
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Lei Nie | + 3169 word(s) | 3169 | 2021-05-12 08:49:06 | | | |
| 2 | Peter Tang | Meta information modification | 3169 | 2021-05-17 10:16:35 | | |
Video Upload Options
Hydroxyapatite (HA) and HA-based nanocomposites have been recognized as ideal biomaterials in hard tissue engineering because of their compositional similarity to bioapatite. Three-dimensional (3D) printing has been shown to provide a fast, precise, controllable, and scalable fabrication approach for the synthesis of HA-based scaffolds.
1. Introduction
Based on the integration of principles of cell biology, medical science, materials science, and biological engineering, tissue engineering (TE) aims to develop biological substitutes for the restoration or replacement of damaged and diseased tissue. TE has been applied in orthopedics, skin, cartilage, and neurons and organ reconstruction. A scaffold is essential for hard tissue regeneration. The scaffold needs to provide a suitable surface and space for the adhesion, proliferation, migration, and differentiation of cells. Hydroxyapatite (Ca10(PO4)6(OH)2, HA) is one of the essential inorganic components from bones and teeth. HA has been used as a bone-substitute material in hard TE due to its structural and functional similarity to human bones and teeth. HA are also widely applied in biomedical engineering due to their characteristic excellent biocompatibility, bioactivity, osteointegrity and osteoconductive properties and HA’s similarity to the inorganic component of human beings [1][2]. Notably, 65% of human bone is composed of HA-like compounds [3]. Crucially however HA has poor mechanical properties, leading to the need for the development of suitable HA-composites that retain the aforementioned benefits of HA while having improved mechanical properties.
These HA-based composites (in combination with other biomaterials such as polymers or other inorganic materials) have been used to fabricate scaffolds with desired properties, including biocompatibility, interconnected porous morphology, adequate mechanical properties, biodegradability, and appropriate surface chemistry for cell attachment and proliferation. In addition to the ‘biological’ benefits of the use of such biomaterials, the use of these biomaterials will reduce the need for synthetics (fossil sourced materials) in the biomedical industry leading to overall improved environmental outcomes [4][5]. Biomaterials based on HA have therefore been under intense study for TE by several researchers in the last ten years (Figure 1).
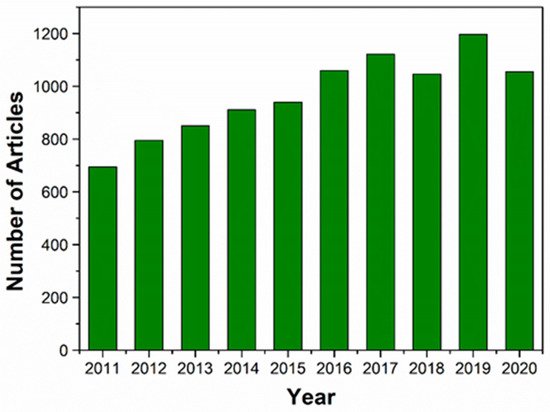
Figure 1. The number of articles published on HA and HA-based nanocomposites for TE in the last 10 years (January 2011 to December 2020). The data were obtained from Web of Science using the terms “hydroxyapatite” or “hydroxyapatite nanocomposites” and “TE”.
According to the literature there are several methods that may be employed in the preparation of HA-based composites [6][7]. Some of these techniques include biomimetic mineralization [8], electrochemical deposition [9], lyophilization [10], electrospinning [11], self assembling [12], and chemical vapor deposition [13]. These techniques for HA-composite preparation are summarized and briefly described in Table 1.
Table 1. Some major methods employed in fabricating hydroxyapatite (HA) composites.
