Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Wei Li | + 1240 word(s) | 1240 | 2021-05-08 11:15:56 | | | |
| 2 | Catherine Yang | Meta information modification | 1240 | 2021-05-18 04:17:17 | | | | |
| 3 | Conner Chen | Meta information modification | 1240 | 2021-08-04 11:20:19 | | | | |
| 4 | Conner Chen | Meta information modification | 1240 | 2021-09-22 02:46:48 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Li, W. Retinopathy of Prematurity. Encyclopedia. Available online: https://encyclopedia.pub/entry/9724 (accessed on 07 February 2026).
Li W. Retinopathy of Prematurity. Encyclopedia. Available at: https://encyclopedia.pub/entry/9724. Accessed February 07, 2026.
Li, Wei. "Retinopathy of Prematurity" Encyclopedia, https://encyclopedia.pub/entry/9724 (accessed February 07, 2026).
Li, W. (2021, May 17). Retinopathy of Prematurity. In Encyclopedia. https://encyclopedia.pub/entry/9724
Li, Wei. "Retinopathy of Prematurity." Encyclopedia. Web. 17 May, 2021.
Copy Citation
Retinopathy of prematurity (ROP) is an ocular vascular disease affecting premature infants, characterized by pathological retinal neovascularization (RNV), dilated and tortuous retinal blood vessels, and retinal or vitreous hemorrhages that may lead to retinal detachment, vision impairment and blindness.
retinopathy of prematurity
oxygen-induced retinopathy
1. Introduction
Retinopathy of prematurity (ROP) with pathological retinal neovascularization (RNV) is the most common cause of blindness in children, primarily afflicting preterm infants [1]. During ROP, abnormal retinal blood vessels form sprouts and branches that extend not only over the retinal surface but also into the vitreous as they progress to pathological RNV and intravitreal neovascularization (IVNV) [2]. In contrast to other ocular neovascular diseases, including proliferative diabetic retinopathy (DR) with RNV and wet age-related macular degeneration (AMD) with choroidal neovascularization (CNV), ROP in the developing retina of premature infants is unique because of the coexistence of physiological and pathological angiogenesis. The former is essential for normal retinal development.
ROP first appeared as a clinical disorder in the 1940s, coincident with the liberal use of supplemental oxygen to improve the survival of preterm infants with respiratory distress syndrome (RDS) [3]. The disease typically occurs in preterm subjects that weigh about 1250 g or less and are born before 31 weeks of gestational age (GA). A full-term human pregnancy has a gestation period of ~40 weeks. Birth weight correlates inversely with the probability of ROP. There were ~14.9 million live premature births globally in 2010 [4], of which an estimated 184,700 developed ROP, including 20,000 with severe visual impairment and/or blindness [5]. Although about 90% of ROP infants sustain only mild visual impairment, subjects with untreated RNV remain at an elevated risk for progression to more severe conditions, including dilated and tortuous retinal vessels (i.e., the “plus” disease), retinal folds, pathological RNV and IVNV, and retinal or vitreous hemorrhage that may lead to retinal detachment, impaired vision and blindness (Figure 1).
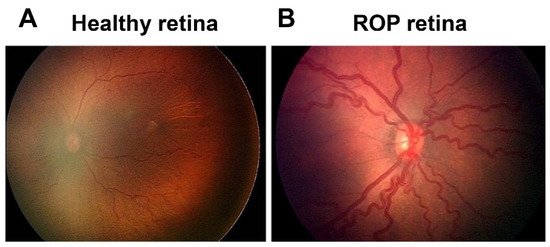
Figure 1. Fundus image of retinopathy of prematurity (ROP): (A) Healthy retina and (B) ROP retina with tortuous arteries and dilated veins as plus disease.
