
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Mahmoud Elsohly | + 3288 word(s) | 3288 | 2021-05-13 10:18:21 | | | |
| 2 | Dean Liu | -10 word(s) | 3278 | 2021-05-18 03:14:20 | | |
Video Upload Options
Cannabis sativa is one of the oldest medicinal plants in the world. It was introduced into Western medicine during the early 19th century. It contains a complex mixture of secondary metabolites, including cannabinoids and non-cannabinoid-type constituents.
1. Introduction
Cannabis sativa L. belongs to the plant family Cannabaceae, which only has one genus (Cannabis) with only one highly variable species, C. sativa. This is one of the oldest plants grown for food, fiber, and medicine. It grows in all habitats, ranging from sea level to temperate to alpine foothills. The plant originated in Western Asia and introduced to western medicine during the early 19th century. Cannabis has a long history of being used as a medicine to treat a variety of ailments, including asthma, epilepsy, fatigue, glaucoma, insomnia, nausea, pain, and rheumatism [1].
Cannabis is primarily a dioecious plant (male and female flowers occur on individual plants); it is only occasionally found as a hermaphrodite (male and female flowers on the same plant). It flowers under a short photoperiod (below 12 h of light) and continues growing vegetatively during the longer photoperiod days.
The plant is a chemically complex species, due to its numerous natural constituents [2]. Cannabinoids, a specific chemical class found in cannabis, are produced in the glandular trichomes of the plant. Among the cannabinoid constituents of cannabis, Δ9-tetrahydrocannabinol (Δ9-THC), which is naturally present in the form of an acid (Δ9-tetrahydrocannabinolic acid, Δ9-THCA), is the main psychoactive constituent of the plant. Decarboxylation of the acid with age or heat is required to form the pharmacologically active Δ9-tetrahydrocannabinol [3]. Cannabidiol (CBD), another cannabinoid of current interest, is reported to be active as an antiepileptic agent, particularly for the treatment of intractable pediatric epilepsy [4][5].
Other than Δ9-THC and CBD, tetrahydrocannabivarin (THCV), cannabinol (CBN), cannabigerol (CBG), and cannabichromene (CBC) are four other major cannabinoids also identified in C. sativa. Modern studies report that the pharmacological effects of phytocannabinoids result from their ability to interact with cannabinoid receptors and/or with other kinds of pharmacological targets, including non-cannabinoid receptors [6]. Thus far, more than 500 constituents have been reported from Cannabis, out of which 125 are classified as cannabinoids. The non-cannabinoid constituents include non–cannabinoid phenols, flavonoids, terpenes, alkaloids and others.
2. Cannabinoids: (125 Compounds)
Cannabinoids are a group of compounds with a characteristic C21 terpenophenolic backbone. This nomenclature can be applied to parent cannabinoids, cannabinoid derivatives, and transformation products. These cannabinoids can be further classified into 11 cannabinoid sub-classes, namely; cannabichromene (CBC), cannabidiol (CBD), cannabielsoin (CBE), cannabigerol (CBG), cannabicyclol (CBL), cannabinol (CBN), cannabinodiol (CBND), cannabitriol (CBT), (−)-Δ8-trans-tetrahydrocannabinol (Δ8-THC), (−)-Δ9-trans-tetrahydrocannabinol (Δ9-THC), and miscellaneous-type cannabinoids.
2.1. (−)-Δ9-Trans-Tetrahydrocannabinol (Δ9-THC) Type (25 Cannabinoids)
The isolation of pure (−)-Δ9-trans-tetrahydrocannabinol (Δ9-THC, 1), from a hexane extract of hashish using column chromatography over florisil followed by alumina was reported by Goani and Mecholum in 1964. A crystalline nitrophenylurethane derivative of THC was prepared, followed by a mild alkaline hydrolysis for further purification of THC. IR and NMR spectroscopic techniques were used to elucidate its chemical structure [7]. In 1967, (−)-Δ9-trans-tetrahydrocannabinolic acid A (Δ9-THCAA, 2) was isolated using a cellulose powder column (eluted with a mixture of hexane and dimethylformamide), followed by preparative thin layer chromatography. The chemical structure of THCAA (2) was elucidated through a combination of UV, IR, and NMR spectroscopic analysis [8]. The isolation of Δ9-THCAA (2) was also reported using an acid–base extraction procedure [9]. The methodology of obtaining both Δ9-THC (1) and Δ9-THCAA (2) from C. sativa plant material was optimized, using an enhanced extraction procedure, and the separation of Δ9-THC (1) was further improved using fractional distillation. For extensive separation, different stationary phases, such as silica, alumina, and C18 silica, were tested to obtain pure Δ9-THC (1) with a high yield. Following these tests, an efficient, preparative C18 HPLC method was developed for the purification of Δ9-THC (1) from the distillate. These steps resulted in an overall reduced cost of production [10].
The isolation of (−)-Δ9-trans-tetrahydrocannabinolic acid B (Δ9-THCAB, 3) was reported from a hashish sole, using a silicic acid column eluted with a mixture of diethyl ether in petroleum ether. The structure of 3 was established by comparing its physical properties (m.p., optical rotation, MS, UV, and IR) with those of THCAA (2) [11]. The crystal structure of Δ9-THCAB (3) was determined in 1975 through slow evaporation of its chloroform solution [12]. The compounds (−)-Δ9-trans-tetrahydrocannabinol-C4 (Δ9-THC-C4, 4) and (−)-Δ9-trans-tetrahydrocannabinolic acid A-C4 (Δ9-THCAA-C4, 5) were identified and characterized through the analysis of ethyl acetate extracts of police confiscated cannabis resins, tinctures, and leaves using GC-MS [13]. In 1971, (−)-Δ9-trans-tetrahydrocannabivarin (Δ9-THCV, 6) was isolated from a cannabis tincture of Pakistani origin, using the counter-current distribution technique to isolate the compound from a light petroleum ether extract and its chemical structure was determined by IR, NMR and MS spectroscopy, and was confirmed by synthesis [14]. The isolation of (−)-Δ9-trans-tetrahydrocannabivarinic acid (Δ9-THCVAA, 7) from fresh C. sativa leaves from South Africa was reported in 1973 [15]. The chemical structure of Δ9-THCVAA (7) was determined in 1977 using IR, UV, and NMR spectral analysis; the structure was confirmed by via MS analysis of the methyl ester of the cannabinoid, yielding a characteristic fragmentation pattern with 28 mass units less [16]. In 1973, (−)-Δ9-trans-tetrahydrocannabiorcol (Δ9-THCO or Δ9-THC1, 8) was identified from the analysis of a light petroleum ether extract of Brazilian marijuana [17]. The GC-MS analysis of different cannabis samples resulted in the identification of (−)-Δ9-trans-tetrahydrocannabiorcolic acid (Δ9-THCOAA, 9) [13].
