Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Xinyi Yang | + 2282 word(s) | 2282 | 2021-05-08 12:52:06 | | | |
| 2 | Catherine Yang | Meta information modification | 2282 | 2021-05-18 04:23:11 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Yang, X. Drought Stress. Encyclopedia. Available online: https://encyclopedia.pub/entry/9735 (accessed on 08 February 2026).
Yang X. Drought Stress. Encyclopedia. Available at: https://encyclopedia.pub/entry/9735. Accessed February 08, 2026.
Yang, Xinyi. "Drought Stress" Encyclopedia, https://encyclopedia.pub/entry/9735 (accessed February 08, 2026).
Yang, X. (2021, May 18). Drought Stress. In Encyclopedia. https://encyclopedia.pub/entry/9735
Yang, Xinyi. "Drought Stress." Encyclopedia. Web. 18 May, 2021.
Copy Citation
Drought is one of the most important factors restricting agricultural production, which seriously affects crop yield.
drought stress
osmotic regulation
LEA protein
1. Introduction
As one of the main restraining factors in the process of plant growth, drought can hinder plant respiration, photosynthesis, and stomatal movement; thus, affecting plant growth and physiological metabolism. In response to drought stress, plants activate their drought response mechanisms, such as morphological and structural changes, expression of drought-resistant genes, synthesis of hormones, and osmotic regulatory substances to alleviate drought stress. To better reveal the mechanism of drought resistance of plants, based on a lot of previous work, we summarized the status quo and progress of studies on the morphological structure, physiological and biochemical mechanism changes, internal signal transduction system, and molecular regulation mechanism of plants under drought stress in recent years. Under drought conditions, plants sense water stress signals and produce signal molecules, such as abscisic acid (ABA), Ca2+, inositol-1, 4, 5-triphosphate (IP3), cyclic adenosine 5′-diphosphate ribose (cADPR), NO, etc., and directly or indirectly lead to the morphological and physiological changes of plants through signal transduction. Indirectly, drought stress signals induce the expression of downstream genes. Functional gene products, such as proline (pro), glycine betaine (GB), soluble sugar (SS), late embryogenesis abundant (LEA) proteins, and aquaporin (AQP) can be involved in plant metabolism and, thus, affect plant state. Regulatory gene products, such as calcium-dependent protein kinases (CDPKs), mitogen-activated protein kinases (MAPKs), HD-zip/bZIP, AP2/ERF, NAC, MYB, and WRKY can cause changes in plant morphology or physiology by regulating signal transduction pathways or acting as transcription factors to regulate the expression of downstream genes, and further enable plants to successfully survive in the arid environment (Figure 1).
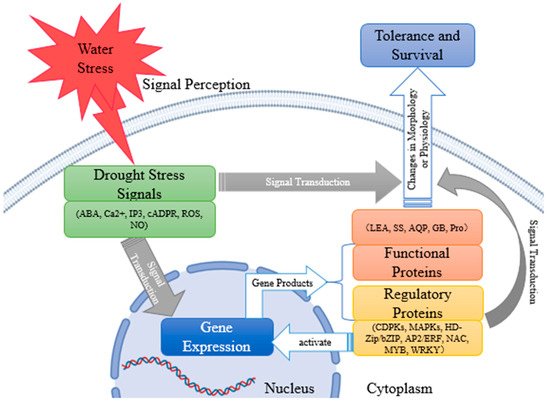
Figure 1. The process of plant drought-tolerance development.
2. Drought Stress Signal Transduction in Plants
The signal transduction process of plants from sensing environmental stimuli to responding to them generally includes three parts: (1) the sensory transduction and response of sensory cells to environmental stimuli, namely the original signal sensory transduction process, producing intercellular messenger; (2) the intercellular messenger is transmitted between cells or tissues, and finally acts on the receptor cell site; (3) the transduction and response of acceptor cells to intercellular messengers lead to physiological, biochemical, and functional changes in the acceptor tissues, which are ultimately reflected in the response of plants to environmental stimuli or adversity [1].
