
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Xavier Escoté | + 2931 word(s) | 2931 | 2020-06-01 10:08:41 | | | |
| 2 | Nicole Yin | -8 word(s) | 2923 | 2020-06-03 08:44:24 | | | | |
| 3 | Nicole Yin | -13 word(s) | 2910 | 2020-11-06 04:45:51 | | |
Video Upload Options
Recently, hesperidin, a flavonone mainly present in citrus fruits, has emerged as a new potential therapeutic agent able to modulate several cardiovascular diseases (CVDs) risk factors.
1. Introduction
Animal and in vitro studies demonstrate beneficial effects of hesperidin and its derived compounds on CVD risk factors. Thus, hesperidin has shown glucose-lowering and anti-inflammatory properties in diabetic models, dyslipidemia-, atherosclerosis-, and obesity-preventing effects in CVDs and obese models, and antihypertensive and antioxidant effects in hypertensive models. However, there is still controversy about whether hesperidin could contribute to ameliorate glucose homeostasis, lipid profile, adiposity, and blood pressure in humans, as evidenced by several clinical trials reporting no effects of treatments with this flavanone or with orange juice on these cardiovascular parameters. In this entry, we focus on hesperidin's beneficial effects on CVD risk factors, paying special attention to the high interindividual variability in response to hesperidin-based acute and chronic interventions, which can be partly attributed to differences in gut microbiota. Based on the current evidence, we suggest that some of hesperidin's contradictory effects in human trials are partly due to the interindividual hesperidin variability in its bioavailability, which in turn is highly dependent on the α-rhamnosidase activity and gut microbiota composition.
2. Effects of Hesperidin on Glucose Homeostasis
Diabetes is one of the major risk factors for developing CVDs. The main complication of diabetes is CVDs, and it is estimated that 65% of diabetic patients die from CVD complications[1]. In this sense, several studies have shown beneficial effects of hesperidin in glucose metabolism at the preclinical level, both in animal and in vitro models.
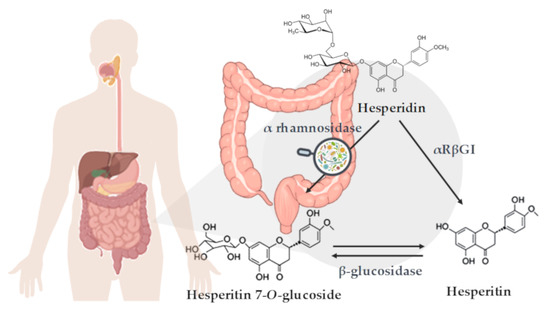
Figure 1. Schematic representation of hesperidin metabolization in the colon. Enzymatic deglycosylation of hesperidin to yield hesperetin: via hesperetin-7-O-glucoside by two specific monoglycosidases, α-rhamnosidase and β-glucosidase, and via one-step deglycosylation through α-rhamnosyl-β-glucosidase (αRβGl).
At the in vitro level, neohesperidin (derived from hesperidin) treatment was shown to increase glucose consumption in the hepatocyte cell line HepG2, which was associated with increased phosphorylation levels of adenosine monophosphate (AMP)-activated protein kinase (AMPK)[2]. Xuguang et al. recently reported attenuated glucose content in culture medium and increased glucose uptake in lipopolysaccharide (LPS)-induced insulin-resistant HepG2 cells treated with hesperidin. These changes seemed to be associated with the regulation of the insulin receptor substrate 1 (IRS1)- glucose transporter (GLUT)-2 pathway via toll-like receptor (TLR)-4[3]. This positive effect over glucose uptake was corroborated in another recent study, showing that both hesperidin and hesperetin exert antidiabetic properties in L6 myotubes by inducing glucose uptake and reducing oxidative stress and advanced glycation end-products (AGEs) formation[4]. Related to AGE formation, Irshad and collaborators recently showed that a combination of trans-resveratrol and hesperetin is able to dampen the rise of methylglyoxal levels caused by high glucose concentrations by increasing the expression of Glyoxalase (Glo)-1 and decreasing the expression of hexokinase (HK)-2 in human aortal endothelial cells[5].
There is accumulating evidence demonstrating the glucose-lowering effects and the improvement in insulin resistance parameters exerted by hesperidin both in Type-1 diabetes (T1D)[6][7][8][9][10][11] and Type-2 diabetes (T2D)[12][13][14][15][16] rodent experimental models, thus demonstrating the antidiabetic properties of hesperidin. These effects were shown to be achieved by the modulation of key glucose regulation enzymes, such as an upregulation of glucokinase (involved in glycolysis) or a downregulation of the gluconeogenic enzyme glucose-6-phosphatase[6][10][12][13][14]. Other effects of hesperidin treatment in diabetic animals include a reduction in inflammatory parameters, such as tumor necrosis factor alpha (TNFα), interleukin (IL)-6 or IL-1β, and the reduction of oxidative stress associated with diabetes[8][9][15]. Akiyama and collaborators also reported a recovery of adiponectin levels mediated by hesperidin both in T1D and T2D models[6][14]. In addition to the effects observed in diabetic models, improvement of glucose metabolism and insulin resistance were also described by our group and others in other animal models of human diseases that are associated with alterations in glucose metabolism, such as metabolic syndrome (MetS) and obesity[17][18][19][20][21].
Despite all evidence at the preclinical level, the effects of hesperidin consumption on glucose metabolism in humans are not conclusive. In a recent randomized, double‑blind, placebo‑controlled clinical trial, Yari et al. reported that daily consumption of hesperidin capsules (500 mg) for 12 weeks significantly decreased fasting glucose levels, both compared with basal levels and with placebo group in patients with MetS[22]. Decreases in insulin levels and in the homeostatic model assessment for insulin resistance (HOMA-IR) index were also reported, although no significant differences vs. the placebo group were observed in these parameters[22]. Ribeiro et al. reported a decrease of 18% in insulin levels and a reduction of 33% in HOMA-IR index after 12 weeks of daily consumption of 500 mL of orange juice (OJ) in obese individuals compared to control group[23]. Lima and collaborators also reported significant decreases in blood glucose and insulin fasting levels, as well as in HOMA-IR index after daily consumption of 300 mL of OJ during 60 days in a non-placebo-controlled clinical trial in healthy women[24].