In 2015, (−)-Δ9-trans-tetrahydrocannabinal (Δ9-THC aldehyde, 10) was isolated from a high potency variety of C. sativa by applying VLC (Vacuum Liquid Chromatography), silica gel column chromatography, and HPLC chromatographic techniques and identified by 1D and 2D NMR [18]. Eight cannabinoid esters (11–17) were isolated from the hexane extract of the same high potency variety of C. sativa seven years earlier, using various chromatographic techniques, such as vacuum liquid chromatography (VLC), C18 semi-preparative HPLC, and chiral HPLC. The compounds were identified as β-fenchyl (−)-Δ9-trans-tetrahydrocannabinolate (11), α-fenchyl (−)-Δ9-trans-tetrahydrocannabinolate (12), epi-bornyl (−)-Δ9-trans-tetrahydrocannabinolate (13), bornyl (−)-Δ9-trans-tetrahydrocannabinolate (14), α-terpenyl (−)-Δ9-trans-tetrahydrocannabinolate (15), 4-terpenyl (−)-Δ9-trans-tetrahydrocannabinolate (16), α-cadinyl (−)-Δ9-trans-tetrahydrocannabinolate (17), and γ-eudesmyl (−)-Δ9-trans-tetrahydrocannabinolate (18) by spectroscopic analysis (1H NMR, 13C NMR, 2D NMR) and GC-MS analysis [19]. Three additional cannabinoid-type compounds were isolated and identified in 2015, namely 8α-hydroxy-(−)-Δ9-trans-tetrahydrocannabinol (19), 8β-hydroxy-(−)-Δ9-trans-tetrahydro cannabinol (20), and 11-acetoxy- (−)-Δ9-trans-tetrahydrocannabinolic acid A (21) from high potency C. sativa. Multiple chromatographic techniques were used, including silica gel VLC, C18-solid phase extraction (SPE), and HPLC [20]. The compound 8-oxo-(−)-Δ9-trans-tetrahydrocannabinol (22) was also isolated from the same high potency C. sativa variety [18]. The chemical structures of compounds 19–20 were elucidated based on HRESIMS and NMR analysis [18][20].
Cannabisol (23) was isolated from a group of illicit cannabis samples received under the cannabis potency monitoring program, with a high CBG content using flash silica gel chromatography eluted with hexane/CHCl3 (1:1). The structure of 23 was unambiguously deduced by HRESIMS, GCMS, and NMR spectroscopy. GC-MS analysis of these samples indicated the dimeric nature of the compound, displaying two molecular ion peaks at m/z 314 and m/z 328, corresponding to Δ9-THC and 2-methyl-Δ9-THC, respectively [21].
Two new heptyl and hexyl homologs of Δ9-THC namely, (−)-Δ9-trans-tetrahydrocannabiphorol (24) and (−)-Δ9-trans-tetrahydrocannabihexol (25) were recently isolated from the hexane extract of C. sativa inflorescences of an Italian origin (strain CIN-RO). The hexane extract was cooled at −20 °C for 48 h to remove waxes by precipitation. The dewaxed extract was subjected to semi-preparative liquid chromatography on a C18 stationary phase column to isolate compounds 24 and 25 after heating the corresponding acids at 120 °C for 2 h as clear oil. 1 H and 13C NMR, circular dichroism (CD) and UV absorption spectroscopy, along with LC-HRMS were used to determine their chemical structure. Both compounds were prepared synthetically using stereoselective synthesis to confirm their chemical structures [22][23]. All of the Δ9-THC type cannabinoids are shown in Figure 1.
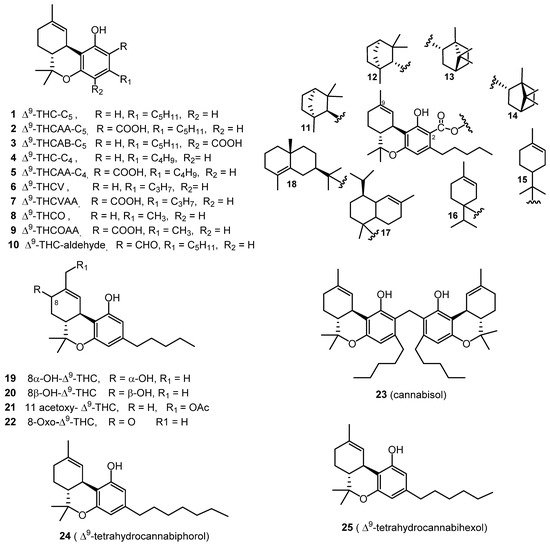
Figure 1. Δ9-THC-Type Cannabinoids.
2.2. (−)-Δ8-Trans-Tetrahydrocannabinol (Δ8-THC) Type (Five Cannabinoids)
The cannabinoid (−)-Δ8-trans-tetrahydrocannabinol (Δ8-THC, 26) was isolated in 1966 from the leaves and flowers of Cannabis grown in Maryland. Δ8-THC was purified from the petroleum ether extract through silicic acid column chromatography using benzene and an eluent [24]. Nine years later, its carboxylic acid, Δ8- trans-tetrahydrocannabinolic acid A (Δ8-THCA, 27) was isolated as the methyl ester from a Cannabis plant of Czechoslovakian origin (Figure 2) [25]. NMR analysis was used to determine the chemical structure of [26]. In 2015, three hydroxylated Δ8-THC-type cannabinoids were isolated from high-potency C. sativa. The structural elucidation for 10α-hydroxy-Δ8-tetra-hydrocannabinol (28), 10β-hydroxy-Δ8-tetra-hydrocannabinol (27) and 10a-α-hydroxy-10-oxo-Δ8-tetrahydrocannabinol (30) was carried out using 1D and 2D NMR spectra [18][20]. The Δ8-THC type cannabinoids are shown in Figure 2.