ROP is currently treated by laser therapy or cryotherapy that provide only limited efficacy and significant risk of adverse side effects. Anti-vascular endothelial growth factor (VEGF) drugs have been used off-label in the U.S. but are not FDA approved because of the potential for adverse side effects caused by indiscriminate inhibition of all forms of angiogenesis in the developing retina. The recent discovery by our group that secretogranin III (Scg3) is a disease-selective angiogenic factor presents a unique opportunity to selectively target and alleviate ROP safely without affecting physiological angiogenesis and normal retinal development.
2. Molecular Mechanisms of ROP
2.1. Oxygen
After low GA and birth weight, oxygen is the single most important risk factor for ROP. The liberal use of supplement oxygen in the 1940s resulted in a high incidence of ROP, first manifested as retrolental fibroplasia [3]. The subsequent development of technologies to better monitor and regulate oxygen exposure to premature infants decreased the risk of ROP, but reduction of supplemental oxygen also increased preterm infant mortality rates [6][7]. Clinical findings show that fluctuations of blood oxygen saturation (SpO2) of preterm babies during the first few weeks of life also contribute to the risk of ROP, and a high incidence of intermittent hypoxia is associated with severe ROP [8][9].
2.2. VEGF
VEGF is the most intensely studied angiogenic factor and widely considered to be the central player in both physiological and pathological angiogenesis of ROP as well as numerous other neovascular disorders. It was initially discovered as a vascular permeability factor and subsequently characterized as a potent mitogenic factor that stimulates endothelial proliferation and angiogenesis [10]. The mammalian VEGF family comprises five members, including VEGF-A, VEGF-B, VEGF-C, VEGF-D and placental growth factor (PlGF). Human VEGF-A undergoes alternative exon splicing into multiple variants, including VEGF111, VEGF121, VEGF145, VEGF165 and VEGF189 [10]. Of these isoforms, VEGF165 is the most active in terms of receptor stimulation and bioavailability. VEGF binds to 3 receptor tyrosine kinases, including VEGF receptor 1–3 (VEGFR1–3) encoded by Flt1, Kdr and Flt4 genes, respectively [10]. VEGFR2 is the principal conduit that promotes endothelial proliferation and vascular permeability.
VEGF levels are significantly elevated in the vitreous of ROP infants [11][12]. Similarly, in mice with oxygen-induced retinopathy (OIR), a surrogate animal model of ROP, VEGF expression is suppressed by hyperoxia in Phase 1 but markedly upregulated by the conditions of relative hypoxia imposed in Phase 2 [13][14].
Oxygen tension regulates the expression of VEGF and multiple other angiogenic factors via hypoxia-inducible transcription factors (HIFs) [15]. HIF-1, the main regulator of responses to hypoxia consists of two subunits, HIF-1α and HIF-1β, both of which are required to activate target genes and are expressed constitutively. Oxygen tensions in the normal range (normoxia) or hyperoxia, drive prolyl hydroxylation of HIF-1α via prolyl hydroxylase domain proteins. Hydroxylated HIF-1α is sequestered to the proteasome for ubiquitination and degradation such that HIF-1 regulation is inactivated by normal (aerobic) or elevated oxygen pressure [15]. Under hypoxia, HIF-1α is stabilized and translocated to the nucleus where it heterodimerizes with HIF-1β, and the complex binds to hypoxia-responsive elements (HREs) of target genes, such as VEGF, to activate transcription. HIF-1 is induced during physiological angiogenesis as well as in Phase 2 of ROP but suppressed by supplemental oxygen during Phase 1 [16][17]. HIFs are central to both physiological and pathological angiogenesis in many organs [18].
2.3. IGF-1
IGF-1 is a non-oxygen regulated factor that has been extensively studied in ROP [19]. Clinical studies indicated that serum levels of IGF-1 in premature babies correlate inversely with the severity of clinical ROP [20]. Administration of recombinant human IGF-1 reduces the risk of OIR in mice [21]. Absence of IGF-1 in IGF-1−/− mice delays normal retinal vascular development [20]. IGF-1 is a permissive factor for VEGF-dependent vascular endothelial cell growth [20][22][23].