However, to date, there are several clinical trials performed in different populations (healthy, obese, diabetic, or MetS) reporting no differences in glucose or insulin levels after chronic hesperidin or OJ consumption[25][26][27][28][29][30]. One study reported an increase in glucose levels in OJ-treated obese or overweight individuals, both in low and high hesperidin concentrations, which could be attributed to the daily addition to the diet of 500 mL dietary OJ during 12 weeks or to a decrease in insulin levels, which was also observed after the intervention[31].
3. Effects of Hesperidin on Lipid Profile and Adiposity
The dysregulation of lipid and lipoprotein metabolism contributes to the pathogenesis of multitude of human diseases, including CVDs[32]. Several therapeutic strategies exist to modulate lipid metabolism and prevent the development of metabolic diseases, but these strategies present some inherent limitations. For instance, statin drugs, which have been widely used to improve lipid profile and reduce atherosclerotic risk, present well recognized side-effects such as myalgia, arthralgia, and temporary gastrointestinal upset[33]. Those patients presenting dyslipidemia associated with MetS are unable to reach their lipid treatment goals by the administration of statin drugs[34]. Considering this, flavonoids including hesperidin have emerged as new therapeutic agents that could prevent alterations regarding lipid metabolism. In this sense, hesperidin has been shown to be especially effective in modulating dyslipidemia associated with MetS, which is considered a major risk for atherosclerosis, by exerting lipid-lowering properties in animal models and humans[17][24][28][35][36][37][38][39]. Jung et al. investigated the effects of hesperidin on lipid regulation in C57BL/KsJ-db/db mice, a well-established model of obesity-induced T2D. The results of this study demonstrated that hesperidin (0.2 g hesperidin/kg diet) was effective in lowering the plasma free fatty acids (FFAs) and plasma and hepatic triglyceride levels after five weeks. Additionally, hesperidin reduced the hepatic fatty acid oxidation and carnitine palmitoyl transferase activity. Hesperidin effects on lipid regulation were attributable to a suppression of the hepatic fatty acid synthase, glucose-6-phosphate dehydrogenase, and phosphatidate phosphohydrolase activities and to an increase in the fecal triglycerides[13]. Furthermore, it was also demonstrated that hesperidin administration led to a decrease in plasma and hepatic cholesterol levels through a downregulation of the hepatic 3-hydroxy-3-methylglutaryl-coenzyme (HMG-CoA) reductase and acyl CoA: cholesterol acyltransferase (ACAT) activities[13]. Wu et al. demonstrated similar lipid-regulating effects with neohesperidin. Neohesperidin showed a potent hypolipidemic effect in HepG2 cells loaded with FFAs and reversed the pathological changes of lipid in the acute or chronic dyslipidemia mouse model. They suggested that neohesperidin regulates lipid metabolism in vivo and in vitro via fibroblast growth factor 21 (FGF21) and AMP-activated protein kinase/Sirtuin type1/Peroxisome proliferator-activated receptor gamma coactivator 1α signaling axis[21]. Hesperidin treatment has also been shown to reduce lipid accumulation in adipocytes derived from human mesenchymal stem cells by reducing lipogenesis and activating lipolysis[40]. Similar in vitro antiadipogenic effects have been observed in 3T3-L1 preadipocytes[41]. In addition, and related to lipid metabolism, Kim et al. have recently shown that hesperidin treatment increases Uncoupling protein 3 (UCP3) expression in differentiated C2C12 myocytes, thus boosting energy consumption from lipids[42].
The beneficial effect of hesperidin on atherosclerosis development was demonstrated in a study conducted by Sun et al. using LDL receptor deficient (LDLr−/−) mice. The authors observed that hesperidin ameliorated high fat diet (HFD)-induced hyperlipidemia and suppressed HFD-induced hepatic steatosis, atherosclerotic plaque area, and macrophage foam cell formation. According to these results, Sun et al. suggested that hesperidin reduced atherosclerosis in part via amelioration of lipid profiles, inhibition of macrophage foam cell formation, its antioxidative effect, and anti-inflammatory action[17].
Therefore, results from in vitro and animal studies demonstrate a beneficial effect of hesperidin treatment on lipid profile, but these findings are in contrast with some human intervention studies. Thus, while the administration of glucosyl hesperidin to hypertriglyceridemic subjects for 24 weeks resulted in a clear reduction in plasma triglycerides and apolipoprotein B levels[43], in other studies, the administration of hesperidin capsules did not affect plasma total cholesterol, LDL-cholesterol, or triglyceride levels in moderately hypercholesterolemic individuals[44].
Adipose tissue plays an important role in storing lipid in the form of triglycerides, as well as secreting a variety of adipokines and cytokines[45]. However, adipose tissue dysfunction is a determinant cause for the development of obesity, an independent risk factor for CVDs[45][46]. In this sense, there are several studies demonstrating that hesperidin exerts beneficial effects on lipid accumulation and adiposity[41][42][47][48]. In animal models of obesity or MetS, a body-weight-reducing effect has been widely reported in response to hesperidin treatment[17][18][19][20][21], as well as a reduction in adipose tissue weight[49][18][20][21]. In contrast, Mosqueda-Solis et al. reported no significant changes in body weight after a daily hesperidin administration (100 mg/kg body weight) for eight weeks in Western-diet-fed rats, although hesperidin treatment resulted in a decreased size of adipocytes[48].