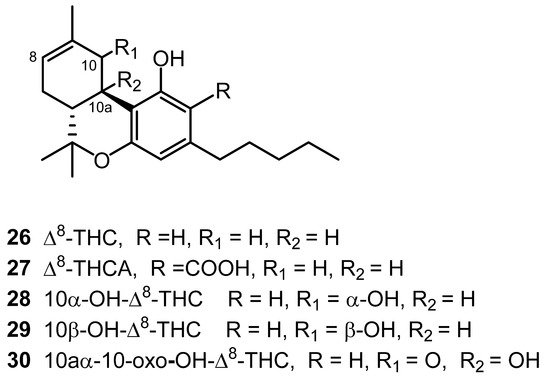
Figure 2. Δ8-THC-Type Cannabinoids.
2.3. Cannabigerol (CBG) Type (16 Cannabinoids)
Sixteen cannabinoids (31–46) were classified as CBG-type cannabinoids (Figure 3). In 1964, cannabigerol ((E)-CBG, 31) was isolated from cannabis resin, using florisil chromatography; the chemical structure of (E)-CBG (31) was confirmed through synthesis [7]. Cannabigerolic acid (CBGAA, 32) and the monomethyl ether of CBGAA (CBGAM, 34) were isolated in 1975, proving that CBGA (32) is the first cannabinoid synthesized in the biosynthetic pathway of Δ9-THCAA (2) [27]. The monomethyl ether of (E)-CBG (CBGM, 33) was isolated from a benzene extract of hemp by heating the extract with toluene for seven hours and purifying using column chromatography (silica-gel column) with benzene as the eluent [28]. Cannabigerovarin (CBGV, 35) and cannabigerovarinic acid (CBGVA, 36) were isolated from a benzene extract of “Meao variant” cannabis from Thailand; the chemical structures for CBGV (35) and CBGVA (36) were determined through IR, NMR, and UV spectroscopies [16][29]. In 1995, cannabinerolic acid ((Z)CBGA, 37) was isolated from an acetone extract of the leaves of a Mexican strain of C. sativa, using silica-gel column chromatography. The chemical structure of (Z)CBGA (37) was determined through FAB-MS and NMR spectroscopies and confirmed by the synthesis of the cannabinoid [30]. Another cannabinoid derivative, 5-acetyl-4-hydroxy-cannabigerol (38), was isolated from the buds of high potency C. sativa using normal phase HPLC of the polar fractions [31]. Two esters of CBGA (32), γ-eudesmyl-cannabigerolate (37) and α-cadinyl-cannabigerolate (38), were also isolated from high potency C. sativa, using chiral HPLC and their chemical structures were established by GC/MS, HRESIMS, 1D NMR, and 2D NMR [19]. In 2008, four epoxy cannabigerol derivatives, (±)-6,7-trans-epoxycannabigerolic acid (41), (±)-6,7-cis-epoxycannabigerolic acid (42), (±)-6,7-cis-epoxycannabigerol (43), and (±)-6,7-trans-epxoycannabigerol (44), were isolated from high potency C. sativa (grown in Mississippi) by the application of various chromatographic techniques (VLC, flash chromatography, and HPLC). The chemical structures of the four epoxy cannabigerol derivatives (41–44) based on NMR and HRESIMS spectroscopic analyses [32]. The polar dihydroxycannabigerol derivative (camagerol, 45) was isolated from the aerial parts of a C. sativa strain, Carma, using reverse-phase (C18) silica-gel column chromatography, followed by normal-phase silica gel column chromatography and, finally, normal phase (NP)-HPLC. The wax of the aerial parts of the Carma strain was hydrolyzed and purified, using silica and alumina column chromatography, resulting in waxy and non-waxy fractions. The farnesyl prenylogue of cannabigerol (sesquicannabigerol, 46) was isolated from one of the waxy fractions. Its structure was established on the basis of NMR spectroscopic analysis (1H-NMR, 13C-NMR) and semisynthesis from cannabigerol (31) [33]. The CBG type cannabinoids are shown in Figure 3.
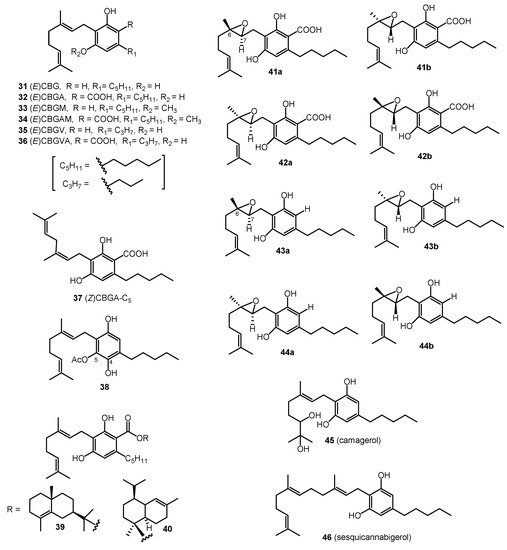
Figure 3. CBG-Type Cannabinoids.
2.4. Cannabidiol (CBD) Type (10 Cannabinoids)
Cannabidiol (CBD, 47) was isolated from an ethanolic extract (red oil) of Minnesota wild hemp. It was purified as a bis-3,5-dinitrobenzoate crystalline derivative [34]. The absolute configuration of CBD (47), (−)-trans-(1R,6R), was determined in 1969 through synthesis from (+)-cis- and (+)-trans-p-menthadien-(2,8)-ol and olivetol [35]. CBDA-C5 (48) was isolated from the fresh tops and leaves of C. sativa after extraction with benzene and identified by comparing its UV spectrum with that of CBD derivatives [36]. Cannabidiol monomethylether (CBDM-C5, 49) was isolated from the decarboxylated ethanol extract of hemp leaves on using florisil and silica gel column chromatography. CBDM (49) was identified by comparing its physical properties with an authentic sample YA [37]. CBD-C4 (50) was obtained from the ethyl acetate extract of cannabis resin and leaves after derivatization; it was characterized through GC-FID and GC-MS analyses [13]. Cannabidivarin (CBDV-C3, 51) was reported from hashish through silica gel chromatography and was identified by spectroscopic analysis (UV, IR, NMR and EIMS) [38].
Shoyama et al. isolated cannabidivarinic acid (CBDVA, 52) from the benzene extract of Thai cannabis, which was chromatographed on a polyamide column eluted with H2O:MeOH (1:1–1:6). Its structure was elucidated using UV, IR, and 1H NMR spectroscopic analysis [16]. Cannabidiorcol (CBD-C1, 53) was identified in the hexane extract of Lebanese hashish by combined gas chromatography–mass spectrometry [39].
