, a superior valve leaflet has been generated, preventing retrograde blood flow, and by 5 dpf, the heart valve is fully functional, the epicardium fully covers the myocardium, and there is extensive trabeculation (reviewed in [10]). It is important to mention here that zebrafish embryos, unlike mammals or birds, are capable of surviving without circulation for up to 7 dpf [11]. This has enabled research into the contribution of blood flow to cardiovascular development and manipulating blood flow is a key tool in the zebrafish developmental field.
2. The Endothelium in Heart Regeneration
Coronary blood vessel obstruction caused by atherosclerotic plaque rupture or embolism can leave the heart with insufficient blood supply and result in myocardial infarction (MI), more commonly referred to as a heart attack. It is estimated that 1 billion cardiomyocytes are lost during a heart attack in humans [12]. Fibrotic tissue is produced in place of the functional cardiac tissue lost during the MI; this results in cardiac remodelling, hypertrophy, and eventually, heart failure. These devastating complications of heart attacks contribute to 31 % of deaths worldwide, making coronary artery disease the global leading cause of death [13]. Understanding the biological and cellular mechanisms taking place in animal models that can achieve full regeneration of the heart after cardiac injury may identify therapeutic targets to help prevent progression to heart failure or even treat patients. Zebrafish exhibit remarkable regenerative capabilities and can regenerate appendages and organs, including the heart. Full regeneration was shown to be dependent on vessel restoration to the damaged tissue. There are several distinct experimental approaches to induce cardiac regeneration in adult zebrafish which are reviewed elsewhere [14]. Generally, heart injuries are caused by either surgically removing the tip of the ventricle (apical resection), by using a probe cooled in liquid nitrogen (cryoinjury), or genetic ablation approaches which cause extensive and diffuse CM apoptosis. The length of time necessary to complete regeneration depends on the model used, with the regeneration following cryoinjury taking longer than the resection or the genetic ablation models because of the need to clear the necrotic tissue and fibrotic scar.
To achieve complete regeneration and restore full cardiac function following MI, the reestablishment of the coronary vessel network is critical, as the delivery of oxygen and nutrients is essential to cater to the high metabolic demands of the heart [15][16]. In adult zebrafish hearts, endothelial cells (EC) represent 37 % of all cardiac cells, nearly equalling the number of cardiomyocytes (CM) (39 %). These numbers are comparable to the relative proportions of CM and EC in mice, nevertheless, the relative ventricular surface area covered by endocardial cells is significantly greater in zebrafish [17]. This highlights the importance of the endothelium and endocardium in the maintenance and regeneration of the myocardium following injury.
The cellular mechanisms and signalling pathways that have been reported to play essential roles in the restoration of the coronary vascular network and endocardial response in the regenerating zebrafish heart are summarised in Figure 1.
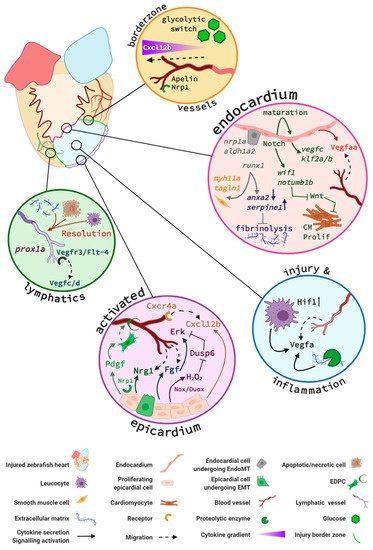
Figure 1. Key signalling pathways in zebrafish endocardium and cardiac vessels during regeneration. Vessels at the injury border (orange circle) undergo a glycolytic switch and use glucose as a primary energy source. Border zone vessels express Apelin and Neuropilin 1 (Nrp1) and respond to Cxcl12b signals that originate at least in part from the activated epicardium. The activated endocardium (pink circle) expresses Notch, which regulates expression of endocardial maturation genes, endothelial differentiation, integrity, and pro-angiogenic genes (e.g., vegfc, klf2a/b). Additionally, endocardial Notch induces expression of Wnt antagonists wif1 and notum1b that in turn promote cardiomyocyte (CM) proliferation (Prolif). Activated endocardial cells express nrp1, aldh1a2, and runx1. A subpopulation of Runx1-positive cells expresses smooth muscle cell genes myh11a and taglnI. Runx1 also regulates anxa2 and serpine1 and limits fibrinolysis of scar tissue. Within the injury (blue circle), it is hypothesised that recruited macrophages and neutrophils combined with increased Hif1 activity upregulate Vegfa expression. An additional Vegfa source is thought to arise from proteolytic enzyme activity within the injury that releases extracellular matrix (ECM)–bound Vegfa. The activated epicardium (purple circle) secretes Cxcl12b chemokines that bind to the Cxcr4a receptor of endothelial cells in infiltrating vessels. Endothelial cell proliferation is further promoted by epicardial expression of Duox and Nox enzymes that catalyse the generation of hydrogen peroxide (H2O2). This subsequently inhibits Dusp6 activity in endothelial cells, thus relieving Dusp6 suppression of Erk signalling and enhancing endothelial proliferation. Fgf signalling promotes revascularisation and epicardial activation. Pdgf and Nrp1 promote epicardial activation, a subpopulation of epicardial cells undergo epicardial to mesenchymal transition (EpiMT). These epicardial-derived cells (EPDCs) become fibroblasts secreting ECM and perivascular cells. Nrg1 expression by EPDCs further promotes angiogenesis. Prox1a-expressing lymphatic vessels are detected in the injury and respond to Vegfc/d via Vegfr3. Inflammatory cells, necrotic and apoptotic cells, and extracellular matrix debris are removed via the lymphatic vessels (green circle) to aid the regenerative process. Figure generated with BioRender.com.
