
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ayse T. Kendi | + 1168 word(s) | 1168 | 2021-04-26 11:02:09 | | | |
| 2 | Vicky Zhou | Meta information modification | 1168 | 2021-05-18 09:03:26 | | | | |
| 3 | Vicky Zhou | + 105 word(s) | 1273 | 2021-05-25 10:08:13 | | |
Video Upload Options
177Lu-PSMA (prostate specific membrane antigen) therapy is used for metastatic castration-resistant prostate cancer (mCRPC). Although there are some different approaches regarding the use of 177Lu-PSMA therapy in different countries, this type of therapy is generally safe, with a low toxicity profile. From the oncological point of view, a PSA (prostate specific antigen) decline of ≥50% was seen in 10.6–69% of patients with mCRPC; whereas progression-free survival (PFS) was reported to be 3–13.7 months in different studies. Consequently, 177Lu-PSMA therapy is a promising treatment in patients with mCRPC, with good clinical efficacy, even in heavily pretreated patients with multiple lines of systemic therapy. Currently, there are ongoing clinical trials in the United States, including a phase III multicenter FDA registration trial.
1. Introduction
Prostate cancer (PCa) is the most common cancer and the second cause of death related to cancer in the United States [1]. For 2020, it was estimated that 191,930 new cases would be diagnosed; whereas a total of 33,340 cases would have died due to PCa [1]. Depending on the relationship between serum PSA (prostate specific antigen) levels and applied treatment according to the location of the disease, the natural course of PCa might be evaluated in four distinct disease states. In the initial state, disease is localized to the prostate and curative treatment options such as radical prostatectomy (RP) or radiotherapy (RT) are available. If the patient was not cured at the initial phase, a non-castrate rising PSA phase follows. The remaining states are non-castrate metastatic and metastatic castration-resistant PCa (mCRPC), respectively. It is estimated that the metastatic state of PCa leads to death in 30% of the patients within 5 years; whereas the survival of patients with mCRPC is only 14 months [1][2].
For the metastatic disease state, the backbone treatment option is hormonal. Briefly, removing the androgens (androgen deprivation therapy (ADT)) leads to the inhibition of progression of PCa for a period of 18–24 months [3]. Despite appropriate ADT, if the disease progresses, it is defined as mCRPC [4]. For this disease state, many agents given in conjunction with ADT are available with different survival benefits. For example, abiraterone, enzalutamide and apalutamide interfere with androgenic stimulation of the PCa growth by blocking androgen synthesis [5]. Meanwhile, systemic taxane-based chemotherapies, such as docetaxel for patients with high metastatic burden, or cabazitaxel after treatment with docetaxel, are also available. Immunotherapy with sipueleucel-T and bone-targeted radiopharmaceutical therapy with radium-223 (223Ra) are also in use, although less frequent [6]. Lastly, the use of theranostic agents such as 177Lu or 225Ac-PSMA is an emerging therapy in patients with mCRPC, available for the clinical care of patients in many countries outside North America and in a multicenter phase III clinical trial in the United States. This review aims to summarize the theranostic benefits of 177Lu-PSMA in mCRPC.
2. Therapy-Related Issues
2.1. Performing Therapy
At the start of therapy, patients are advised to be well hydrated by oral intake. Oral hydration before, on the day and two days after therapy is encouraged. Injection of 177Lu-PSMA is administered intravenously over one to two minutes. Meanwhile, in patients with low cardiovascular risk, 1000–2000 mL 0.9% NaCl can be given after the therapy [7][8]. Corticosteroids and antiemetics can be used. Despite being controversial, ice-packs could be used for the external cooling of salivary glands, 30 min before and up to four hours after the therapy [9][10]. Due to the renal excretion of 177Lu-PSMA, patients should follow the radiation safety rules for contamination risk. To reduce the radiation exposure, patients are advised to void frequently or are catheterized if the condition of patient is not well enough to void [11].