Recently, two new CBD homologues with n-hexyl and n-heptyl side chain were isolated from the hexane extract of C. sativa and their chemical structures were assigned as cannabidihexol (CBDH, 54) and cannabidiphorol (CBDP, 55). The two compounds were purified using a semi-preparative C18 HPLC using a mixture of ACN/0.1 aqueous formic acid as a mobile phase. The fractions containing 52 and 53 were analyzed by HRESMS. The chemical structures of CBDH and CBDP were determined by HNMR, C-NMR, UV, and HR-ESI-MS and confirmed by stereoselective synthesis [22][23].
In 2020, a CBD dimer was isolated from the hexane extract of hemp and named Cannabitwinol, (CBDD, 56). The hexane extract was chromatographed on a Sigel column, which was eluted with hexane/CH2Cl2 followed by semi preparative C18-HPLC using a mixture of ACN/H2O/formic acid (7:3:0.1) as the mobile phase. The chemical structure of CBDD (56) was determined by applying a plethora of 1D and 2D NMR at −30 °C and was confirmed by HRESIMS and MS/MS spectrometry. The authors confirmed the chemical structure of 56 as two units of CBD connected by a methylene bridge and suggested that the dimerization of CBD occurred as a result of enzymatic reaction medicated by a one-carbon donor enzyme like methylene tetrahydrofolate [40]. All of the CBD type cannabinoids are shown in Figure 4.
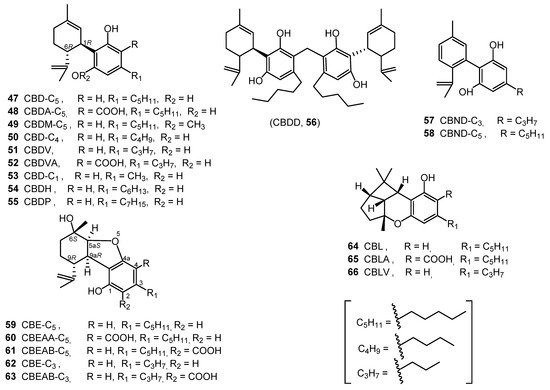
Figure 4. CBD, CBND, CBE and CBL-Type Cannabinoids.
2.5. Cannabinodiol (CBND) Type (Two Cannabinoids)
Only two cannabinodiol (CBND) type cannabinoids (57 and 58) have been reported (Figure 4). In 1972, cannabinodivirin (CBND-C3, 57) was detected in hashish by GC-MS analysis [41]. In 1977, cannabinodiol (CBND-C5, 58) was isolated from Lebanese hasish, using silica-gel column chromatography. The structure of CBND-C5 (58) was determined by 1H-NMR and confirmed by the phytochemical transformation of cannabinol into cannabinodiol [42].
2.6. Cannabielsoin (CBE) Type (Five Compounds)
In 1973, cannabielsoin (CBE-C5, 59) was detected in the ethanolic extract of Lebanese hashish. The extract was subjected to counter current distribution followed by GCMS analysis [43]. In 1974, its configuration was determined to be 5aS, 6S, 9R, 9aR [44]. Both cannabielsoin acid A (CBEAA, 60) and cannabielsoin acid B (CBEAB, 61) were isolated from hashish; the structural elucidation was carried by NMR spectroscopy and chemical transformations [45]. Cannabielsoin-C3 (CBE-C3, 62) and Cannabielsoic acid B-C3 (CBEAB-C3, 63) were also reported from cannabis [2][46]. The structures of these five cannabinoids (59–63) can be found in Figure 4.
2.7. Cannabicyclol (CBL) Type Three3 Compounds)
Krote and Sieper isolated cannabicyclol (CBL, 64) from hashish by thin layer chromatography, but its structure was correctly elucidated by Mechoulam and Gaoni in 1967, based on spectral data [47][48]. The relative configuration of CBL (64) was determined by X-ray analysis in 1970 [49]. Cannabicyclolic acid (CBLA, 65) was obtained from the benzene extract of cannabis. The benzene extract was chromatographed on a polyamide column using methanol water as a mobile phase. CBLA was isolated as a methylated derivative and considered to be an artifact formed when CBCA is naturally irradiated during storage [50]. Cannabicyclovarin (CBLV, 66) was detected in the ether extract of Congo marihuana and was identified by GLC and GCMS [51]. CBLA (65) was determined to be produced during the natural irradiation of cannabichromenic acid (CBCA, 68), proving that CBLA is not a natural cannabinoid [50]. The chemical structures of CBL-type cannabinoids are shown in Figure 4.
2.8. Cannabichromene (CBC) Type (Nine Compounds)
Cannabichromene (CBC, 67) was isolated from the hexane extract of hashish, using column chromatography (florisil column) in 1966 [52]. The carboxylic acid derivative of CBC (67), cannabichromenic acid (CBCA, 68) was isolated from the benzene extract of hemp, using silica-gel column chromatography. The chemical structure of CBCA (68) was determined using IR, NMR, and UV spectroscopies [27]. Cannabivarichromene (± CBCV, 69) was identified through GC-MS analysis, and both cannabichromevarin (+ CBCV, 70) and cannabichromevarinic acid (CBCVA, 71) were isolated from a benzene extract of the “Meao” variant of cannabis from Thailand [27]. Three cannabichromene derivatives, (±)-4-acetoxycannabichromene (72), (±)-3”-hydroxy-Δ4”-cannabichromene (73) and (–)-7-hydroxycannabichromane (74), were isolated from high potency C. sativa using silica-gel VLC, normal-phase silica HPLC, and reverse-phase silica (C18) HPLC. These derivatives (72–74) were chemically identified using 1D and 2D NMR spectroscopic techniques [31]. In 1984, the CBC-C3 derivative (75) was reported, and using mass spectral analysis, the chemical structure of the CBC-C3 derivative (75) was determined to be 2-methyl-2-(4-methyl-2-pentyl)-7-propyl-2H-1-benzopyran-5-ol [53]. The structures of these nine CBC-type cannabinoids (67–75) are shown in Figure 5.