IGF-1 binds the IGF-1 receptor (IGF-1R), and elimination of IGF-1R reduced RNV in OIR mice [24]. Insulin-like growth factor-binding proteins 1–6 (IGFBP1–6) bind and sequester >99% of available IGF-1, thereby regulating bioavailability. IGFBP-3, the most abundant IGFBP, sequesters 75–90% of IGF-1 protein [25][26]. IGFBP-3 concentrations are decreased in infants with ROP [27]. Deletion of the IGFBP-3 gene in mice resulted in a dose-dependent increase of OIR-related vessel loss, while administration of IGFBP-3 promoted vessel regrowth in wild-type mice and reduced vaso-obliteration in OIR mice [26][27].
2.4. Other Molecular Regulators
Erythropoietin, a key hematopoietic cytokine that regulates the formation of red blood cells during hematopoiesis, is also a pleiotropic growth factor that confers growth stimulation and cytoprotection to numerous cells, including endothelial cells. Chen et al. reported that EPO levels were suppressed in Phase 1 of OIR mice and markedly elevated in Phase 2 [28]. Exogenous EPO protected OIR retina in Phase 1 but worsened pathological RNV in Phase 2. EPO siRNA effectively suppressed pathological RNV in OIR mice [29]. Recombinant human EPO (rhEPO) used to treat anemia of prematurity is a significant independent risk factor for developing ROP [30].
ω-3 and ω-6 polyunsaturated fatty acids (PUFAs) are essential fatty acids required for optimal health and cannot be synthesized by the body. They are structural components of the plasma membrane and critical for optimal fluidity of photoreceptor membranes, retinal integrity and visual function [31]. ω-3 PUFAs include alpha-linolenic acid (ALA) and docosahexaenoic acid (DHA), while arachidonic acid (AA) is a ω-6 PUFA. DHA accounts for ~20% of total lipid in photoreceptor cells [32] and can be metabolized to neuroprotectin D1, resolvin D1 and resolvin E1, potent lipid-derived cytokines that confer protection against OIR-induced RNV [33]. Blockade of the peroxisome proliferator-activated receptor gamma (PPARγ) abrogated ω-3-mediated inhibition of pathological RNV through a VEGF-independent pathway [34], suggesting that ω-3 regulates angiogenesis through PPARγ. A clinical study revealed that low postnatal levels of serum AA are strongly associated with ROP development [35].
References
- Hellström, A.; Smith, L.E.H.; Dammann, O. Retinopathy of prematurity. Lancet 2013, 382, 1445–1457.
- Hartnett, M.E. Pathophysiology and mechanisms of severe retinopathy of prematurity. Ophthalmology 2015, 122, 200–210.
- Hartnett, M.E.; Lane, R.H. Effects of oxygen on the development and severity of retinopathy of prematurity. J. Am. Assoc. Pediatric Ophthalmol. Strabismus 2013, 17, 229–234.
- Blencowe, H.; Lawn, J.E.; Vazquez, T.; Fielder, A.; Gilbert, C. Preterm-associated visual impairment and estimates of retinopathy of prematurity at regional and global levels for 2010. Pediatr. Res. 2013, 74 (Suppl. S1), 35–49.
- Brown, A.C.; Nwanyanwu, K. Retinopathy of Prematurity. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, January 2021.
- Bolton, D.P.; Cross, K.W. Further observations on cost of preventing retrolental fibroplasia. Lancet 1974, 1, 445–448.
- Saugstad, O.D.; Aune, D. Optimal oxygenation of extremely low birth weight infants: A meta-analysis and systematic review of the oxygen saturation target studies. Neonatology 2014, 105, 55–63.
- Di Fiore, J.M.; Bloom, J.N.; Orge, F.; Schutt, A.; Schluchter, M.; Cheruvu, V.K.; Walsh, M.; Finer, N.; Martin, R.J. A higher incidence of intermittent hypoxemic episodes is associated with severe retinopathy of prematurity. J. Pediatr. 2010, 157, 69–73.