References
- Kimmel, C.B.; Ballard, W.W.; Kimmel, S.R.; Ullmann, B.; Schilling, T.F. Stages of embryonic development of the zebrafish. Dev. Dyn. 1995, 203, 253–310.
- Antinucci, P.; Hindges, R. A crystal-clear zebrafish for in vivo imaging. Sci. Rep. 2016, 6, 29490.
- Krauss, J.; Astrinidis, P.; Astrinides, P.; Frohnhöfer, H.G.; Walderich, B.; Nüsslein-Volhard, C. Transparent, a gene affecting stripe formation in Zebrafish, encodes the mitochondrial protein Mpv17 that is required for iridophore survival. Biol. Open 2013, 2, 703–710.
- White, A.J.; Boffa, M.B.; Jones, B.; Petkovich, M. A zebrafish retinoic acid receptor expressed in the regenerating caudal fin. Developement 1994, 120, 1861–1872.
- Isogai, S.; Horiguchi, M.; Weinstein, B.M. The Vascular Anatomy of the Developing Zebrafish: An Atlas of Embryonic and Early Larval Development. Dev. Biol. 2001, 230, 278–301.
- Milgrom-Hoffman, M.; Harrelson, Z.; Ferrara, N.; Zelzer, E.; Evans, S.M.; Tzahor, E. The heart endocardium is derived from vascular endothelial progenitors. Developement 2011, 138, 4777–4787.
- Nakano, A.; Nakano, H.; Smith, K.A.; Palpant, N.J. The developmental origins and lineage contributions of endocardial endothelium. Biochim. Biophys. Acta (BBA) Bioenerg. 2016, 1863, 1937–1947.
- Sharma, B.; Ho, L.; Ford, G.H.; Chen, H.I.; Goldstone, A.B.; Woo, Y.J.; Quertermous, T.; Reversade, B.; Red-Horse, K. Alternative Progenitor Cells Compensate to Rebuild the Coronary Vasculature in Elabela- and Apj-Deficient Hearts. Dev. Cell 2017, 42, 655–666.
- Chen, H.I.; Sharma, B.; Akerberg, B.N.; Numi, H.J.; Kivelä, R.; Saharinen, P.; Aghajanian, H.; McKay, A.S.; Bogard, P.E.; Chang, A.H.; et al. The sinus venosus contributes to coronary vasculature through VEGFC-stimulated angiogenesis. Developement 2014, 141, 4500–4512.
- Staudt, D.; Stainier, D. Uncovering the Molecular and Cellular Mechanisms of Heart Development Using the Zebrafish. Annu. Rev. Genet. 2012, 46, 397–418.
- Sehnert, A.J.; Huq, A.; Weinstein, B.M.; Walker, C.; Fishman, M.; Stainier, D.Y.R. Cardiac troponin T is essential in sarcomere assembly and cardiac contractility. Nat. Genet. 2002, 31, 106–110.
- Murry, C.E.; Reinecke, H.; Pabon, L.M. Regeneration gaps: Observations on stem cells and cardiac repair. J. Am. Coll. Cardiol. 2006, 47, 1777–1785.
- Ritchey, M.D.; Wall, H.K.; George, M.G.; Wright, J.S. US trends in premature heart disease mortality over the past 50 years: Where do we go from here? Trends Cardiovasc. Med. 2020, 30, 364–374.
- González-Rosa, J.M.; Burns, C.E. Zebrafish heart regeneration: 15 years of discoveries. Regeneration 2017, 4, 105–123.
- Das, S.; Goldstone, A.B.; Wang, H.; Farry, J.; D’Amato, G.; Paulsen, M.J.; Eskandari, A.; Hironaka, C.E.; Phansalkar, R.; Sharma, B.; et al. A Unique Collateral Artery Development Program Promotes Neonatal Heart Regeneration. Cell 2019, 176, 1128–1142.e18.
- Zangi, L.; Lui, K.O.; Von Gise, A.; Ma, Q.; Ebina, W.; Ptaszek, L.M.; Später, D.; Xu, H.; Tabebordbar, M.; Gorbatov, R.; et al. Modified mRNA directs the fate of heart progenitor cells and induces vascular regeneration after myocardial infarction. Nat. Biotechnol. 2013, 31, 898–907.
- Patra, C.; Kontarakis, Z.; Kaur, H.; Rayrikar, A.; Mukherjee, D.; Stainier, D.Y.R. The zebrafish ventricle: A hub of cardiac endothelial cells for in vitro cell behavior studies. Sci. Rep. 2017, 7, 2687.