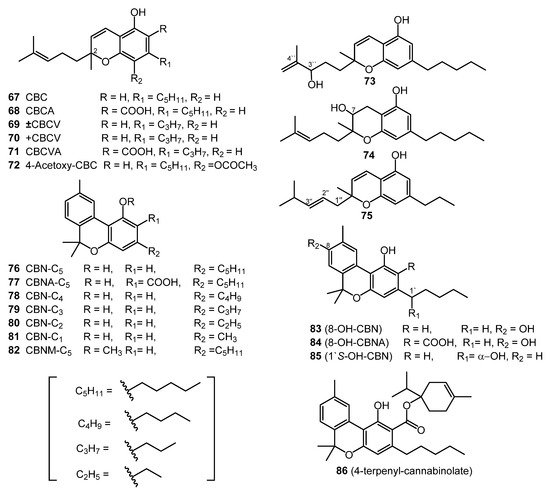
Figure 5. CBC and CBN-Type Cannabinoids.
2.9. Cannabinol (CBN) Type (Eleven Compounds)
The chemical structure and the isolation details of seven cannabinol derivatives (76–82, Figure 5) have been reported in 1980 [3]. Both 8-hydroxycannabinol (83) and 8-hydroxy cannabinolic acid A (84) were isolated from the high potency variety of C. sativa and chemically identified based on NMR and high-resolution mass (HR-MS) analysis in 2009 [31]. The compounds 1′S-hydroxy-cannabinol (85) and 4-terpenyl cannabinolate (86) were isolated from the same cannabis variety (high potency C. sativa), and their chemical structures were confirmed by GC-MS analysis [18].
2.10. Cannabitriol (CBT) Type (Nine Compounds)
Nine CBT-type cannabinoids, including (−)-trans-CBT-C5 (87), (+)-trans-CBT-C5 (88), (±)-cis-CBT-C5 (89), (±)-trans-CBT-C3 (90), CBT-C3-homologue (91), (−)-trans-CBT-OEt-C5 (92), (–)-trans-CBT-OEt-C3 (93), 8,9-Di-OH-CBT-C5 (94), and CBDA-C5 9-OH-CBT-C5 ester (95) (Figure 6), have been isolated from cannabis. Cannabitriol (87) was originally reported in 1966 from Japanese hemp [28], but its chemical structure was elucidated in 1976 [54]. The configuration of this compound was later determined by X-ray analysis [55]. The compounds (+)-trans-CBT-C5 (88) and (–)-trans -CBT-OEt-C5 (92) were isolated by ElSohly et al. from the ethanolic extract of cannabis, which was chromatographed on a silica gel column [56] and identified by GCMS.
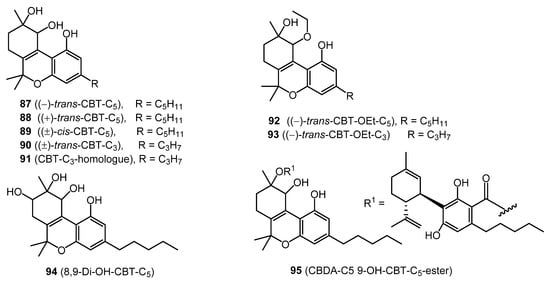
Figure 6. CBT-Type Cannabinoids.
The two ethoxy cannabitriols (92–93) are most likely artifacts, since ethanol was used in their isolation from Cannabis [3][57].
2.11. Miscellaneous Types Cannabinoids (30 Compounds)
A total of thirty miscellaneous type cannabinoids (Figure 7 and Figure 8) have been isolated from cannabis, including dehydrocannabifuran (DCBF-C5, 96), cannabifuran (CBF-C5, 97), 8-hydroxy-isohexahydrocannabivirin (OH-iso-HHCV-C3, 98), 10-oxo-Δ6a(10a)-tetrahydro-cannabinol (OTHC, 99), cannabicitran (100), (–)-Δ9-cis-(6aS,10aR)-tetrahydro-cannabinol (cis-Δ9-THC, 101), cannabicoumaronone (CBCON-C5, 102), cannabiripsol (CBR, 103), cannabitetrol (CBTT, 104), cannabichromanone-C5 (CBCN-C5, 105), cannabichromanone-C3 (CBCN-C3, 106),(±)-Δ7-cis-isotetrahydrocannabivarin-C3 (cis-iso-Δ7-THCV,107), (–)-Δ7-trans-(1R,3R,6R)-isotetrahydrocannabi- varin-C3 (trans-iso-Δ7-THCV,108), and (–)-Δ7-trans-(1R,3R,6R)-isotetrahydrocannabinol-C5 (trans-iso-Δ7-THC, 109). The cyclohexane-methanol extract of Afghan hashish afforded dehydrocannabifuran (DCBF-C5, 96), cannabifuran (CBF-C5, 97), OTHC (99), and cannabichromanone-C5 (CBCN-C5, 105) after micropreparative GC and TLC. Their chemical structures were determined by mass and NMR spectroscopic analysis [58]. Cannabicitran (CBT, 104) was isolated from an ethanolic extract of Lebanese hashish. It was purified by counter-current distribution and silica gel chromatography. Its chemical structure was determined by GCMS, IR and 1H NMR analyses [59]. The isolation of cis-Δ9-THC (101) was reported from a petroleum extract of marihuana by Smith and Kampfert in 1977. The extract was purified on a florsil column followed by preparative TLC [60].
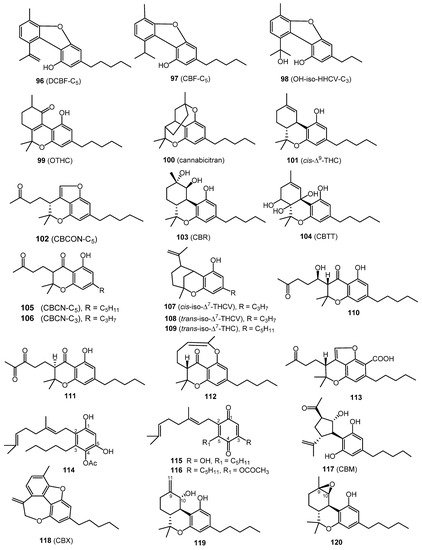
Cannabiripsol (CBR, 103) was isolated from a South African Cannabis variant after hexane extraction and chromatography on silica and polyamide columns. Its chemical structure was determined by spectral means (IR, GCMS, UV, 1H NMR) and by synthesis [61]. Cannabitetrol (CBTT, 104) was obtained and identified from the hexane extract of Mexican marijuana grown in Mississippi using silica gel column chromatography [62]. ElSohly and Slade reported the details of the isolation and chemical identification of compounds 106–109 [2].