|
HA-Composites Fabrication Methods |
Brief Description |
Sources |
|---|---|---|
|
Biomimetic mineralization |
In this approach, the composite material is decorated in a solution of bioactive substances or simulated electrolyte body fluid solution (SBF). In such a solution, the increased concentration of calcium ions induces the nucleation of hydroxyapatite crystals on the selected composite material. |
[14] |
|
Electrochemical deposition |
In this method, the hydroxyapatite composite is deposited onto the surface of a conductor using an electrolysis processes such that the solution contains the calcium ions and phosphate ions for (HA) and the relevant composite candidate (i.e., dissolved chitosan). |
[15] |
|
Lyphilisation |
The composite materials (i.e., graphene and HA) are dispersed in an organic solvent after which the mixture is frozen. Sublimation of the frozen solution is subsequently achieved by reducing the pressure. |
[16] |
|
Electrospinning |
This approach is employed when there is a need to develop fibrous scaffolds that can mimic the extracellular matrix of native tissue. Such fibers are prepared by electrospinning a precursor mixture containing ions (i.e., calcium ions in Ca(NO3)2·4H2O and phosphate ions in (C2H5O)3PO) and polymer additive, followed by thermal treatment. |
|
|
Self-assembling |
This is a self-aggregation process that involves the spontaneous aggregation to form the target composites. During the self-assembling process, the organic phase (i.e., collagen) is made to interact with the mineral phase (i.e., hydroxyapatite) via the use of suitable precursors (i.e., Ca(OH)2 for Ca2+ and H3PO4 for PO43−). |
[12] |
|
Chemical vapor deposition |
In this method, the film is deposited on the surface of the substrate through chemical reaction from gas-phase or vapor-phase precursor (i.e., Fe2O3/HA + H2 as carrier gas). |
[13] |
|
Hydrothermal |
In this approach, a mixture composed of suitable precursors containing calcium ions (i.e., calcium nitrate tetrahydrate) and phosphate ions (diammonium hydrogen phosphate solutions) is used in dispersing the composite candidate material (i.e., graphene) at a high temperature condition (i.e., 180 °C).The hydrothermal method is also employed in the fabrication of three-dimensional reduced graphene oxide/hydroxyapatite (HA)/gelatin scaffolds. |
|
|
Solvothermal Synthesis |
HA nanoparticles are crystalized via a two-state solvothermal method at the high temperature of 180 °C. Calcium nitrate tetrahydrate and diammonium hydrogen phosphate are used as calcium and phosphate precursors, respectively. |
[21] |
Hull, a graduate of university of Colorado, USA displayed the first uses of additive manufacturing (AM) in 1984 [22][23]. AM technologies have been applied in many fields, including medical devices and TE [24][25]. Nowadays, 3D printing is considered as a promising technology to fabricate sophisticated biological products, including biological scaffolds, tissues, organs, and personalized medical devices, via printing biological materials, living cells, and signaling molecules.
2. 3D Printing Technologies for HA-Based Nanocomposites
The procedure employed in 3D printing, based on the deposition of exact layers using biomaterials, occurs with or without encapsulating cells. The whole process mainly includes the preparatory phase, printing phase, and post-handling phase [26]. Computer graphics software (such as CAD/CAM) and the selection of biomaterials are employed in the preparatory phase. The post-handling process is involved in tissue maturation in a bioreactor, in vitro transplantation, or animal implantation. Inkjet-based 3D printing, stereolithography (SLA)-based 3D printing, extrusion-based 3D printing, and laser-based 3D printing are the four commonly used 3D printing strategies [27][28][29].
3. Hydroxyapatite (HA) and HA-Based Nanocomposites via 3D Printing
3.1. Hydroxyapatite
Hydroxyapatite (HA), (Ca10(PO4)6(OH)2), is characterized with a hexagonal crystallographic structure, as illustrated in Figure 2a. Figure 2a shows that a unit cell of HA contains Ca. PO4 moieties are arranged such that four Ca atoms are surrounded by nine O atoms of the PO4 moieties, while the other six Ca atoms are surrounded by the remaining six O atoms of the PO4 moieties. Pure HA has the stoichiometric Ca/P ratio of 1.67, with lattice parameters of a-axis of 0.9422 nm and c-axis of 0.688 nm [30]. The chemical structure of HA is similar to the mineralized constituents of bone [31]. In addition, HA has excellent physicochemical properties, including osteoconductivity, bioactivity, re-sorbability, and slow decaying properties [32][33]. Furthermore, nanometer-sized HA can also increase intracellular uptake and reduce cell viability in vitro [34].