Similar to what has been observed in glucose and lipid metabolism, hesperidin or OJ treatment in obese or overweight individuals do not clearly reflect the effects observed in obesogenic animal models. Although Aptekmann and Cesar reported a significant reduction in body weight after daily consumption of OJ over 13 weeks in hypercholesterolemic subjects, no significant differences were observed between the intervention and control groups[38]. Rangel-Huerta and collaborators also observed a significant reduction in body weight after daily consumption of OJ over 12 weeks in obese or overweight subjects in a nonplacebo-controlled clinical trial[31]. By contrast, at least three studies reported no significant changes between control group and hesperidin or OJ groups in obese subjects[23][37][50].
4. Effects of Hesperidin on Blood Pressure and Endothelial Function
High blood pressure is one of the most significant risk factors for developing CVDs in all age groups[51]. In fact, it is known that a reduction of 10 and 5 mmHg in systolic blood pressure (SBP) and diastolic blood pressure (DBP), respectively, significantly decreases the relative risk of all major cardiovascular outcomes[52]. An extensive number of animal studies evaluating the cardioprotective role of hesperidin have shown its beneficial effects on high blood pressure[53][54][55][56][57]. The hypotensive effect after acute administration of hesperidin derivatives, hesperetin and glucosyl hesperidin (G-hesperidin), was demonstrated by Yamamoto et al.[58] in spontaneously hypertensive rats (SHR). In this study, a single oral dose of G-hesperidin (10 to 50 mg/kg) induced a dose-dependent reduction in SBP in SHR, but had no effect in control Wistar Kyoto rats (WKY), discarding possible hypotensive effects under normotensive conditions. The antihypertensive effect of hesperidin was suggested to be mediated by the vascular nitric oxide (NO) synthase pathway. Similar effects were reported by Liu et al., observing an increase in NO production in hesperetin-treated human endothelial cells[59]. In this sense, Ikemura et al.[54] reported a preventive effect of hesperidin and G-hesperidin against age-related increase in blood pressure. This preventive effect of hesperidin seemed to be mediated by an important increase in NO production in the groups supplemented with hesperidin or G-hesperidin and by an improvement in the endothelial function[54]. The long-term effects of hesperidin and G-hesperidin on blood pressure were also evaluated when administered to SHR and to WKY. Chronic oral administration for 25 weeks of hesperidin and G-hesperidin resulted in a decrease in blood pressure after 15 weeks of administration in SHR, while no changes occurred in WKY[53]. Recently, it was also demonstrated that chronic administration of hesperidin for eight weeks resulted in a significant reduction in SBP in cafeteria-fed rats, a well-stablished animal model for diet-induced hypertension[60][61]. They observed that chronic administration of hesperidin in these animals presenting diet-induced hypertension also resulted in lower secretion of inflammation and oxidative stress-related metabolites. A reduction in inflammation and oxidative stress could be the underlying mechanisms involved in hesperidin effects on blood pressure in these animals[55].
These findings suggest that a potential mechanism whereby hesperidin and its derivatives, including G-hesperidin and hesperetin, exert their beneficial effects on hypertension through their demonstrated antioxidant effect[54][62][63], enhancing NO bioavailability and protecting endothelial function from reactive oxygen species. Besides, several studies indicate that not only NO enhancement is involved in the antihypertensive effect exerted by hesperidin. The administration of hesperidin in SHR reduced blood pressure by reducing oxidative stress by the suppression of the renin–angiotensin system cascade[56]. In addition, hesperidin improved the reported oxidative stress observed under hypertensive conditions as a consequence of an overexpression of NADPH oxidase via suppression of this enzyme, which results in enhanced NO bioavailability[56][64][65].
Despite the beneficial effects observed in animal and in vitro studies, the results shown by human interventional studies are not consistent. Asgary et al. demonstrated that consumption of 500 mL/day of OJ decreased SBP and DBP in healthy subjects after four weeks[66]. Similar results were also reported by Morand et al. when evaluating the effect of daily consumption of OJ for four weeks in healthy volunteers[30]. In this study, it was stated that the beneficial effects on blood pressure maintenance induced by daily OJ consumption could be due to an improvement on endothelial function[30]. In another study, it was also demonstrated that six‐week consumption of hesperidin improved blood pressure in T2D patients. The authors suggested that hesperidin exerts its beneficial effects via anti‐inflammatory activity[67]. Furthermore, our group carried out a clinical study in which the beneficial effects of the consumption of OJ with natural hesperidin content and a hesperidin-enriched OJ on risk factors associated with CVDs, including its antihypertensive effects, in pre- and grade-1 hypertensive individuals were evaluated (submitted for its publication). However, the results of a crossover study that included individuals with MetS presenting prehypertension did not reveal changes in blood pressure after three-week supplementation with hesperidin[27]. Besides, a systematic review and meta-analysis of randomized controlled trials that evaluated the efficacy of hesperidin supplementation on blood pressure concluded that hesperidin intake is not associated with significant changes in blood pressure[68]. Similar results were reported by Plà et al.[69], concluding that hesperidin consumption effects on blood pressure were no conclusive.
There are many hypotheses that could explain why hesperidin lacks a significant effect on blood pressure in humans, including its metabolization and absorption. In this sense, Yamamoto et al.[70] reported that the hesperidin metabolite hesperetin-7-O-glucuronide, but not hesperetin-3-O-glucuronide, was the responsible agent for the demonstrated antihypertensive effect. Therefore, not all hesperidin metabolites present the same biological effects when administered. In addition, few studies are available to clarify the pharmacokinetics of hesperidin[71]. In consequence, it might be possible that hesperidin does not reach the sufficient circulating concentrations that are needed for the regulation of blood pressure.