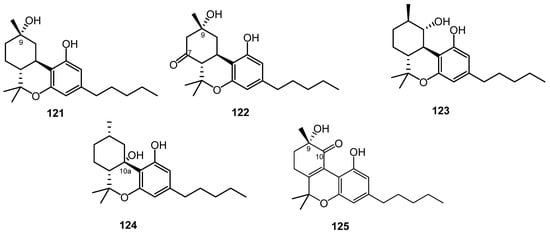
From a high potency variety of C. sativa, three cannabichromanones were isolated, namely cannabichromanone B (110), C (111), and D (112). The absolute configurations of these three cannabinoids were assigned on the basis of the Mosher ester and inspection of their circular dichroism spectra. The isolation of these compounds was performed using semi-preparative C18 HPLC [63]. Additionally, (–)-(7R)-cannabicoumarononic acid (113), 4-acetoxy-2-geranyl-5-hydroxy-3-n-pentylphenol (114), and 2-geranyl-5-hydroxy-3-n-pentyl-1,4-benzoquinone (115) were isolated from the buds and leaves of the same variety of cannabis (high potency C. sativa) using several chromatographic techniques, including silica-gel VLC, solid-phase extraction, reverse-phase columns (C18 SPE), and normal-phase HPLC. In addition, 5-acetoxy-6-geranyl-3-n-pentyl-1,4-benzoquinone (116) was isolated on silica gel column chromatography followed by normal-phase HPLC [32]. In 2010, a new cannabinoid named cannabimovone (CBM, 117) was isolated from a non-psychotropic variety of C. sativa. This unusual metabolite is presumably formed from CBD and was isolated from a polar fraction of hemp by using flash chromatography over reverse-phase C18 silica gel followed by normal-phase HPLC. The chemical identity of CBM (117) was revealed by a combination of 1D and 2D NMR along with ESI-MS spectroscopic techniques [64]. A tetracyclic cannabinoid, cannabioxepane, (CBX, 118) was isolated in 2011 from a cannabis variety called Carmagnola by applying many chromatographic techniques including RP-18 column, silica gel column chromatography, and NP-HPLC chromatography. Its chemical structure was established using MS and NMR data [65]. Seven more cannabinoids (119–125) were isolated from a high potency variety of C. sativa (Figure 7 and Figure 8) and chemically elucidated by 1D and 2D NMR and HRMS analyses as 10α-hydroxy-Δ9,11-hexahydrocannabinol (119), 9β,10β-epoxyhexahydrocannabinol (120), 9α-hydroxyhexahydrocannabinol (121), 7-oxo-9α-hydroxyhexa-hydrocannabinol, (122) 10α-hydroxyhexahydrocannabinol (123), 10aR-hydroxyhexahydrocannabinol (124), and 9α-hydroxy-10-oxo-Δ6a,10a-THC (125) [18][20].
References
- Zuardi, A.W. History of cannabis as a medicine: A review. Braz. J. Psychiatry 2006, 28, 153–157.
- ElSohly, M.A.; Slade, D. Chemical constituents of marijuana: The complex mixture of natural cannabinoids. Life Sci. 2005, 78, 539–548.
- Turner, C.E.; Elsohly, M.A.; Boeren, E.G. Constituents of Cannabis sativa L. XVII. A review of the natural constituents. J. Nat. Prod. 1980, 43, 169–234.
- Mechoulam, R.; Carlini, E.A. Toward drugs derived from cannabis. Naturwissenschaften 1978, 65, 174–179.
- Cunha, J.M.; Carlini, E.; Pereira, A.E.; Ramos, O.L.; Pimentel, C.; Gagliardi, R.; Sanvito, W.; Lander, N.; Mechoulam, R. Chronic administration of cannabidiol to healthy volunteers and epileptic patients. Pharmacology 1980, 21, 175–185.
- Cascio, M.G.; Pertwee, R.G.; Marini, P. The pharmacology and therapeutic potential of plant cannabinoids. In Cannabis Sativa L.-Botany and Biotechnology; Springer: Cham, Switzerland, 2017; pp. 207–225.
- Gaoni, Y.; Mechoulam, R. Isolation, structure, and partial synthesis of an active constituent of hashish. J. Am. Chem. Soc. 1964, 86, 1646–1647.
- Yamauchi, T.; Shoyama, Y.; Aramaki, H.; Azuma, T.; Nishioka, I. Tetrahydrocannabinolic acid, a genuine substance of tetrahydrocannabinol. Chem. Pharm. Bull. 1967, 15, 1075–1076.
- Verwey, A.; Witte, A. A rapid method of preparation of 1-THC by isolation of 1-THC acid from hashish. Pharm. Weekbl. 1972, 107, 415.
- Elsohly, M.A.; Ross, S.A. Method of Preparing Delta-9-Tetrahydrocannabinol. U.S. Patent US6365416B1, 2 April 2002.
- Mechoulam, R.; Ben-Zvi, Z.; Yagnitinsky, B.; Shani, A. A new tetrahydrocannabinolic acid. Tetrahedron Lett. 1969, 10, 2339–2341.
- Rosenqvist, E.; Ottersen, T. The crystal and molecular structure of Δ9-tetrahydrocannabinolic acid B. Acta Chem. Scand. B. 1975, 29, 379–384.
- Harvey, D.J. Characterization of the butyl homologs of Δ1-tetrahydrocannabinol, cannabinol and cannabidiol in samples of cannabis by combined gas chromatography and mass spectrometry. J. Pharm. Pharm. 1976, 28, 280–285.
- Gill, E. Propyl homologue of tetrahydrocannabinol: Its isolation from Cannabis, properties, and synthesis. J. Chem. Soc. C Org. 1971, 579–582.
- Paris, M.; Ghirlanda, C.; Chaigneau, M.; Giry, L. Δ1-Tetrahydrocannabivarolic acid, new constituent of Cannabis sativa. C. R. Acad. Sci. Ser. C 1973, 276, 205–207.
- Shoyama, Y.; Hirano, H.; Makino, H.; Umekita, N.; Nishioka, I. Cannabis. X. The isolation and structures of four new propyl cannabinoid acids, tetrahydrocannabivarinic acid, cannabidivarinic acid, cannabichromevarinic acid and cannabigerovarinic acid, from Thai Cannabis, ‘Meao variant’. Chem. Pharm. Bull. 1977, 25, 2306–2311.