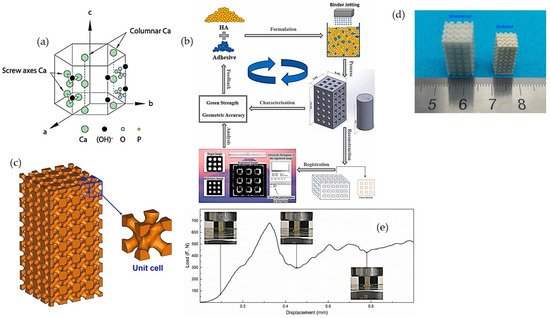
Figure 2. (a) Hexagonal crystallographic structure of the HA crystal [31]; (b) schematic diagram of fabrication of porous constructs via HA/adhesive powder mixtures [35]; (c) 3D models and objects of parts; (d) green body and as-sintered body; and (e) compression test of porous printed scaffolds [36].
Although HA is extensively being considered for hard tissue regeneration because of its presence in the native extracellular matrix (ECM) of bone tissue, extensive research has not been undertaken regarding pure-HA printed materials, due to the lack of bonding and flowability for the printing process [37]. Thus, various types of sacrificial materials and polymers are used as binders in the process of 3D printing to print neat HA constructs. To overcome the poor reactivity between HA powder with standard water-based ink, Zhou et al. [35] investigated different water-soluble adhesives to increase the 3D printability of HA powder, such as maltodextrin and polyvinyl alcohol (PVA) (Figure 2b). Zhou et al. showed that, by using a high molecular weight of PVA at 30 wt.% as adhesive, the printed formulation could achieve a geometrical accuracy of ~>85% and an excellent green compressive strength of 5.63 ± 0.27 MPa [35]. The curing, de-binding, and sintering parameters of the printing process influence the mechanical properties, porosity, and shrinkage of the sintered samples. Liu et al. [36] fabricated HA bone scaffold using the DLP technique; the printed HA bone scaffolds displayed pore sizes of 300–600 μm, the porosity of around 49.8%, and compressive strength of 15.25 MPa, showing potential for bone repair applications (Figure 2c–e) [36]. Similarly, Seitz et al. [38] employed modified HA powder to print scaffolds; the polymer binder was removed after the consolidation of the printed ceramic green bodies at a temperature of 1250 °C in ambient air [38].
3.2. Hydroxyapatite (HA)/Polymer-Based Nanocomposites
The addition of a polymer to HA nanoparticles can enhance the printability of HA constructs [39][40][41]. Due to the suitability and compatibility to cellular environments, various polymers could be used to fabricate (no matter how complex) constructs in ambient or relatively mild chemical and environmental conditions [42]. Many synthetic and natural polymers can be used to reinforce HA scaffold via 3D printing for TE applications, as shown in Figure 3.
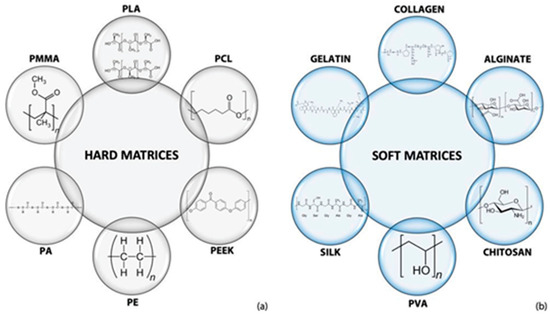
Figure 3. Polymers are used to composite with HA to build (a) hard and (b) soft matrices fabricated via 3D printing for TE [31].