2.1. Plant Drought Stress Signal
The decrease of soil water content caused the change of leaf water status, and then affected the physiological function of plants. Leaf water potential reflects plant water status and is related to specific stress degrees. The decrease of leaf water potential and turgor pressure affected the synthesis, transportation, and distribution of plant hormones, such as ABA and cytokinin. Changes in turgor pressure caused by cell water loss may be the reason for cell perception of water stress, which is also known as the hydraulic signal of plant drought stress [2]. Besides hydraulic signal, the electrical signal also plays an important role in plant signal transduction under drought stress. Fromm et al. proposed through their study on maize in dry soil that electrical signals play an important role in the communication between roots and shoots of water-deficient plants [3]. What is more, when plants feel the initial drought signal, the osmotic stress signal is converted into an intracellular chemical signal by the membrane receptor, which triggers the downstream effector to produce the second messenger. Then the signal is amplified gradually through the cascade transmission of the signal. In the process of signal transduction of dry early stress, the second messengers involved in signal transduction mainly included plant hormone signals, Ca2+, IP3, phosphatidic acid, and ROS signals.
Plant hormones are a kind of chemical signal molecules that regulate plant growth. They often play a regulatory role in a low concentration. They can transmit cell signals in different parts of plants and among cells so that the remote transmission of plant signals can be realized. When soil water content decreases, some physiologically active substances act as chemical signals, and their content increases, which is called a positive signal. For example, under drought stress, the content of IAA, ABA, and ethylene increases. In contrast, a decrease in a biologically active substance is called a negative signal, such as cytokinins.
ABA is a small molecule lipophilic plant hormone, which is a crucial signal molecule in plant water stress. As a kind of plant hormone, ABA can control plant growth, inhibit seed germination and promote aging. In addition to regulating plant growth and development, ABA is also involved in regulating plant responses to various external stresses, embodied in content increasing greatly when the plant is in drought, high salt, low temperature, and other adversities. Moreover, ABA plays a pivotal role in the information connection between the aboveground and underground parts of plants. When plants are under drought stress, ABA produced in the rhizosphere can be used as a positive signal to regulate the physiological activities of aboveground parts. When plants are under water stress, root cells are the first to experience environmental changes and produce ABA, which transmits the signal to other organs and tissues of plants through vascular bundles, causing senescence of leaves and stomatal closure, so as to reduce water loss. ABA can be transported from the underground part to the aboveground part through the xylem, leading to increased ABA content in the leaves. In fact, ABA induces a wide range of downstream signaling factor responses, including kinases, phosphatases, G-proteins, and proteins in the ubiquitin pathway.
ABA has multiple receptors, such as ABAR/CHLH, •GCR2, •GTG1/2, and PYR/PYL/RCAR. These receptor proteins have the activity of protein kinases, which can be activated by binding ABA molecules to change the protein structure, and then activate or inhibit the activity of downstream signaling proteins to transmit signals between cells. Research on ABA receptors is still ongoing, and the exact function of the different receptors remains questionable. ABAR/CHLH is a magnesium ion chelatase H subunit located in plant cyto-plastids/chloroplasts. It not only catalyzes the synthesis of chlorophyll in cells but also participates in the reverse signal transfer between plastids/chloroplasts and the nucleus under stress conditions [4][5]. GCR2 protein is a G protein coupled receptor located in the plasma membrane of the cell. The C-terminal of GCR2 protein can interact with the A subunit of G protein (GPA1) to form a complex. The specific binding of ABA and GCR2 protein induces the release of G protein. The G protein is then separated into Gα and Gβγ dimer, and the signal response of ABA is regulated by the downstream effector of GCR2 protein [6]. G protein, consisting of Gα, Gβ, and Gγ subunits, plays an important role in response to plant hormone signaling by synergistic G-protein coupled receptors and their downstream effectors. GTG1/2 was first identified and named by Pandey et al. through bioinformatics analysis. In the ABA signal transduction pathway model with GTG1/2 as the receptor, GPA1–GTP promoted GTG–GTP to maintain a high level by inhibiting GTG1/2 protease activity, thus reducing the binding probability of GTG–GDP and ABA. On the contrary, the binding of GTGSGDP to ABA can lead to the configuration change and then initiate ABA signaling response, but the specific molecular mechanism has not been clarified. The PYR/PYL/RCAR protein binds to ABA molecules outside the cell membrane, which in turn binds and inhibits the phosphatase activity of the downstream protein phosphatase PP2C [7].