- Turner, C.E.; Hadley, K.W.; Fetterman, P.S.; Doorenbos, N.J.; Quimby, M.W.; Waller, C. Constituents of Cannabis sativa L. IV: Stability of cannabinoids in stored plant material. J. Pharm. Sci. 1973, 62, 1601–1605.
- Ahmed, S.A.; Ross, S.A.; Slade, D.; Radwan, M.M.; Khan, I.A.; ElSohly, M.A. Minor oxygenated cannabinoids from high potency Cannabis sativa L. Phytochemistry 2015, 117, 194–199.
- Ahmed, S.A.; Ross, S.A.; Slade, D.; Radwan, M.M.; Zulfiqar, F.; ElSohly, M.A. Cannabinoid Ester Constituents from High-Potency Cannabis sativa. J. Nat. Prod. 2008, 71, 536–542.
- Radwan, M.M.; ElSohly, M.A.; El-Alfy, A.T.; Ahmed, S.A.; Slade, D.; Husni, A.S.; Manly, S.P.; Wilson, L.; Seale, S.; Cutler, S.J.; et al. Isolation and pharmacological evaluation of minor cannabinoids from high-potency Cannabis sativa. J. Nat. Prod. 2015, 78, 1271–1276.
- Zulfiqar, F.; Ross, S.A.; Slade, D.; Ahmed, S.A.; Radwan, M.M.; Ali, Z.; Khan, I.A.; ElSohly, M.A. Cannabisol, a novel Δ9-THC dimer possessing a unique methylene bridge, isolated from Cannabis sativa. Tetrahedron Lett. 2012, 53, 3560–3562.
- Citti, C.; Linciano, P.; Russo, F.; Luongo, L.; Iannotta, M.; Maione, S.; Laganà, A.; Capriotti, A.L.; Forni, F.; Vandelli, M.A.; et al. A novel phytocannabinoid isolated from Cannabis sativa L. with an in vivo cannabimimetic activity higher than Δ9-tetrahydrocannabinol: Δ9-Tetrahydrocannabiphorol. Sci. Rep. 2019, 9, 1–13.
- Linciano, P.; Citti, C.; Russo, F.; Tolomeo, F.; Laganà, A.; Capriotti, A.L.; Luongo, L.; Iannotta, M.; Belardo, C.; Maione, S.; et al. Identification of a new cannabidiol n-hexyl homolog in a medicinal cannabis variety with an antinociceptive activity in mice: Cannabidihexol. Sci. Rep. 2020, 10, 1–11.
- Hively, R.; Mosher, W.; Hoffman, F. Isolation of trans-delta-6-THC from mari huana. J. Am. Chem. Soc. 1966, 88, 1832.
- Krejcí, Z.; Šantavý, F. Isolation of two new cannabinoid acids from Cannabis sativa L. of Czechoslovak origin. Acta Univ. Olomuc Fac. Med. 1975, 74, 161–166.
- Archer, R.A.; Boyd, D.B.; Demarco, P.V.; Tyminski, I.J.; Allinger, N.L. Structural studies of cannabinoids. Theoretical and proton magnetic resonance analysis. J. Am. Chem. Soc. 1970, 92, 5200–5206.
- Shoyama, Y.; Yagi, M.; Nishioka, I.; Yamauchi, T. Biosynthesis of cannabinoid acids. Phytochemistry 1975, 14, 2189–2192.
- Obata, Y.; Ishikawa, Y. Studies on the constituents of hemp plant (Cannabis sativa L.) Part III. Isolation of a gibbs-positive compound from Japanese hemp. Agric. Biol. Chem. 1966, 30, 619–620.
- Shoyama, Y.; Hirano, H.; Oda, M.; Somehara, T.; Nishioka, I. Cannabichromevarin and cannabigerovarin, two new propyl homologues of cannabichromene and cannabigerol. Chem. Pharm. Bull. 1975, 23, 1894–1895.
- Taura, F.; Morimoto, S.; Shoyama, Y. Cannabinerolic acid, a cannabinoid from Cannabis sativa. Phytochemistry 1995, 39, 457–458.
- Radwan, M.M.; ElSohly, M.A.; Slade, D.; Ahmed, S.A.; Khan, I.A.; Ross, S.A. Biologically active cannabinoids from high-potency Cannabis sativa. J. Nat. Prod. 2009, 72, 906–911.
- Radwan, M.M.; Ross, S.A.; Slade, D.; Ahmed, S.A.; Zulfiqar, F.; ElSohly, M.A. Isolation and characterization of new cannabis constituents from a high potency variety. Planta Med. 2008, 74, 267.
- Appendino, G.; Giana, A.; Gibbons, S.; Maffei, M.; Gnavi, G.; Grassi, G.; Sterner, O. A polar cannabinoid from Cannabis sativa var. Carma. Nat. Prod. Comm. 2008, 3, 1977–1980.
- Adams, R.; Hunt, M.; Clark, J. Structure of cannabidiol, a product isolated from the marihuana extract of Minnesota wild hemp. I. J. Am. Chem. Soc. 1940, 62, 196–200.
- Petrzilka, T.; Haefliger, W.; Sikemeier, C. Synthese von Haschisch-Inhaltsstoffen. 4. Mitteilung. Helv. Chim. Acta 1969, 52, 1102–1134.
- Krejci, Z.; Santavy, F. Isolation of other substances from the leaves of Indian hemp. Acta Univ. Palacki Olomuc 6 1955, 59, 66.
- Shoyama, Y.; Kuboe, K.; Nishioka, I.; Yamauchi, T. Cannabidiol monomethyl ether. A new neutral cannabinoid. Chem. Pharm. Bull. 1972, 20, 2072.
- Vollner, L.; Bieniek, D.; Korte, F. Hashish. XX. Cannabidivarin, a new hashish constituent. Tetrahedron Lett. 1969, 3, 145–147.
- Vree, T.; Breimer, D.; Van Ginneken, C.; Van Rossum, J. Identification in hashish of tetrahydrocannabinol, cannabidiol and cannabinol analogues with a methyl side-chain. J. Pharm. Pharm. 1972, 24, 7–12.
- Chianese, G.; Lopatriello, A.; Schiano-Moriello, A.; Caprioglio, D.; Mattoteia, D.; Benetti, E.; Ciceri, D.; Arnoldi, L.; De Combarieu, E.; Vitale, R.M.; et al. Cannabitwinol, a dimeric phytocannabinoid from hemp, Cannabis sativa L.; is a selective thermo-TRP modulator. J. Nat. Prod. 2020, 83, 2727–2736.