2.2. Release of Patients
After the administration of 177Lu-PSMA, the patient-specific radiation dose decreases below 25 μSv/hour at 1 m, which allows treatment in outpatient settings in Australia; whereas in Europe, this requires the admission of patients to specialized shielded hospital radiation wards for 1–3 days [11][12][13]. Patients are warned to stay away from children, and especially pregnant women, for approximately 3 days after the therapy, to follow the hygiene rules for contamination risk, and are encouraged to maintain hydration, void frequently and shower daily. In the United States, 177Lu-PSMA therapy is performed under clinical trials and the treatments are performed usually in outpatient settings, with home release exposure below 5 mSv/hour and guidance given to patients. This guidance is similar to the 177Lu-DOTATATE therapy home release guidance [14].
2.3. Post-Therapy Imaging
A whole body scan can be performed 24–48 h post-injection to confirm tumoral uptake. SPECT/CT (single-photon emission computed tomography) can be added to whole body imaging (4, 24 and 96 h post-injection) for dosimetry analysis. In addition to PSA response, patients may be evaluated for interim therapy response with 68Ga-PSMA imaging after two cycles of 177Lu-PSMA therapy, for determining lesion response (Figure 1). The patients can be evaluated with 68Ga-PSMA imaging after finishing four cycles of therapy, for evaluating therapy response (Figure 2).
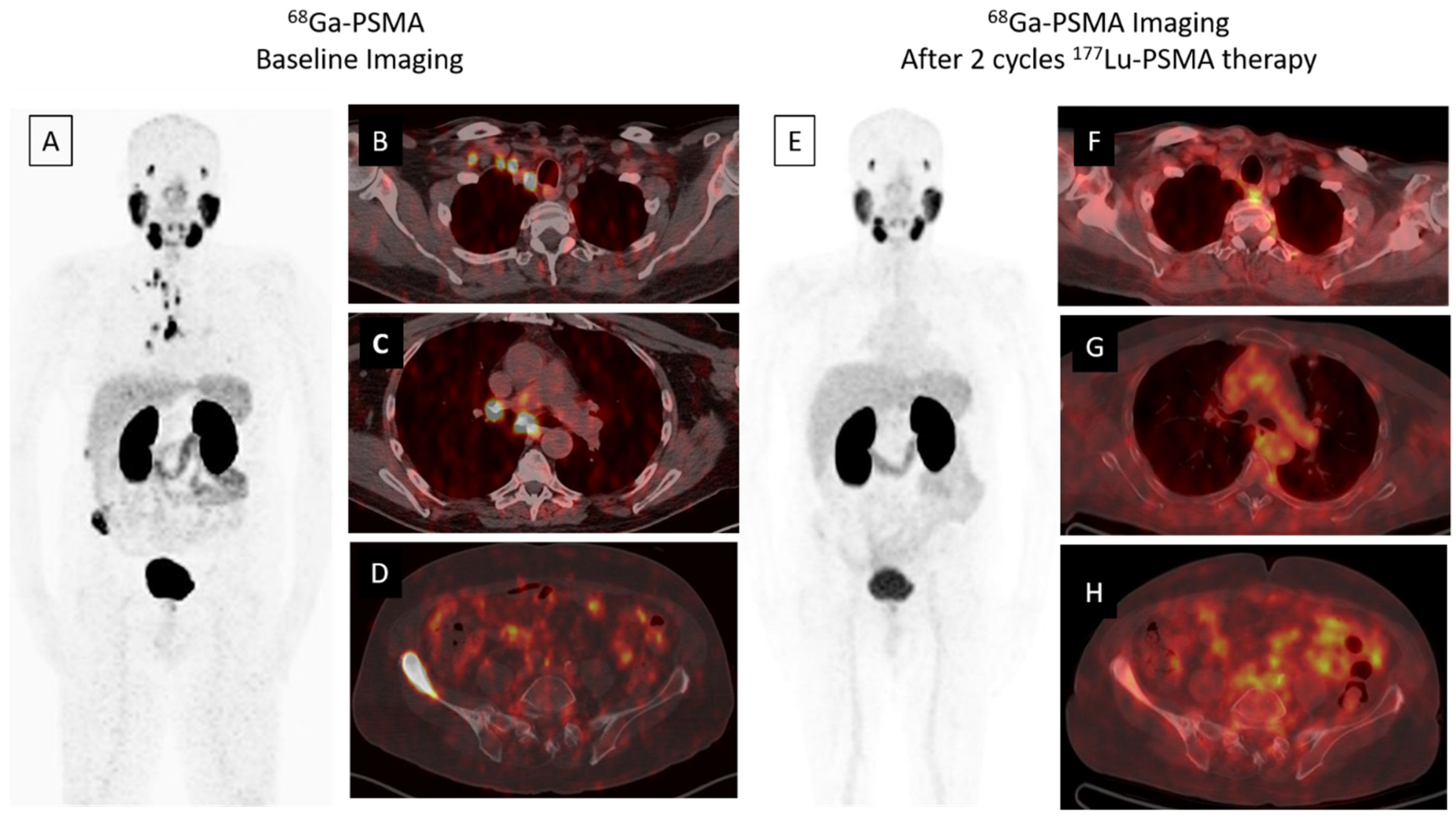