3.3. Hydroxyapatite (HA)-Based Ceramics
β-tricalcium phosphate (β-TCP) is of low mechanical strength and degrades too quickly in a physiological environment. These properties may however be altered/improved via its combination with HA [43][44][45]. BCP has been used to fabricate bone graft materials for 30 years; BCP-based ceramics have proven efficacy in clinical indications [46][47]. Although many 3D printing approaches could be used to fabricate complex BCP-based ceramics, including inkjet printing, SLA, selective laser sintering, and DLP, the performance of BCP and BCP-based ceramics and their printable properties as bio-inks need to be further viewed [48]. Huang et al. [49] fabricated porous BCP ceramics using extrusion-based 3D printing with a motor-assisted micro-syringe (MAM) system; the morphology, pore size, and porosity of printed BCP scaffolds could be precisely controlled to optimize their mechanical properties [49]. Li et al. [46] obtained BCP scaffolds with a complex geometric structure using a slurry-based microscale mask image projection SLA. The BCP-based photocurable suspension with complex geometry was obtained first. After that, the curing performance and physical properties of BCP suspension were investigated to optimize the scaffold composite. The printed BCP scaffold presented excellent biocompatibility, as well as possessed sufficient mechanical strength compared to a long bone for surgery [46]. Wang et al. printed BCP scaffolds using inkjet 3D printing; 0.6 wt.% PVA solution and 0.25 wt.% Tween 80 were used as a binder to prepare BCP bio-ink. The printed BCP scaffold with HA/β-TCP mass ratio at 60:40 showed the best biocompatibility [45]. It is clear that the binder used will vary significantly depending on the researcher, printing technique, etc. Thus, in addition to the binder employed by Wang et al., some other examples of binders and the associated ceramic 3D printing techniques reported in the literature are highlighted in Table 2.
Table 2. Some binders employed in major 3D printing technologies.
|
3D Printing Technology |
Binder |
Some Notes |
Source |
|---|---|---|---|
|
DIW writing |
Polymethylsilsesquioxane |
This binder has been shown to be viable in the fabrication of ceramic matrix composite. In the study, polymethylsilsesquioxane and ceramics were used in the preparation of a preceramic polymer. Using this binder and 3D printing technology, complex ceramic matrix composite structures with porosity and compressive strength of ~75% and ~4 MPa were fabricated. |
[50] |
|
Inkjet-based 3D printing |
Polymethylsilsesquioxane |
This binder was employed in the 3D printing with β-TCP and a polysiloxane to manufacture bulk β-TCP with a silica coating. The mechanical strength of the final sintered porous structures was within the range of that of trabecular bones, in the order of 0.1–16 MPa. |
[51] |
|
Inkjet-based 3D printing |
Colloidal silica |
In this study, the focus was to demonstrate and assess the possibility of using the inkjet-based 3D printing technique and the colloidal silica binder in the fabrication of porous ceramic-based composite parts. Information regarding the mechanical strength of the composite was however not presented. |
[52] |
|
DLP 3D printing |
Silicon resin |
In this work, a DLP-based 3D printing technique was used in fabricating a ceramic composite while also employing silicon resin as the binder. The study showed that the compressive strength and elastic modulus values 3D-structured ceramic based lattice were 5.12 and 2.1 MPa, respectively. |
[53] |
|
Extrusion-based 3D printing |
PVA |
In this study, PVA was employed as a binder in the fabrication of structures of HA composites. The study showed that, at 7–14% of the polymer, HA composites are well extruded and presented a mechanical strength of ~4 MPa after hardening. |
[54] |
|
Selective laser sintering |
Schelofix, Polymeric binder |
In this study, water soluble Schelofix was employed as a binder in the fabrication of HA based composited for 3D printing of scaffolds. A structure with mechanical strength of 22 MPa via the printing technique was achieved. |
[38] |
|
Selective laser sintering |
Polyvinyl alcohol |
In this study, water-soluble PVA was employed as a binder, in the fabrication of ceramic based composites. The study showed that, by using the binder in conjunction with the selective laser sintering, the resulting structure has an average flexural strength of 363.5 MPa and a relative density of 98%. |
[55] |
|
SLA based 3D printing |
Photopolymer binder such as (meth)acrylate monomer/oligomers |
In the study [56] 1,6-hexanediol diacrylate was used as an acrylate-based monomer as the photopolymer binder with a ceramic content of 50 vol% to enable the fabrication of structures with high relative density of 99.95% and high flexural strength of 1008.5 MPa. |
[57] |
4. Applications of HA-Based Nanocomposites
HA-based nanocomposites fabricated using 3D printing serve as 3D templates to provide support for cells to attach, proliferate, and maintain their differentiated function in tissue regeneration [58]. HA-based constructs have been used in several applications, including bone, cartilage, dental, skin, and drug delivery (Figure 4).