In conclusion, the results from in vivo and in vitro studies point out that hesperidin represents a promising agent for the prevention and/or the treatment of CVDs. From these studies, it could be concluded that the potential mechanisms by which hesperidin exerts its beneficial effects include the regulation of gene expression and enzymatic activity of key proteins involved in pathways related to lipid and glucose metabolism, blood pressure control, and obesity development. Furthermore, it has been demonstrated the antioxidant and anti-inflammatory activities of hesperidin that may explain, at least in part, the observed beneficial effects on CVDs. However, despite all the evidences from in vitro and animal models, there is still controversy about whether hesperidin and their derivatives could contribute to ameliorating glucose homeostasis, lipid profile, adiposity, and blood pressure and thus reduce the cardiovascular risk, especially in humans (Figure 2). A possible explanation for the lack of conclusive results from human studies might be related to the presence of several important factors, including interindividual differences and external factors that impact the effectiveness of hesperidin in humans, including interindividual differences and external factors, with the variability of hesperidin bioavailability due to differences in intestinal microbiota composition and activity among individuals being a major factor. Although a low bioavailability of hesperidin has been described in animal studies[72][73], the variability in studies with experimental animals is much lower than that observed in clinical studies due to inbreeding, leading to a phenotypic uniformity between the animals. Furthermore, external factors such as diet, physical activity, or seasonality are much more controlled than in human studies, leading to lower variability in intestinal microbiota composition and activity. Further well-designed clinical trials to specifically examine the effects of hesperidin on CVD risk factors, considering the variability that exists in the response to treatment with hesperidin in humans, are necessary. In this sense, intestinal microbiota may play a role in this interindividual variability, as it has been shown to have a direct effect on the absorption and bioavailability of polyphenols, such as hesperidin.
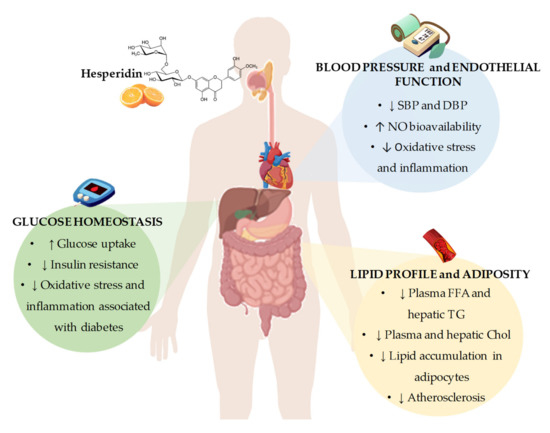
Figure 2. Summary of the most representative effects of hesperidin consumption and its derivatives on cardiovascular risk factors, including glucose homeostasis, blood pressure and endothelial function, and lipid profile and adiposity. SBP: systolic blood pressure; DBP: diastolic blood pressure; NO: nitric oxide; FFA: free fatty acids; TG: triglycerides; Chol: cholesterol.
References
- Carlos A. Alvarez; Ildiko Lingvay; Valerie Vuylsteke; Robin L. Koffarnus; Darren K. McGuire; Cardiovascular Risk in Diabetes Mellitus: Complication of the Disease or of Antihyperglycemic Medications.. Clinical Pharmacology & Therapeutics 2015, 98, 145-161, 10.1002/cpt.143.
- Jiukai Zhang; Chongde Sun; Youyou Yan; Qingjun Chen; Fenglei Luo; Xiaoyan Zhu; Xian Li; Kunsong Chen; Purification of naringin and neohesperidin from Huyou (Citrus changshanensis) fruit and their effects on glucose consumption in human HepG2 cells. Food Chemistry 2012, 135, 1471-1478, 10.1016/j.foodchem.2012.06.004.
- Hu Xuguang; Tian Aofei; Liu Tao; Zhou Longyan; Bei Weijian; Guo Jiao; Hesperidin ameliorates insulin resistance by regulating the IRS1-GLUT2 pathway via TLR4 in HepG2 cells.. Phytotherapy Research 2019, 33, 1697-1705, 10.1002/ptr.6358.
- R. Dhanya; P. Jayamurthy; In vitro evaluation of antidiabetic potential of hesperidin and its aglycone hesperetin under oxidative stress in skeletal muscle cell line. Cell Biochemistry and Function 2020, null, null, 10.1002/cbf.3478.
- Zehra Irshad; Mingzhan Xue; Amal Ashour; James R. Larkin; Paul J. Thornalley; Naila Rabbani; Activation of the unfolded protein response in high glucose treated endothelial cells is mediated by methylglyoxal. Scientific Reports 2019, 9, 7889, 10.1038/s41598-019-44358-1.
- Satoko Akiyama; Shin-Ichi Katsumata; Kazuharu Suzuki; Yoshiko Ishimi; Jian Wu; Mariko Uehara; Dietary Hesperidin Exerts Hypoglycemic and Hypolipidemic Effects in Streptozotocin-Induced Marginal Type 1 Diabetic Rats. Journal of Clinical Biochemistry and Nutrition 2009, 46, 87-92, 10.3164/jcbn.09-82.
- Binit Kumar; Suresh Kumar Gupta; B.P. Srinivasan; Tapas C. Nag; Sushma Srivastava; Rohit Saxena; Hesperetin ameliorates hyperglycemia induced retinal vasculopathy via anti-angiogenic effects in experimental diabetic rats. Vascular Pharmacology 2012, 57, 201-207, 10.1016/j.vph.2012.02.007.
- Asjad Visnagri; Amit Kandhare; Shalendra Chakravarty; Pinaki Ghosh; S.L. Bodhankar; Hesperidin, a flavanoglycone attenuates experimental diabetic neuropathy via modulation of cellular and biochemical marker to improve nerve functions. Pharmaceutical Biology 2014, 52, 814-828, 10.3109/13880209.2013.870584.
- Wanthanee Hanchang; Aree Khamchan; Navinee Wongmanee; Chananchida Seedadee; Hesperidin ameliorates pancreatic β-cell dysfunction and apoptosis in streptozotocin-induced diabetic rat model.. Life Sciences 2019, 235, 116858, 10.1016/j.lfs.2019.116858.
- Sundaram Ramalingam; E. Nandhakumar; H. Haseena Banu; Sundaram R; Nandhakumar E; Haseena Banu H.; Hesperidin, a citrus flavonoid ameliorates hyperglycemia by regulating key enzymes of carbohydrate metabolism in streptozotocin-induced diabetic rats.. Toxicology Mechanisms and Methods 2019, 29, 644-653, 10.1080/15376516.2019.1646370.