Figure 1. A 68-year-old male was diagnosed, with a Gleason score of 3 + 4 = 7, with prostate adenocarcinoma in 2001, who then underwent external beam radiation therapy with medical castration therapy. He had biochemical recurrence starting in November 2014. He started medical castration therapy and finally his PSA (prostate specific antigen) level slowly progressed to 7.5 ng/mL in December 2018. 68Ga-PSMA-617 PET/CT maximum intensity projection (MIP) image (A) demonstrates widespread abnormal activity in the supraclavicular (B) and mediastinal (C) lymph nodes, pelvic lymph nodes, prostate gland and bones (D). The patient was evaluated after two cycles of 177Lu-PSMA-617 therapy for response to radioligand therapy. A significant response in skeletal and lymph node metastases was detected in 68Ga PSMA-617 PET-CT MIP (E) and axial fused images (F–H) and serum PSA value decreased by approximately 80% (PSA value decreased from 7.5 to 1.53 ng/mL).
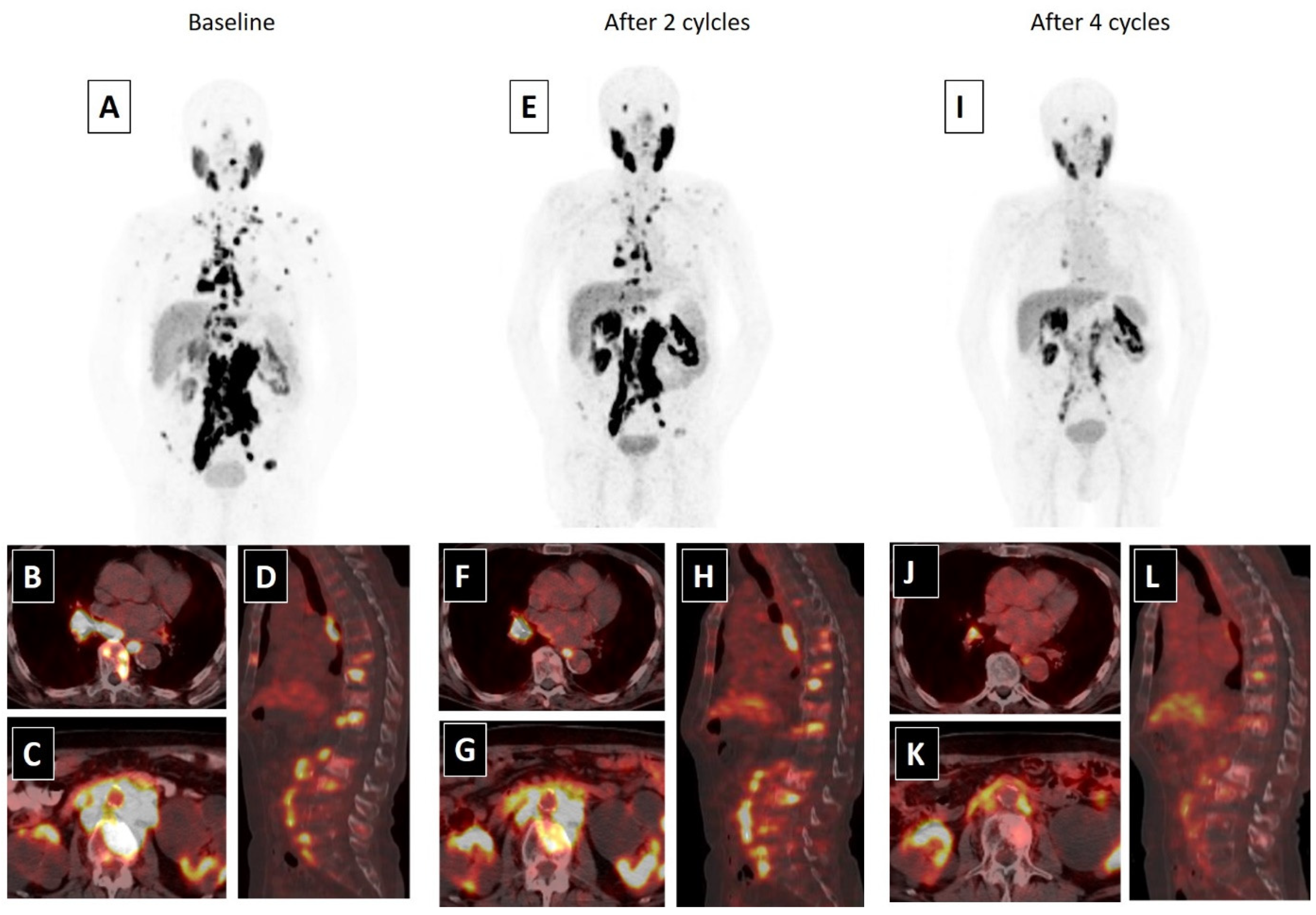
Figure 2. An 80-year-old male with mCRPC, having a Gleason score of 4 + 5 = 9 and a serum PSA value of 293.5 ng/mL, was not responsive to the systemic therapies, such as docetaxel, abiraterone and cabazitaxel. He was also suffering from pain (5/10) and fatigue (ECOG score 2). 68Ga-PSMA-617 PET-CT, 99mTc-MAG3 renography and routine laboratory tests were performed to evaluate the patient for 177Lu-PSMA-617 therapy. In 68Ga-PSMA-617 PET-CT (A), an intense PSMA uptake was detected in supra-infra diaphragmatic metastatic lymph nodes and sclerotic bone metastases (axial fused: (B,C); sagittal fusion: (D)). Laboratory tests were within normal limits except for a high-normal serum creatinine level (1.4 mg/dL). 