- Van Ginneken, C.; Vree, T.; Breimer, D.; Thijssen, H.; Van Rossum, J. Cannabinodiol, a new hashish consituent, identified by gaschromatography-mass spectrometry. In Proceedings of the International Symposium on Gas Chromatography-Mass Spectometery Isle of Elba, San Marino, Italy, 17–19 May 1972; pp. 110–129.
- Ch, L.R.J.; Bercht, C.L.; van Ooyen, R.; Spronck, H.J. Cannabinodiol: Conclusive identification and synthesis of a new cannabinoid from Cannabis Sativa. Phytochemistry 1977, 16, 595–597.
- Bercht, C.; Lousberg, R.; Küppers, F.; Salemink, C.; Vree, T.; Van Rossum, J. Cannabis: VII. Identification of cannabinol methyl ether from hashish. J. Chromatogr. A 1973, 81, 163–166.
- Uliss, D.B.; Razdan, R.K.; Dalzell, H.C. Stereospecific intramolecular epoxide cleavage by phenolate anion. Synthesis of novel and biologically active cannabinoids. J. Am. Chem. Soc. 1974, 96, 7372–7374.
- Shani, A.; Mechoulam, R. Cannabielsoic acids: Isolation and synthesis by a novel oxidative cyclization. Tetrahedron 1974, 30, 2437–2446.
- Grote, H.; Spiteller, G. Neue cannabinoide—III: Die struktur des cannabicumaronons und analoger verbindungen. Tetrahedron 1978, 34, 3207–3213.
- Korte, F.; Sieper, H. Zur chemischen klassifizierung von pflanzen: XXIV. Untersuchung von Haschisch-Inhaltsstoffen durch Dünnschichtchromatographie. J. Chromatogr. A 1964, 13, 90–98.
- Mechoulam, R.; Mechoulam, R.; Gaoni, Y.; Gaoni, Y. Recent advances in the chemistry of hashish. In Fortschritte der Chemie organischer Naturstoffe/Progress in the Chemistry of Organic Natural Products; Springer Science and Business Media LLC: Wien, Austria, 1967; pp. 175–213.
- Begley, M.; Clarke, D.; Crombie, L.; Whiting, D. The X-ray structure of dibromocannabicyclol: Structure of bicyclomahanimbine. J. Chem. Soc. D Chem. Comm. 1970, 22, 1547–1548.
- Shoyama, Y.; Yamauchi, T.; Oku, R.; Nishioka, I. Cannabis. VI. Cannabicyclolic acid. Chem. Pharm. Bull. 1972, 20, 1927–1930.
- Vree, T.; Breimer, D.; Van Ginneken, C.; Van Rossum, J. Identification of cannabicyclol with a pentyl or propyl side-chain by means of combined as chromatography—Mass spectrometry. J. Chromatogr. 1972, 74, 124–127.
- Gaoni, Y.; Mechoulam, R. Cannabichromene, a new active principle in hashish. Chem. Comm. 1966, 1, 20–21.
- Morita, M.; Ando, H. Analysis of hashish oil by gas chromatography/mass spectrometry. Kagaku Keisatsu Kenkyusho Hokoku Hokagaku Hen 1984, 37, 137–140.
- Chan, W.; Magnus, K.; Watson, H. The structure of cannabitriol. Experientia 1976, 32, 283–284.
- McPhail, A.T.; ElSohly, H.N.; Turner, C.E.; ElSohly, M.A. Stereochemical assignments for the two enantiomeric pairs of 9, 10-dihydroxy-Δ6a (10a)-tetrahydrocannabinols. X-Ray crystal structure analysis of (±) trans-cannabitriol. J. Nat. Prod. 1984, 47, 138–142.
- ElSohly, M.; El-Feraly, F.; Turner, C. Isolation and characterization of (+) cannabitriol and (-)-10 ethoxy 9 hydroxy delta 6a tetrahydrocannabinol: Two new cannabinoids from Cannabis sativa L. extract. Lloydia 1977, 275–280.
- Harvey, D. Examination of a 140 Year Old Ethanolic Extract of Cannabis: Identification of New Cannabitriol Homologues and the Ethyl Homologue of Cannabinol, Marihuana’84; Harvey, D.J., Paton, W., Nahas, G.G., Eds.; IRL Press: Oxford, UK, 1985.
- Friedrich-Fiechtl, J.; Spiteller, G. Neue cannabinoide—1. Tetrahedron 1975, 31, 479–487.
- Bercht, C.A.L.; Paris, M.R. Oil of Cannabis sativa. Bull. Tech. Gattefosse Sfpa 1974, 68, 87–90.
- Smith, R.M.; Kempfert, K.D. Delta1–3, 4-cis-tetrahydrocannabinol in Cannabis sativa. Phytochemistry 1977, 1088–1089.
- Boeren, E.; Elsohly, M.; Turner, C.; Salemink, C. β-Cannabispiranol: A new non-cannabinoid phenol from Cannabis sativa L. Experientia 1977, 33, 848.
- ElSohly, H.N.; Boeren, E.G.; Turner, C.E.; ElSohly, M.A. Constituents of Cannabis sativa L. XXIIII: Cannabitetrol, a New Polyhydroxylated Cannabinoid; Academic Press Inc.: Orlando, FL, USA, 1984.
- Ahmed, S.A.; Ross, S.A.; Slade, D.; Radwan, M.M.; Khan, I.A.; ElSohly, M.A. Structure determination and absolute configuration of cannabichromanone derivatives from high potency Cannabis sativa. Tetrahedron Lett. 2008, 49, 6050–6053.
- Taglialatela-Scafati, O.; Pagani, A.; Scala, F.; De Petrocellis, L.; Di Marzo, V.; Grassi, G.; Appendino, G. Cannabimovone, a cannabinoid with a rearranged terpenoid skeleton from hemp. Eur. J. Org. Chem. 2010, 11, 2023.
- Pagani, A.; Scala, F.; Chianese, G.; Grassi, G.; Appendino, G.; Taglialatela-Scafati, O. Cannabioxepane, a novel tetracyclic cannabinoid from hemp, Cannabis sativa L. Tetrahedron 2011, 67, 3369–3373.