As an essential mineral element in plants, Ca2+ plays an important role in maintaining the stability of cell membrane and cell wall structure and participating in intracellular homeostasis and regulation of growth and development in terms of cell structure and physiological functions. Wang et al. found that extracellular Ca2+ can activate the increase of intracellular Ca2+ concentration through the calcium-sensing receptor (CAS) on the plasma membrane of guard cells of Arabidopsis thaliana, thus confirming the role of extracellular Ca2+ as the first messenger [8]. In addition, as mentioned above, in response to drought, plants synthesize the hormone ABA, which causes stomatal closure to reduce water loss. During stomatal closure, the concentration of Ca2+ in the cytoplasm increases, and Ca2+ acts as the second messenger in osmotic stress response [9]. Drought-induced transient increase of intracellular Ca2+ in guard cells promotes stomatal closure, maintains plant water, improves water use efficiency, and ultimately enhances plant adaptation to drought by interacting with or without ABA signaling pathways and downstream signal transduction mechanisms. In stomatal closure, the ABA-dependent Ca2+ signaling pathway is the main pathway. ABA activates plasma membrane calcium channels in various ways and stimulates intracellular calcium reservoirs to release Ca2+. More Ca2+ will inhibit the inward potassium channel and further affect the anion channel. The phenomenon of anion outflow and depolarization will block the inward potassium channel and promote the outward potassium channel, leading to potassium ion outflow [10]. The guard cells are under low turgor pressure due to a large outflow of anions and potassium ions, making the stomata close gradually. IP3 and cyclic adenosine 5′-diphosphate ribose (cADPR) are also key second messengers in guard cells that can regulate Ca2+ concentration. IP3 and cADPR can release Ca2+ in guard cells and increase the concentration of Ca2+, while ABA can rapidly increase IP3 and cADPR in guard cells. These three second messengers initiate calcium channels to transfer calcium ions into the cytoplasm and accumulate in large quantities, causing ion channels to interact with each other to produce a series of effects that promote stomatal closure [11][12]. Ca2+ transmits stress signals downstream by interacting with protein receptors. Major Ca2+ signal transduction pathways are involved in calcium-regulated kinase-mediated phosphorylation, including the regulation of downstream gene expression by Ca2+ regulating transcription factors and Ca2+ sensitive promoter elements [13]. Calcium-dependent protein kinases (CDPKs), calmodulin (CaM), and calcineurin B-like proteins (CBLs), which have been identified in plants, can recognize specific Ca2+ and rely on these calcium signals to transmit downstream to adapt to drought stress.
A certain amount of ROS produced under stress can be used as signal molecules to activate relevant active substances or defense systems, and mitigate the damage caused by abiotic stress [14]. Among ROS, H2O2 is mostly used as an important signal molecule for animal and plant cells to respond to various stresses because H2O2 is a very stable ROS with the longest half-life and strong diffusivity. Different plant organelles have different responses to cellular REDOX signals under drought stress. Although H2O2 is produced faster in peroxisomes and chloroplasts, mitochondria are the most vulnerable organelles to oxidative damage [15][16]. Increased mitochondrial production of H2O2 may be an important alarm signal, up-regulating the antioxidant defense system or triggering programmed cell death when oxidative stress intensifies. Studies have shown that H2O2 can regulate calcium mobilization, protein phosphorylation, and gene expression. Pei et al. found that H2O2 can regulate Ca2+ influx in protoplasts and increase of [Ca2+]cyt in guard cells by activating Ca2+ channels in the plasma membrane of guard cells of Arabidopsis thaliana. In addition, they further proposed that ABA-induced H2O2 production and H2O2−activated Ca2+ channels are important mechanisms of ABA-induced stomatal closure [17]. Mori et al. also reported an inevitable link between ROS signaling and stomatal closure in plants [18]. Yan et al. also reached the same conclusion: ABA can promote the production of ROS, and the ROS produced can act as signal molecules to regulate stomatal closure [19]. In addition, H2O2 also induces the phosphorylation of mitogen-activated protein kinase (MAPK), which is involved in multiple signal transduction cascades that regulate downstream gene expression [20].