177Lu-PSMA-617 therapy was planned with a dosimetric approach and he received two cycles of 177Lu-PSMA-617 therapy (cumulative dose: 12.5 Gbq) without any significant adverse effect. A partial response in skeletal and lymph node metastases were detected in 68Ga-PSMA-617 PET-CT and his serum PSA value decreased by 76.2% (PSA: 69.5 ng/mL) after two cycles of therapy (MIP image: (E); axial fused: (F,G); sagittal fusion: (H)). His pain significantly resolved (3/10) and his quality of life improved (ECOG: 1). Accordingly, the patient completed four cycles of 177Lu-PSMA-617 therapy (cumulative dose: 25 Gbq), without any serious side effects. After the end of 177Lu-PSMA-617 therapy, an approximately 95% PSA decline (PSA: 13.5 ng/mL) was detected and a marked response was observed in post-therapy 68Ga-PSMA-617 PET-CT (MIP image: (I); axial fused: (J,K); sagittal fusion: (L)). The patients’ pain was relieved for 15 months and he was alive at 18 months of the 177Lu-PSMA-617 therapy.
3. Conclusions
References
- Miller, K.D.; Siegel, R.L.; Khan, R.; Jemal, A. Cancer Statistics. Cancer Rehabil. 2018, 70, 7–30.
- Kirby, M.; Hirst, C.; Crawford, E.D. Characterising the castration-resistant prostate cancer population: A systematic review. Int. J. Clin. Pract. 2011, 65, 1180–1192.