- Hatice Iskender; Eda Dokumacioglu; Tugba Mazlum Sen; Imran Ince; Ali Dokumacioğlu; Yalcin Kanbay; Elif Erbas; Sinan Saral; The effects of hesperidin and quercetin on serum tumor necrosis factor-alpha and interleukin-6 levels in streptozotocin-induced diabetes model. Pharmacognosy Magazine 2018, 14, 167-173, 10.4103/pm.pm_41_17.
- Un Ju Jung; Mi-Kyung Lee; Kyu-Shik Jeong; Myung Suk Choi; The Hypoglycemic Effects of Hesperidin and Naringin Are Partly Mediated by Hepatic Glucose-Regulating Enzymes in C57BL/KsJ-db/db Mice. The Journal of Nutrition 2004, 134, 2499-2503, 10.1093/jn/134.10.2499.
- Un Ju Jung; Mi-Kyung Lee; Yong Bok Park; Mi Ae Kang; Myung Suk Choi; Effect of citrus flavonoids on lipid metabolism and glucose-regulating enzyme mRNA levels in type-2 diabetic mice. The International Journal of Biochemistry & Cell Biology 2006, 38, 1134-1145, 10.1016/j.biocel.2005.12.002.
- Satoko Akiyama; Shin-Ichi Katsumata; Kazuharu Suzuki; Yumi Nakaya; Yoshiko Ishimi; Mariko Uehara; Hypoglycemic and Hypolipidemic Effects of Hesperidin and Cyclodextrin-Clathrated Hesperetin in Goto-Kakizaki Rats with Type 2 Diabetes. Bioscience, Biotechnology, and Biochemistry 2009, 73, 2779-2782, 10.1271/bbb.90576.
- Ayman M. Mahmoud; Mohamed B. Ashour; A. Abdel Moneim; Osama M. Ahmed; Hesperidin and naringin attenuate hyperglycemia-mediated oxidative stress and proinflammatory cytokine production in high fat fed/streptozotocin-induced type 2 diabetic rats. Journal of Diabetes and its Complications 2012, 26, 483-490, 10.1016/j.jdiacomp.2012.06.001.
- Sheng Jia; Ying Hu; Wenna Zhang; Xiaoyong Zhao; Yanhong Chen; Chongde Sun; Xian Li; Kunsong Chen; Hypoglycemic and hypolipidemic effects of neohesperidin derived from Citrus aurantium L. in diabetic KK-Aymice. Food & Function 2015, 6, 878-886, 10.1039/c4fo00993b.
- Ye-Zi Sun; Jian-Fei Chen; Li-Min Shen; Ji Zhou; Cui-Fang Wang; Anti-atherosclerotic effect of hesperidin in LDLr −/− mice and its possible mechanism. European Journal of Pharmacology 2017, 815, 109-117, 10.1016/j.ejphar.2017.09.010.
- Jordi Mayneris-Perxachs; Juan Maria Alcaide-Hidalgo; Esther De La Hera; Josep Maria Del Bas; Lluís Arola; Antoni Caimari; Supplementation with biscuits enriched with hesperidin and naringenin is associated with an improvement of the Metabolic Syndrome induced by a cafeteria diet in rats. Journal of Functional Foods 2019, 61, , 10.1016/j.jff.2019.103504.
- Kanwal Rehman; Syeda Mehak Munawar; Muhammad Sajid Hamid Akash; Manal Ali Buabeid; Tahir Ali Chohan; Muhammad Tariq; Komal Jabeen; El-Shaimaa A. Arafa; Correction: Hesperidin improves insulin resistance via down-regulation of inflammatory responses: Biochemical analysis and in silico validation. PLOS ONE 2020, 15, e0229348, 10.1371/journal.pone.0229348.
- Pu, P.; Protection mechanisms of hesperidin on mouse with insulin resistance. China Journal of Chinese Materia Medica 2016, 41, 3290–3295, 10.4268/cjcmm20161728.
- Haoshu Wu; Yunxi Liu; Xiaobing Chen; Difeng Zhu; Jian Ma; Youyou Yan; Meimei Si; Xian Li; Chongde Sun; Bo Yang; Qiaojun He; Kunsong Chen; Neohesperidin Exerts Lipid-Regulating Effects in vitro and in vivo via Fibroblast Growth Factor 21 and AMP-Activated Protein Kinase/Sirtuin Type 1/Peroxisome Proliferator-Activated Receptor Gamma Coactivator 1α Signaling Axis. Pharmacology 2017, 100, 115-126, 10.1159/000452492.
- Zahra Yari; Mina Movahedian; Hossein Imani; Seyed Moayed Alavian; Mehdi Hedayati; Azita Hekmatdoost; The effect of hesperidin supplementation on metabolic profiles in patients with metabolic syndrome: a randomized, double-blind, placebo-controlled clinical trial.. European Journal of Nutrition 2019, null, 1-9, 10.1007/s00394-019-02105-2.
- Carolina Ribeiro; Grace Dourado; Thais Cesar; Orange juice allied to a reduced-calorie diet results in weight loss and ameliorates obesity-related biomarkers: A randomized controlled trial. Nutrition 2017, 38, 13-19, 10.1016/j.nut.2016.12.020.
- Ana Carolina Delgado Lima; Clara Cecatti; Melaine Priscila Fidélix; Maria Angela Tallarico Adorno; Isabel Kimiko Sakamoto; Thais Cesar; Katia Sivieri; Effect of Daily Consumption of Orange Juice on the Levels of Blood Glucose, Lipids, and Gut Microbiota Metabolites: Controlled Clinical Trials. Journal of Medicinal Food 2019, 22, 202-210, 10.1089/jmf.2018.0080.