2.2. Intracellular Transduction Pathways and Regulation Mechanisms of Plant Drought Stress Signals
Drought stress signal transduction can be divided into two pathways. The first pathway is the ROS-activated MAPK cascade pathway. MAPK cascade regulates antioxidant defense system and osmotic regulation system in plants. Furthermore, the damage caused by drought stress can be relieved by removing ROS and changing the osmotic potential of cells. The other pathway is Ca2+-dependent stress signaling bypass mediated by calmodulin-dependent protein kinase (CDPK). Ca2+ signal is produced under drought stress, and Ca2+ signal further regulates the expression of plant protective proteins, such as LEA protein through CDPK, which is involved in the late response to drought stress, and ultimately enhances the drought resistance of plants.
Mitogen-activated protein kinases (MAPKs) are a class of important protein kinases involved in signal transduction, which play an extremely important role in plant growth, development, and stress response [21]. The MAPK cascade consists of three components: MAPK, MAPKK (MAPK kinase), and MAPKKK (MAPK kinase kinase). When the first member of this pathway, MAPKKK, is activated, the other two components undergo sequential phosphorylation and are activated in turn. The reason is that MAPKKK can double phosphorylate the serine (Ser) and serine/threonine (Ser/Thr) in MAPKK, thus activating it. The protein kinase of MAPK containing n conservative district and a very conservative TXY motif between the VII and the III subregion [22]. MAPKK initiates MAPKK by dual phosphorylation of threonine (T) and tyrosine (Y) residues at both ends of the X site [23]. As a result, MAPKK phosphorylates MAPKK and MAPKK phosphorylates MAPKK. Activated MAPKK can activate transcription factors and also cause cellular signaling responses through interactions with other proteins.
The full name of CDPK is calmodulin-dependent/calmodulin-independent protein kinase or calmodulin-like domain protein kinase. It belongs to Ser/Thr type protein kinases and is a large family encoded by multiple genes. Under the stimulation of external signals, plant cells showed changes in Ca2+ concentration and then activated CDPK. CDPK regulates downstream gene expression and product activity through the phosphorylation cascade. These products play an important role in the regulation of gene expression, enzyme metabolism, ion, and water transmembrane transport, and other microscopic aspects so that plants show macroscopic changes such as growth and development, stress resistance changes [24].
References
- Davies, W.J.; Zhang, J.H. Root Signals and the Regulation of Growth and Development of Plants in Drying Soil. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1991, 42, 55–76.
- Chazen, O.; Neumann, P.M. Hydraulic Signals from the Roots and Rapid Cell-Wall Hardening in Growing Maize (Zea mays L.) Leaves Are Primary Responses to Polyethylene Glycol-Induced Water Deficits. Plant Physiol. 1994, 104, 1385–1392.
- Fromm, J.; Fei, H. Electrical signaling and gas exchange in maize plants of drying soil—ScienceDirect. Plant Sci. 1998, 132, 203–213.
- Walker, J.C.; Willows, D.R. Mechanism and regulation of Mg-chelatase. Biochem. J. 1997, 327, 321–333.
- Mochizuki, N.; Brusslan, J.A.; Larkin, R. Arabidopsis genomes uncoupled 5 (GUN5) mutant reveals the involvement of Mg-chelatase H subunit in plastid-to-nucleus signal transduction. Proc. Natl. Acad. Sci. USA 2001, 98, 2053–2058.
- Liu, X.; Yue, Y.; Li, B.; Nie, Y.; Li, W.; Wu, W.H.; Ma, L. A G protein-coupled receptor is a plasma membrane receptor for the plant hormone abscisic acid. Science 2007, 315, 1712–1716.
- Yin, P.; Fan, H.; Hao, Q.; Yuan, X.; Wu, D.; Pang, Y.; Yan, C.; Li, W.; Wang, J.; Yan, N. Structural insights into the mechanism of abscisic acid signaling by PYL proteins. Nat. Struct. Mol. Biol. 2009, 16, 1230–1237.