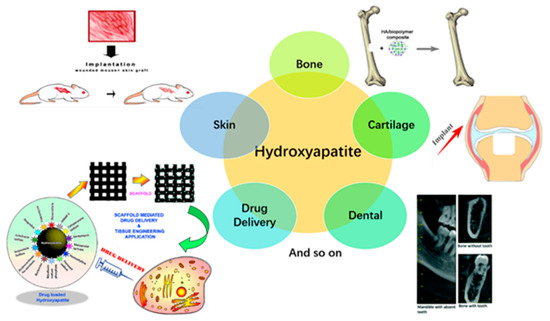
The application of these HA-based nanocomposites constructs in bone, cartilage, dental, skin, and drug delivery are discussed briefly below.
4.1. HA-Based Nanocomposites Constructs in Bone TE
The HA-based construct constitutes an excellent candidate as an orthopedic implant during prosthesis revision surgery since natural bone contains 70 wt.% of HA [64]. Compared to autografts and allografts, artificially-engineered bone scaffold with a complex hierarchical structure can avoid the risk of infection, disease transmission, and immune response [58]. The ideal scaffold as an engineered bond scaffold should meet various criteria, such as biocompatibility, osteoconduction, osteoinduction, mechanical properties, without compromising the interconnected porous structure [65][66][67]. The interconnected porous pure HA scaffold can be prepared using 3D gel-printing approach. Shao et al. [68] obtained the HA scaffold with a pore size of over 350 μm, a porosity of 52.26%, and maximum compressive strength and compressive modulus of the scaffold of 16.77 ± 0.38 and 492 ± 11 MPa, respectively [68]. The incorporation of the HA-based nanocomposites constructs in bone TE can be achieved via the development of hierarchical porous HA scaffold with micropores and macropores via combining 3D printing and microwave sintering [69]. These hierarchical HA scaffold can be readily integrated with the native bone. This approach was demonstrated by Song et al. [70], who reported the fabrication of hierarchical HA scaffold with interconnected pores by combining freeze-casting and extrusion-based 3D printing, such that the structure of human bone was mimicked from the microscopic (below 10 μm) to macroscopic (submillimeter to the millimeter) perspective.
4.2. HA-Based Nanocomposites Constructs in Cartilage TE
HA-based nanocomposites could also be employed in cartilage TE. Articular cartilage, covering the bone ends in diarthrodial joints, is viscoelastic connective tissue, which provides an efficient aqueous lubrication system with high load-bearing and low-friction properties [71]. Unfortunately, once a lesion or injury of articular cartilage occurs, it is difficult to heal due to its limited capacity to repair, and artificial cartilage is required in the clinic [72]. The 3D printing technique can be used to fabricate constructs with high structural complexity and flexibility, such as hydrogels, which exhibit the advantage of individualized precision customization, making the construct perfectly fit with the defective surface in the area of cartilage repair [73]. The application of HA-based nanocomposites constructs in cartilage TE has been demonstrated. For instance, Yuan et al. [61] prepared a composite hydrogel consisting of bovine serum albumin/sodium alginate and HA nanowires, and the in vivo results confirm that the hydrogel can promote the generation of new cartilage. Additionally, Hsieh et al. [74] printed biomimetic scaffolds consisting of HA/PCL and glycidyl–methacrylate–hyaluronic acid for healing osteochondral defects. The scaffolds were implanted in the knees of a miniature pig for a period of 12 months. The hematoxylin and eosin staining and computer tomography (CT) results indicate that the cartilage was partially matured, and the hyaline cartilage was regenerated [74].
4.3. HA-Based Nanocomposites Constructs in Dental Applications
In the last few years, 3D printing for dental applications has notably increased, especially in the areas of oral and maxillofacial surgery, endodontics, orthodontics, prosthodontics, and periodontics. The possibility of individualized dental products drives 3D printing in this area [75]. Metal-, ceramic-, and polymer-based materials are common in the fabrication of dental prosthesis and crowns. Indeed, 3D printing is currently utilized to replace missing teeth [76]. The mechanical properties of constructs for prosthodontics application needs to be improved and issues with porosity avoided to ensure a denser and more compact structure. Such denser and more compact structure may be achieved via ink-jet printing rather than SLS or SLA printing [76]. Furthermore, the development of 3D printing, has promoted the development of regenerative endodontic procedures due to the improved precision and accuracy, while simultaneously ameliorating patient comfort [77]. It is reported that controlling infection is the key to the success of apical inducing angioplasty in the root canal. However, HA itself lacks bactericidal properties; HA-based nanocomposites with antibacterial properties can therefore inhibit the growth of microorganisms in the root canal [78].