- Makan Cheraghpour; Hossein Imani; Shahrzad Ommi; Seyed Moayed Alavian; Elahe Karimi‐Shahrbabak; Mehdi Hedayati; Zahra Yari; Azita Hekmatdoost; Hesperidin improves hepatic steatosis, hepatic enzymes, and metabolic and inflammatory parameters in patients with nonalcoholic fatty liver disease: A randomized, placebo-controlled, double-blind clinical trial.. Phytotherapy Research 2019, 33, 2118-2125, 10.1002/ptr.6406.
- Fatemeh Homayouni; Fatemeh Haidari; Mehdi Hedayati; Mehrnoosh Zakerkish; Kambiz Ahmadi; Hesperidin Supplementation Alleviates Oxidative DNA Damage and Lipid Peroxidation in Type 2 Diabetes: A Randomized Double-Blind Placebo-Controlled Clinical Trial. Phytotherapy Research 2017, 31, 1539-1545, 10.1002/ptr.5881.
- Stefano Rizza; Ranganath Muniyappa; Micaela Iantorno; Jeong-A Kim; Hui Chen; Philomena Pullikotil; Nicoletta Senese; Manfredi Tesauro; Davide Lauro; Carmine Cardillo; Michael Quon; Citrus polyphenol hesperidin stimulates production of nitric oxide in endothelial cells while improving endothelial function and reducing inflammatory markers in patients with metabolic syndrome.. The Journal of Clinical Endocrinology & Metabolism 2011, 96, E782-92, 10.1210/jc.2010-2879.
- Bouke N. Salden; Freddy J Troost; Eric De Groot; Yala R Stevens; Marta Garcés-Rimón; Sam Possemiers; Bjorn Winkens; A. A. Masclee; Randomized clinical trial on the efficacy of hesperidin 2S on validated cardiovascular biomarkers in healthy overweight individuals. The American Journal of Clinical Nutrition 2016, 104, 1523-1533, 10.3945/ajcn.116.136960.
- Joël Constans; Catherine Bennetau-Pelissero; Jean-François Martin; Edmond Rock; Andrzej Mazur; Aurélie Bedel; Christine Morand; Annie M. Bérard; Marked antioxidant effect of orange juice intake and its phytomicronutrients in a preliminary randomized cross-over trial on mild hypercholesterolemic men. Clinical Nutrition 2015, 34, 1093-1100, 10.1016/j.clnu.2014.12.016.
- Christine Morand; Claude DuBray; Agan Milenkovic; Delphine Lioger; Jean François Martin; Augustin Scalbert; Andrzej Mazur; Hesperidin contributes to the vascular protective effects of orange juice: a randomized crossover study in healthy volunteers. The American Journal of Clinical Nutrition 2010, 93, 73-80, 10.3945/ajcn.110.004945.
- Oscar D Rangel-Huerta; Concepción María Aguilera; Maria V Martin; Maria J Soto; Maria C Rico; Fernando Vallejo; Francisco Abraham Tomás-Barberán; Antonio J Perez-De-La-Cruz; Angel Gil; María-Dolores Mesa; et al. Normal or High Polyphenol Concentration in Orange Juice Affects Antioxidant Activity, Blood Pressure, and Body Weight in Obese or Overweight Adults. The Journal of Nutrition 2015, 145, 1808-1816, 10.3945/jn.115.213660.
- B. Isomaa; Peter Almgren; Tiinamaija Tuomi; Björn Forsén; Kaj Lahti; Michael Nissén; Marja-Riitta Taskinen; Leif Groop; Cardiovascular morbidity and mortality associated with the metabolic syndrome.. Diabetes Care 2001, 24, 683-689, 10.2337/diacare.24.4.683.
- Jack Stewart; Gavin Manmathan; Peter Wilkinson; Primary prevention of cardiovascular disease: A review of contemporary guidance and literature. JRSM Cardiovascular Disease 2017, 6, 2048004016687211, 10.1177/2048004016687211.
- Gissette Reyes-Soffer; Carlos Rondon-Clavo; Henry N Ginsberg; Combination therapy with statin and fibrate in patients with dyslipidemia associated with insulin resistance, metabolic syndrome and type 2 diabetes mellitus. Expert Opinion on Pharmacotherapy 2011, 12, 1429-1438, 10.1517/14656566.2011.563506.
- Julia M. Assini; Erin E. Mulvihill; Murray W. Huff; Citrus flavonoids and lipid metabolism. Current Opinion in Lipidology 2013, 24, 34-40, 10.1097/mol.0b013e32835c07fd.
- Haijun Xiong; Jin Wang; Qian Ran; Guanhua Lou; Chengyi Peng; Qingxia Gan; Ju Hu; Jilin Sun; Renchuan Yao; Qinwan Huang; Hesperidin: A Therapeutic Agent For Obesity. Drug Design, Development and Therapy 2019, 13, 3855-3866, 10.2147/DDDT.S227499.
- Elizabeth J. Simpson; B. Mendis; Ian A. Macdonald; Orange juice consumption and its effect on blood lipid profile and indices of the metabolic syndrome; a randomised, controlled trial in an at-risk population. Food & Function 2016, 7, 1884-1891, 10.1039/c6fo00039h.
- Nancy P. Aptekmann; Thais Cesar; Orange juice improved lipid profile and blood lactate of overweight middle-aged women subjected to aerobic training. Maturitas 2010, 67, 343-347, 10.1016/j.maturitas.2010.07.009.
- Erin E. Mulvihill; Murray W. Huff; Citrus flavonoids and the prevention of atherosclerosis.. Cardiovascular & Hematological Disorders-Drug Targets 2012, 12, 84-91, 10.2174/1871529x11202020084.
- Saioa Gomez-Zorita; Arrate Lasa; Naiara Abendaño; Alfredo Fernández-Quintela; Andrea Mosqueda-Solís; Maria Pilar Garcia-Sobreviela; Jose M. Arbones-Mainar; Maria P. Portillo; Phenolic compounds apigenin, hesperidin and kaempferol reduce in vitro lipid accumulation in human adipocytes. Journal of Translational Medicine 2017, 15, 237, 10.1186/s12967-017-1343-0.