- Wang, W.H.; Yi, X.Q.; Han, A.D.; Liu, T.W.; Chen, J.; Wu, F.H.; Dong, X.J.; He, J.X.; Pei, Z.M.; Zheng, H.L. Calcium-sensing receptor regulates stomatal closure through hydrogen peroxide and nitric oxide in response to extracellular calcium in Arabidopsis. J. Exp. Bot. 2012, 63, 177–190.
- Case, R.M.; Eisner, D.; Gurney, A.; Jones, O.; Muallem, S.; Verkhratsky, A. Evolution of calcium homeostasis: From birth of the first cell to an omnipresent signalling system. Cell Calcium 2007, 42, 345–350.
- Kim, T.H.; Böhmer, M.; Hu, H.; Nishimura, N.; Schroeder, J.I. Guard Cell Signal Transduction Network: Advances in Understanding Abscisic Acid, CO2, and Ca2+ Signaling. Annu. Rev. Plant Biol. 2010, 61, 561–591.
- Li, S.; Assmann, S.M.; Albert, R. Predicting essential components of signal transduction networks: A dynamic model of guard cell abscisic acid signaling. Plos Biol. 2006, 4, e312.
- McAdam, S.A.M.; Brodribb, T.J. Separating active and passive influences on stomatal control of transpiration. Plant Physiol. 2014, 164, 1578–1586.
- Kudla, J.; Batistic, O.; Hashimoto, K. Calcium Signals: The Lead Currency of Plant Information Processing. Plant Cell 2010, 22, 541–563.
- Miller, G.; Shulaev, V.; Mittler, R. Reactive oxygen signaling and abiotic stress. Physiol. Plant. 2008, 133, 481–489.
- Kohli, S.K.; Khanna, K.; Bhardwaj, R.; Abd Allah, E.F.; Ahmad, P.; Corpas, F.J. Assessment of Subcellular ROS and NO Metabolism in Higher Plants: Multifunctional Signaling Molecules. Antioxidants 2019, 8, 641.
- Singh, A.; Kumar, A.; Yadav, S.; Singh, I.K. Reactive oxygen species-mediated signaling during abiotic stress. Plant Gene 2019, 18, 100173.
- Pei, Z.M.; Murata, Y.; Benning, G.; Thomine, S.; Klüsener, B.; Allen, G.J.; Grill, E.; Schroeder, J.I. Calcium channels activated by hydrogen peroxide mediate abscisic acid signalling in guard cells. Nature 2000, 406, 731–734.
- Mori, I.C.; Schroeder, J.I. Reactive oxygen species activation of plant Ca2+ channels. A signaling mechanism in polar growth, hormone transduction, stress signaling, and hypothetically mechanotransduction. Plant Physiol. 2004, 135, 702–708.
- Yan, J.; Tsuichihara, N.; Etoh, T.; Iwai, S. Reactive oxygen species and nitric oxide are involved in ABA inhibition of stomatal opening. Plant Cell Environ. 2007, 30, 1320–1325.
- Hossain, M.A.; Bhattacharjee, S.; Armin, S.-M.; Qian, P.; Xin, W.; Li, H.-Y.; Burritt, D.J.; Fujita, M.; Tran, L.-S.P. Hydrogen peroxide priming modulates abiotic oxidative stress tolerance: Insights from ROS detoxification and scavenging. Front. Plant Sci. 2015, 6, 420.
- Colcombet, J.; Hirt, H. Arabidopsis MAPKs: A complex signalling network involved in multiple biological processes. Biochem. J. 2008, 413, 217–226.
- Hirt, H. Multiple roles of MAP kinases in plant signal transduction. Trends Plant Sci. Trends Plant Sci 1997, 2, 11–15.
- Payne, D.M.; Rossomando, A.J.; Martino, P.; Erickson, A.K.; Sturgill, T.W. Identification of the regulatory phosphorylation sites in pp42/mitogen-activated protein kinase (MAP kinase). Embo J. 1991, 10, 885–892.
- Tuteja, N.; Mahajan, S. Calcium signaling network in plants: An overview. Plant Signal. Behav. 2007, 2, 79–85.
More
Information
Subjects:
Horticulture
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.1K
Revisions:
2 times
(View History)
Update Date:
18 May 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