4.4. HA-Based Nanocomposites Constructs in Drug Delivery Applications
HA has been used as a composite with biopolymer (e.g., alginate) matrices for a more precise and sustained drug release. For instance, Venkatasubbu et al. [79] loaded the drug ciprofloxacin onto a nano-HA composite with alginate. In their study, the drug was pre-adsorbed onto the ceramic particle before the formation of composite. They showed that the integration of HA-based nanocomposites prolonged the sustained release of ciprofloxacin compared to the ciprofloxacin-loaded HA only. HA/sodium alginate/chitosan (HA/SA/CS) composite microspheres were prepared using an emulsion crosslink technique while using calcium ions as a cross-linking agent [80]. According to Bi et al. [80], the drug loading and encapsulation efficiency of the HA composite was improved compared to when only HA nanoparticles were used. Nie et al. explored silver-doped HA/alginate and HA/β-TCP/alginate microparticles snd micro-clusters, which had excellent antibacterial properties. The obtained microparticles of the HA composite were shown to be useful as drug carriers for the controlled release of doxorubicin [81][82].
In summary, the significance of HA-composites cannot be overemphasized. Moreover, recent studies of 2021 have demonstrated their utilization in human studies. For instance, Kim and Kim [83] employed 3D strontium-substituted HA (Sr-HA) ceramic scaffolds to promote rapid cell proliferation, osteogenic differentiation, and cellular mineralization in human cells. They demonstrated the functionality of Sr-HA scaffold application as new bone graft substitutes in humans. Krzysztof et al. [84] assessed the manufacture of PEEK/HA composite via FFF to determine its suitability for orthopedic implants. They showed that the composite presented comparable mechanical properties to human femoral cortical bone. Huang et al. [85] also demonstrated the development of gelatin/HA hybrid materials which were applied in the fabrication of scaffold for human umbilical cord blood-derived mesenchymal stem cells. The scaffold could effectively support the growth and proliferation of stem cells as well as their adhesion while also inducing chondrogenic differentiation in vitro. Based on the reported success, it is anticipated that future work will further explore the utilization of HA-composites in humans. The next section provides some future expectations of HA composite applications in TE.
References
- Gopi, D.; Kavitha, L.; Ramya, S.; Rajeswari, D. Chemical and green routes for the synthesis of multifunctional pure and substituted nanohydroxyapatite for biomedical applications. In Engineering of Nanobiomaterials; Grumezescu, A.M., Ed.; William Andrew Publishing: Norwich, NY, USA, 2016; Chapter 15; pp. 485–521.
- Lin, K.; Chang, J. 1—Structure and properties of hydroxyapatite for biomedical applications. In Hydroxyapatite (Hap) for Biomedical Applications; Mucalo, M., Ed.; Woodhead Publishing: Cambridge, UK, 2015; pp. 3–19.
- Fernando, S.; McEnery, M.; Guelcher, S.A. 16—Polyurethanes for bone TE. In Advances in Polyurethane Biomaterials; Cooper, S.L., Guan, J., Eds.; Woodhead Publishing: Cambridge, UK, 2016; pp. 481–501.
- Okoro, O.V.; Sun, Z.; Birch, J. Meat processing waste as a potential feedstock for biochemicals and biofuels—A review of possible conversion technologies. J. Clean. Prod. 2017, 142, 1583–1608.
- Okoro, O.V.; Shavandi, A. An assessment of the utilization of waste apple slurry in bio-succinic acid and bioenergy production. Int. J. Environ. Sci. Technol. 2021.
- Shavandi, A.; Bekhit, A.E.-D.A.; Ali, M.A.; Sun, Z. Bio-mimetic composite scaffold from mussel shells, squid pen and crab chitosan for bone tissue engineering. Int. J. Biol. Macromol. 2015, 80, 445–454.
- Ratnayake, J.T.; Gould, M.L.; Shavandi, A.; Mucalo, M.; Dias, G.J. Development and characterization of a xenograft material from N ew Z ealand sourced bovine cancellous bone. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 1054–1062.