- Andrea Mosqueda-Solís; Arrate Lasa; Saioa Gómez-Zorita; Itziar Eseberri; Catalina Pico; María P. Portillo; Screening of potential anti-adipogenic effects of phenolic compounds showing different chemical structure in 3T3-L1 preadipocytes. Food & Function 2017, 8, 3576-3586, 10.1039/c7fo00679a.
- Jeong Kee Kim; Hyun Woo Jeong; A Young Kim; Yong Deog Hong; Ji Hae Lee; Jin Kyu Choi; Jae Sung Hwang; Green satsuma mandarin orange (Citrus unshiu) extract reduces adiposity and induces uncoupling protein expression in skeletal muscle of obese mice. Food Science and Biotechnology 2018, 28, 873-879, 10.1007/s10068-018-0503-1.
- Yoshikatsu Miwa; Hitoshi Mitsuzumi; Takahiro Sunayama; Mika Yamada; Katsuhide Okada; Michio Kubota; Hiroto Chaen; Yasuo Mishima; Masayoshi Kibata; Glucosyl hesperidin lowers serum triglyceride level in hypertriglyceridemic subjects through the improvement of very low-density lipoprotein metabolic abnormality.. Journal of Nutritional Science and Vitaminology 2005, 51, 460-470, 10.3177/jnsv.51.460.
- Isabelle Demonty; Yuguang Lin; Yvonne E. M. P. Zebregs; Mario A. Vermeer; Henk C. M. Van Der Knaap; Martin Jäkel; Elke A. Trautwein; The Citrus Flavonoids Hesperidin and Naringin Do Not Affect Serum Cholesterol in Moderately Hypercholesterolemic Men and Women. The Journal of Nutrition 2010, 140, 1615-1620, 10.3945/jn.110.124735.
- Michele Longo; Federica Zatterale; Jamal Naderi; Luca Parrillo; Pietro Formisano; Gregoryalexander Raciti; Francesco Beguinot; Claudia Miele; Adipose Tissue Dysfunction as Determinant of Obesity-Associated Metabolic Complications.. International Journal of Molecular Sciences 2019, 20, 2358, 10.3390/ijms20092358.
- Jonathan Wells; The evolution of human adiposity and obesity: where did it all go wrong?. Disease Models & Mechanisms 2012, 5, 595-607, 10.1242/dmm.009613.
- Alexa Serino; Gloria Salazar; Protective Role of Polyphenols against Vascular Inflammation, Aging and Cardiovascular Disease. Nutrients 2018, 11, 53, 10.3390/nu11010053.
- Andrea Mosqueda-Solís; J. Sánchez; María P. Portillo; Andreu Palou; Catalina Picó; Combination of Capsaicin and Hesperidin Reduces the Effectiveness of Each Compound To Decrease the Adipocyte Size and To Induce Browning Features in Adipose Tissue of Western Diet Fed Rats. Journal of Agricultural and Food Chemistry 2018, 66, 9679-9689, 10.1021/acs.jafc.8b02611.
- Xinhui Wang; Junichi Hasegawa; Yoshiyuki Kitamura; ZhongZhi Wang; Akiko Matsuda; Waka Shinoda; Norimasa Miura; Koji Kimura; Effects of hesperidin on the progression of hypercholesterolemia and fatty liver induced by high-cholesterol diet in rats.. Journal of Pharmacological Sciences 2011, 117, 129-138, 10.1254/jphs.11097fp.
- Tatsuya Ohara; Koutarou Muroyama; Yoshihiro Yamamoto; Shinji Murosaki; Oral intake of a combination of glucosyl hesperidin and caffeine elicits an anti-obesity effect in healthy, moderately obese subjects: a randomized double-blind placebo-controlled trial.. Nutrition Journal 2016, 15, 6, 10.1186/s12937-016-0123-7.
- Stanley S. Franklin; Systolic blood pressureIt’s time to take control. American Journal of Hypertension 2004, 17, S49–S54, 10.1016/j.amjhyper.2004.08.020.
- Alberto Zanchetti; Lower or higher blood-pressure targets for high-risk patients?. Nature Reviews Cardiology 2016, 13, 637-638, 10.1038/nrcardio.2016.165.
- Kazumasa Ohtsuki; Asaki Abe; Hitoshi Mitsuzumi; Masaru Kondo; Kanae Uemura; Yumiko Iwasaki; Yasuhiro Kondo; Effects of Long-Term Administration of Hesperidin and Glucosyl Hesperidin to Spontaneously Hypertensive Rats.. Journal of Nutritional Science and Vitaminology 2002, 48, 420-422, 10.3177/jnsv.48.420.
- Miyako Ikemura; Y Sasaki; John C. Giddings; J Yamamoto; Preventive Effects of Hesperidin, Glucosyl Hesperidin and Naringin on Hypertension and Cerebral Thrombosis in Stroke-prone Spontaneously Hypertensive Rats. Phytotherapy Research 2012, 26, 1272-1277, 10.1002/ptr.3724.
- Guirro, M.; Gual-Grau, A.; Gibert-Ramos, A.; Alcaide-Hidalgo, J.M.; Canela, N.; Mayneris-Perxachs, J. Metabolomics Elucidates Dose-Dependent Molecular Beneficial E ff ects of Hesperidin Supplementation in Rats Fed an Obesogenic Diet. Antioxidants 2020, 9, 79.
- Chutamas Wunpathe; Prapassorn Potue; Putcharawipa Maneesai; Sarawoot Bunbupha; Parichat Prachaney; Upa Kukongviriyapan; Veerapol Kukongviriyapan; Poungrat Pakdeechote; Hesperidin Suppresses Renin-Angiotensin System Mediated NOX2 Over-Expression and Sympathoexcitation in 2K-1C Hypertensive Rats. The American Journal of Chinese Medicine 2018, 46, 751-767, 10.1142/s0192415x18500398.