- Harris, W.P.; Mostaghel, E.A.; Nelson, P.S.; Montgomery, B. Androgen deprivation therapy: Progress in understanding mechanisms of resistance and optimizing androgen depletion. Nat. Clin. Pract. Urol. 2009, 6, 76–85.
- Cornford, P.; Bellmunt, J.; Bolla, M.; Briers, E.; De Santis, M.; Gross, T.; Henry, A.M.; Joniau, S.; Lam, T.B.; Mason, M.D.; et al. EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part II: Treatment of Relapsing, Metastatic, and Castration-Resistant Prostate Cancer. Eur. Urol. 2017, 71, 630–642.
- Crawford, E.D.; Heidenreich, A.; Lawrentschuk, N.; Tombal, B.; Pompeo, A.C.L.; Mendoza-Valdes, A.; Miller, K.; Debruyne, F.M.J.; Klotz, L. Androgen-targeted therapy in men with prostate cancer: Evolving practice and future considerations. Prostate Cancer Prostatic Dis. 2019, 22, 24–38.
- Lowrance, W.T.; Murad, M.H.; Oh, W.K.; Jarrard, D.F.; Resnick, M.J.; Cookson, M.S. Castration-Resistant Prostate Cancer: AUA Guideline Amendment 2018. J. Urol. 2018, 200, 1264–1272.
- Kratochwil, C.; Fendler, W.P.; Eiber, M.; Baum, R.; Bozkurt, M.F.; Czernin, J.; Bolton, R.C.D.; Ezziddin, S.; Forrer, F.; Hicks, R.J.; et al. EANM procedure guidelines for radionuclide therapy with 177Lu-labelled PSMA-ligands (177Lu-PSMA-RLT). Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 2536–2544.
- Weineisen, M.; Schottelius, M.; Simecek, J.; Baum, R.P.; Yildiz, A.; Beykan, S.; Kulkarni, H.R.; Lassmann, M.; Klette, I.; Eiber, M.; et al. 68Ga- and 177Lu-Labeled PSMA I&T: Optimization of a PSMA-Targeted Theranostic Concept and First Proof-of-Concept Human Studies. J. Nucl. Med. 2015, 56, 1169–1176.
- Ahmadzadehfar, H.; Eppard, E.; Kürpig, S.; Fimmers, R.; Yordanova, A.; Schlenkhoff, C.D.; Gärtner, F.; Rogenhofer, S.; Essler, M. Therapeutic response and side effects of repeated radioligand therapy with 177Lu-PSMA-DKFZ-617 of castrate-resistant metastatic prostate cancer. Oncotarget 2016, 7, 12477–12488.
- Taïeb, D.; Foletti, J.-M.; Bardies, M.; Rocchi, P.; Hicks, R.J.; Haberkorn, U. PSMA-Targeted Radionuclide Therapy and Salivary Gland Toxicity: Why Does It Matter? J. Nucl. Med. 2018, 59, 747–748.
- Emmett, L.; Willowson, K.; Violet, J.; Shin, J.; Blanksby, A.; Lee, J. Lutetium177PSMA radionuclide therapy for men with prostate cancer: A review of the current literature and discussion of practical aspects of therapy. J. Med. Radiat. Sci. 2017, 64, 52–60.
- Kurth, J.; Krause, B.J.; Schwarzenböck, S.M.; Stegger, L.; Schäfers, M.; Rahbar, K. External radiation exposure, excretion, and effective half-life in 177Lu-PSMA-targeted therapies. EJNMMI Res. 2018, 8, 32.
- Demir, M.; Abuqbeitah, M.; Uslu-Beşli, L.; Yıldırım, Ö.; Yeyin, N.; Çavdar, İ.; Vatankulu, B.; Gündüz, H.; Kabasakal, L. Evaluation of radiation safety in 177Lu-PSMA therapy and development of outpatient treatment protocol. J. Radiol. Prot. 2016, 36, 269.
- Kendi, A.T.; Halfdanarson, T.R.; Packard, A.; Dundar, A.; Subramaniam, R.M. Therapy With177Lu-DOTATATE: Clinical Implementation and Impact on Care of Patients with Neuroendocrine Tumors. Am. J. Roentgenol. 2019, 213, 309–317.