- Antebi, B.; Cheng, X.; Harris, J.N.; Gower, L.B.; Chen, X.-D.; Ling, J. Biomimetic Collagen–Hydroxyapatite Composite Fabricated via a Novel Perfusion-Flow Mineralization Technique. Tissue Eng. Part C Methods 2013, 19, 487–496.
- Zhang, J.M.; Lin, C.J.; Feng, Z.D.; Tian, Z.W. Hydroxyapatite/metal composite coatings prepared by multi-step electrodeposition method. J. Mater. Sci. Lett. 1998, 17, 1077–1079.
- Kim, H.-W.; Knowles, J.C.; Kim, H.-E. Hydroxyapatite and gelatin composite foams processed via novel freeze-drying and crosslinking for use as temporary hard tissue scaffolds. J. Biomed. Mater. Res. Part A 2005, 72, 136–145.
- Ito, Y.; Hasuda, H.; Kamitakahara, M.; Ohtsuki, C.; Tanihara, M.; Kang, I.-K.; Kwon, O.H. A composite of hydroxyapatite with electrospun biodegradable nanofibers as a tissue engineering material. J. Biosci. Bioeng. 2005, 100, 43–49.
- Ficai, A.; Andronescu, E.; Voicu, G.; Ghitulica, C.; Vasile, B.S.; Ficai, D.; Trandafir, V. Self-assembled collagen/hydroxyapatite composite materials. Chem. Eng. J. 2010, 160, 794–800.
- Li, H.; Zhao, N.; Liu, Y.; Liang, C.; Shi, C.; Du, X.; Li, J. Fabrication and properties of carbon nanotubes reinforced Fe/hydroxyapatite composites by in situ chemical vapor deposition. Compos. Part A Appl. Sci. Manuf. 2008, 39, 1128–1132.
- Nam, Y.S.; Yoon, J.J.; Park, T.G. A novel fabrication method of macroporous biodegradable polymer scaffolds using gas foaming salt as a porogen additive. J. Biomed. Mater. Res. 2000, 53, 1–7.
- Redepenning, J.; Venkataraman, G.; Chen, J.; Stafford, N. Electrochemical preparation of chitosan/hydroxyapatite composite coatings on titanium substrates. J. Biomed. Mater. Res. Part A 2003, 66, 411–416.
- Nair, M.; Nancy, D.; Krishnan, A.G.; Anjusree, G.S.; Vadukumpully, S.; Nair, S.V. Graphene oxide nanoflakes incorporated gelatin-hydroxyapatite scaffolds enhance osteogenic differentiation of human mesenchymal stem cells. Nanotechnology 2015, 26, 161001.
- Zhou, Y.; Yao, H.; Wang, J.; Wang, D.; Liu, Q.; Li, Z. Greener synthesis of electrospun collagen/hydroxyapatite composite fibers with an excellent microstructure for bone tissue engineering. Int. J. Nanomed. 2015, 10, 3203–3215.
- Wu, Y.; Hench, L.L.; Du, J.; Choy, K.-L.; Guo, J. Preparation of Hydroxyapatite Fibers by Electrospinning Technique. J. Am. Ceram. Soc. 2004, 87, 1988–1991.
- Nosrati, H.; Mamoory, R.S.; Le, D.Q.S.; Bünger, C.E.; Emameh, R.Z.; Dabir, F. Gas injection approach for synthesis of hydroxyapatite nanorods via hydrothermal method. Mater. Charact. 2020, 159, 110071.
- Nosrati, H.; Sarraf Mamoory, R.; Svend Le, D.Q.; Bünger, C.E. Fabrication of gelatin/hydroxyapatite/3D-graphene scaffolds by a hydrogel 3D-printing method. Mater. Chem. Phys. 2020, 239, 122305.
- Nosrati, H.; Sarraf-Mamoory, R.; Le, D.Q.S.; Perez, M.C.; Bünger, C.E. Evaluation of Argon-Gas-Injected Solvothermal Synthesis of Hydroxyapatite Crystals Followed by High-Frequency Induction Heat Sintering. Cryst. Growth Des. 2020, 20, 3182–3189.