- Lukáš Dobiaš; Miriam Petrová; Róbert Vojtko; Viera Kristová; Long-term Treatment with Hesperidin Improves Endothelium-dependent Vasodilation in Femoral Artery of Spontaneously Hypertensive Rats: The Involvement of NO-synthase and KvChannels. Phytotherapy Research 2016, 30, 1665-1671, 10.1002/ptr.5670.
- Masaki Yamamoto; Atsushi Suzuki; Tadashi Hase; Short-term effects of glucosyl hesperidin and hesperetin on blood pressure and vascular endothelial function in spontaneously hypertensive rats.. Journal of Nutritional Science and Vitaminology 2008, 54, 95-98, 10.3177/jnsv.54.95.
- Li Liu; Dong-Mei Xu; Yi-Yu Cheng; Distinct Effects of Naringenin and Hesperetin on Nitric Oxide Production from Endothelial Cells. Journal of Agricultural and Food Chemistry 2008, 56, 824-829, 10.1021/jf0723007.
- Maria Guirro; Andreu Gual-Grau; Albert Gibert-Ramos; Juan María Alcaide-Hidalgo; Núria Canela; Lluís Arola; Jordi Mayneris-Perxachs; Metabolomics Elucidates Dose-Dependent Molecular Beneficial Effects of Hesperidin Supplementation in Rats Fed an Obesogenic Diet. Antioxidants 2020, 9, 79, 10.3390/antiox9010079.
- Z. Pons; L. Guerrero; Maria Margalef; Lluís Arola; Anna Arola-Arnal; Begoña Mugureza; Effect of low molecular grape seed proanthocyanidins on blood pressure and lipid homeostasis in cafeteria diet-fed rats. Journal of Physiology and Biochemistry 2014, 70, 629-637, 10.1007/s13105-014-0329-0.
- Chaoyun Li; Hermann Schluesener; Health-promoting effects of the citrus flavanone hesperidin. Critical Reviews in Food Science and Nutrition 2015, 57, 613-631, 10.1080/10408398.2014.906382.
- Patricia Kelly Wilmsen; Dalla Santa Spada; Mirian Salvador; Antioxidant Activity of the Flavonoid Hesperidin in Chemical and Biological Systems. Journal of Agricultural and Food Chemistry 2005, 53, 4757-4761, 10.1021/jf0502000.
- Masaki Yamamoto; Atsushi Suzuki; Hiroko Jokura; Naoki Yamamoto; Tadashi Hase; Glucosyl hesperidin prevents endothelial dysfunction and oxidative stress in spontaneously hypertensive rats. Nutrition 2008, 24, 470-476, 10.1016/j.nut.2008.01.010.
- Mozhdeh Yousefian; Neda Shakour; Hossein Hosseinzadeh; A.Wallace Hayes; Farzin Hadizadeh; Gholamreza Karimi; The natural phenolic compounds as modulators of NADPH oxidases in hypertension. Phytomedicine 2019, 55, 200-213, 10.1016/j.phymed.2018.08.002.
- Sedigheh Asgary; Mahtab Keshvari; Effects of Citrus sinensis juice on blood pressure. ARYA atherosclerosis 2013, 9, 98-101.
- Fatemeh Homayouni; Fatemeh Haidari; Mehdi Hedayati; Mehrnoosh Zakerkish; Kambiz Ahmadi; Blood pressure lowering and anti-inflammatory effects of hesperidin in type 2 diabetes; a randomized double-blind controlled clinical trial. Phytotherapy Research 2018, 32, 1073-1079, 10.1002/ptr.6046.
- Mohammad Mohammadi; Nahid Ramezani-Jolfaie; Elnaz Lorzadeh; Yadollah Khoshbakht; Amin Salehi-Abargouei; Hesperidin, a major flavonoid in orange juice, might not affect lipid profile and blood pressure: A systematic review and meta-analysis of randomized controlled clinical trials. Phytotherapy Research 2019, 33, 534-545, 10.1002/ptr.6264.
- Laura Pla-Pagà; Judit Companys; L Calderón-Pérez; E Llauradó; R Solà; R M Valls; A Pedret; Effects of hesperidin consumption on cardiovascular risk biomarkers: a systematic review of animal studies and human randomized clinical trials. Nutrition Reviews 2019, 77, 845-864, 10.1093/nutrit/nuz036.
- Masaki Yamamoto; Hiroko Jokura; Koujiro Hashizume; Hideo Ominami; Yusuke Shibuya; Atsushi Suzuki; Tadashi Hase; Akira Shimotoyodome; Hesperidin metabolite hesperetin-7-O-glucuronide, but not hesperetin-3′-O-glucuronide, exerts hypotensive, vasodilatory, and anti-inflammatory activities. Food & Function 2013, 4, 1346, 10.1039/c3fo60030k.
- Ming Ji Jin; Unyong Kim; In Sook Kim; Yuri Kim; Sang Beom Han; Ng-Hyun Kim; Oh-Seung Kwon; Hye Hyun Yoo; Effects of Gut Microflora on Pharmacokinetics of Hesperidin: A Study on Non-Antibiotic and Pseudo-Germ-Free Rats. Journal of Toxicology and Environmental Health, Part A 2010, 73, 1441-1450, 10.1080/15287394.2010.511549.
- Qishu Jiao; Lulu Xu; Lijuan Jiang; Yanyan Jiang; Jiayu Zhang; Bin Liu; Metabolism study of hesperetin and hesperidin in rats by UHPLC-LTQ-Orbitrap MSn. Xenobiotica 2019, 18, 1-27, 10.1080/00498254.2019.1567956.
- Véronique Habauzit; Sandra Maria Sacco; Angel Gil-Izquierdo; Anna Trzeciakiewicz; Christine Morand; Denis Barron; Stéphane Pinaud; Elizabeth A. Offord; Marie-Noëlle Horcajada; Differential effects of two citrus flavanones on bone quality in senescent male rats in relation to their bioavailability and metabolism. Bone 2011, 49, 1108-1116, 10.1016/j.bone.2011.07.030.