- Cunningham, S.; Fleck, B.W.; Elton, R.A.; McIntosh, N. Transcutaneous oxygen levels in retinopathy of prematurity. Lancet 1995, 346, 1464–1465.
- Koch, S.; Claesson-Welsh, L. Signal transduction by vascular endothelial growth factor receptors. Cold Spring Harb. Perspect. Med. 2012, 2, a006502.
- Sato, T.; Kusaka, S.; Shimojo, H.; Fujikado, T. Vitreous levels of erythropoietin and vascular endothelial growth factor in eyes with retinopathy of prematurity. Ophthalmology 2009, 116, 1599–1603.
- Velez-Montoya, R.; Clapp, C.; Rivera, J.C.; Garcia-Aguirre, G.; Morales-Cantón, V.; Fromow-Guerra, J.; Guerrero-Naranjo, J.L.; Quiroz-Mercado, H. Intraocular and systemic levels of vascular endothelial growth factor in advanced cases of retinopathy of prematurity. Clin. Ophthalmol. 2010, 4, 947–953.
- Pierce, E.A.; Avery, R.L.; Foley, E.D.; Aiello, L.P.; Smith, L.E. Vascular endothelial growth factor/vascular permeability factor expression in a mouse model of retinal neovascularization. Proc. Natl. Acad. Sci. USA 1995, 92, 905–909.
- Pierce, E.A.; Foley, E.D.; Smith, L.E. Regulation of vascular endothelial growth factor by oxygen in a model of retinopathy of prematurity. Arch. Ophthalmol. 1996, 114, 1219–1228.
- Koyasu, S.; Kobayashi, M.; Goto, Y.; Hiraoka, M.; Harada, H. Regulatory mechanisms of hypoxia-inducible factor 1 activity: Two decades of knowledge. Cancer Sci. 2018, 109, 560–571.
- Selvam, S.; Kumar, T.; Fruttiger, M. Retinal vasculature development in health and disease. Prog. Retin. Eye Res. 2015, 63, 1–19.
- Sears, J.E.; Hoppe, G.; Ebrahem, Q.; Anand-Apte, B. Prolyl hydroxylase inhibition during hyperoxia prevents oxygen-induced retinopathy. Proc. Natl. Acad. Sci. USA 2008, 105, 19898–19903.
- Zimna, A.; Kurpisz, M. Hypoxia-Inducible Factor-1 in Physiological and Pathophysiological Angiogenesis: Applications and Therapies. Biomed. Res. Int. 2015, 2015, 549412.
- Liegl, R.; Löfqvist, C.; Hellström, A.; Smith, L.E.H. IGF-1 in retinopathy of prematurity, a CNS neurovascular disease. Early Hum. Dev. 2016, 102, 13–19.
- Hellstrom, A.; Perruzzi, C.; Ju, M.; Engstrom, E.; Hard, A.L.; Liu, J.L.; Albertsson-Wikland, K.; Carlsson, B.; Niklasson, A.; Sjodell, L.; et al. Low IGF-I suppresses VEGF-survival signaling in retinal endothelial cells: Direct correlation with clinical retinopathy of prematurity. Proc. Natl. Acad. Sci. USA 2001, 98, 5804–5808.
- Vanhaesebrouck, S.; Daniëls, H.; Moons, L.; Vanhole, C.; Carmeliet, P.; Zegher, F.D. Oxygen-induced retinopathy in mice: Amplification by neonatal IGF-I deficit and attenuation by IGF-I administration. Pediatr. Res. 2009, 65, 307–310.
- Smith, L.E.; Kopchick, J.J.; Chen, W.; Knapp, J.; Kinose, F.; Daley, D.; Foley, E.; Smith, R.G.; Schaeffer, J.M. Essential role of growth hormone in ischemia-induced retinal neovascularization. Science 1997, 276, 1706–1709.
- Smith, L.E.; Shen, W.; Perruzzi, C.; Soker, S.; Kinose, F.; Xu, X.; Robinson, G.; Driver, S.; Bischoff, J.; Zhang, B.; et al. Regulation of vascular endothelial growth factor-dependent retinal neovascularization by insulin-like growth factor-1 receptor. Nat. Med. 1999, 5, 1390–1395.
- Kondo, T.; Vicent, D.; Suzuma, K.; Yanagisawa, M.; King, G.L.; Holzenberger, M.; Kahn, C.R. Knockout of insulin and IGF-1 receptors on vascular endothelial cells protects against retinal neovascularization. J. Clin. Investig. 2003, 111, 1835–1842.
- Firth, S.M.; Baxter, R.C. Cellular actions of the insulin-like growth factor binding proteins. Endocr. Rev. 2002, 23, 824–854.
- Jarajapu, Y.P.R.; Cai, J.; Yan, Y.; Calzi, S.L.; Kielczewski, J.L.; Hu, P.; Shaw, L.C.; Firth, S.M.; Chan-Ling, T.; Boulton, M.E.; et al. Protection of blood retinal barrier and systemic vasculature by insulin-like growth factor binding protein-3. PLoS ONE 2012, 7, e39398.
- Lofqvist, C.; Chen, J.; Connor, K.M.; Smith, A.C.; Aderman, C.M.; Liu, N.; Pintar, J.E.; Ludwig, T.; Hellstrom, A.; Smith, L.E. IGFBP3 suppresses retinopathy through suppression of oxygen-induced vessel loss and promotion of vascular regrowth. Proc. Natl. Acad. Sci. USA 2007, 104, 10589–10594.
- Chen, J.; Connor, K.M.; Aderman, C.M.; Smith, L.E.H. Erythropoietin deficiency decreases vascular stability in mice. J. Clin. Investig. 2008, 118, 526–533.
- Chen, J.; Connor, K.M.; Aderman, C.M.; Willett, K.L.; Aspegren, O.P.; Smith, L.E. Suppression of retinal neovascularization by erythropoietin siRNA in a mouse model of proliferative retinopathy. Investig. Ophthalmol. Vis. Sci. 2009, 50, 1329–1335.
- Romagnoli, C.; Tesfagabir, M.G.; Giannantonio, C.; Papacci, P. Erythropoietin and retinopathy of prematurity. Early Hum. Dev. 2011, 87 (Suppl. S1), S39–S42.
- Querques, G.; Forte, R.; Souied, E.H. Retina and omega-3. J. Nutr. Metab. 2011, 2011, 748361.
- SanGiovanni, J.P.; Chew, E.Y. The role of omega-3 long-chain polyunsaturated fatty acids in health and disease of the retina. Prog. Retin. Eye Res. 2005, 24, 87–138.
- Connor, K.M.; SanGiovanni, J.P.; Lofqvist, C.; Aderman, C.M.; Chen, J.; Higuchi, A.; Hong, S.; Pravda, E.A.; Majchrzak, S.; Carper, D.; et al. Increased dietary intake of omega-3-polyunsaturated fatty acids reduces pathological retinal angiogenesis. Nat. Med. 2007, 13, 868–873.
- Stahl, A.; Sapieha, P.; Connor, K.M.; Sangiovanni, J.P.; Chen, J.; Aderman, C.M.; Willett, K.L.; Krah, N.M.; Dennison, R.J.; Seaward, M.R.; et al. Short communication: PPAR gamma mediates a direct antiangiogenic effect of omega 3-PUFAs in proliferative retinopathy. Circ. Res. 2010, 107, 495–500.
- Löfqvist, C.A.; Najm, S.; Hellgren, G.; Engström, E.; Sävman, K.; Nilsson, A.K.; Andersson, M.X.; Hård, A.L.; Smith, L.E.; Hellström, A. Association of Retinopathy of Prematurity With Low Levels of Arachidonic Acid: A Secondary Analysis of a Randomized Clinical Trial. JAMA Ophthalmol. 2018, 136, 271–277.
More
Information
Subjects:
Ophthalmology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.6K
Revisions:
4 times
(View History)
Update Date:
22 Sep 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