- Ishengoma, F.R.; Mtaho, A.B. 3D printing: Developing countries perspectives. arXiv 2014, arXiv:1410.5349. Available online: (accessed on 27 March 2021).
- Hull, C. Co-Founder and Chief Technology Officer. Ann H.J. Smead Aerosp. Eng. Sci. 2021. Available online: (accessed on 2 February 2021).
- Li, Y.; Wang, J.; Yang, Y.; Shi, J.; Zhang, H.; Yao, X.; Chen, W.; Zhang, X. A rose bengal/graphene oxide/PVA hybrid hydrogel with enhanced mechanical properties and light-triggered antibacterial activity for wound treatment. Mater. Sci. Eng. C 2021, 118.
- Sears, N.A.; Seshadri, D.R.; Dhavalikar, P.S.; Cosgriff-Hernandez, E. A Review of Three-Dimensional Printing in Tissue Engineering. Tissue Eng. Part B Rev. 2016, 22, 298–310.
- Zhang, B.; Gao, L.; Ma, L.; Luo, Y.; Yang, H.; Cui, Z. 3D Bioprinting: A Novel Avenue for Manufacturing Tissues and Organs. Engineering 2019, 5, 777–794.
- Bishop, E.S.; Mostafa, S.; Pakvasa, M.; Luu, H.H.; Lee, M.J.; Wolf, J.M.; Ameer, G.A.; He, T.-C.; Reid, R.R. 3-D bioprinting technologies in TE and regenerative medicine: Current and future trends. Genes Dis. 2017, 4, 185–195.
- Sundaramurthi, D.; Rauf, S.; Hauser, C. 3D bioprinting technology for regenerative medicine applications. Int. J. Bioprint. 2016, 2.
- Zhu, W.; Ma, X.; Gou, M.; Mei, D.; Zhang, K.; Chen, S. 3D printing of functional biomaterials for tissue engineering. Curr. Opin. Biotechnol. 2016, 40, 103–112.
- LeGeros, R.Z. Properties of osteoconductive biomaterials: Calcium phosphates. Clin. Orthop. Relat. Res. 2002, 395, 81–98.
- Milazzo, M.; Contessi Negrini, N.; Scialla, S.; Marelli, B.; Farè, S.; Danti, S.; Buehler, M.J. Additive Manufacturing Approaches for Hydroxyapatite-Reinforced Composites. Adv. Funct. Mater. 2019, 29.
- Zhou, H.; Lee, J. Nanoscale hydroxyapatite particles for bone tissue engineering. Acta Biomater 2011, 7, 2769–2781.
- Damien, E.; Hing, K.; Saeed, S.; Revell, P.A. A preliminary study on the enhancement of the osteointegration of a novel synthetic hydroxyapatite scaffold in vivo. J. Biomed. Mater. Res. Part A 2003, 66A, 241–246.
- Liu, X. Cell responses to two kinds of nanohydroxyapatite with different sizes and crystallinities. Int. J. Nanomed. 2012, 7, 1239–1250.
- Zhou, Z.; Lennon, A.; Buchanan, F.; McCarthy, H.O.; Dunne, N. Binder jetting additive manufacturing of hydroxyapatite powders: Effects of adhesives on geometrical accuracy and green compressive strength. Addit. Manuf. 2020, 101645.
- Liu, Z.B.; Liang, H.X.; Shi, T.S.; Xie, D.Q.; Chen, R.Y.; Han, X.; Shen, L.D.; Wang, C.J.; Tian, Z.J. Additive manufacturing of hydroxyapatite bone scaffolds via digital light processing and in vitro compatibility. Ceram. Int. 2019, 45, 11079–11086.
- Kumar, A.; Kargozar, S.; Baino, F.; Han, S.S. Additive Manufacturing Methods for Producing Hydroxyapatite and Hydroxyapatite-Based Composite Scaffolds: A Review. Front. Mater. 2019, 6, 313.
- Seitz, H.; Rieder, W.; Irsen, S.; Leukers, B.; Tille, C. Three-dimensional printing of porous ceramic scaffolds for bone tissue engineering. J. Biomed. Mater. Res. B Appl. Biomater. 2005, 74, 782–788.
- Balasubramanian, V.; Herranz-Blanco, B.; Almeida, P.V.; Hirvonen, J.; Santos, H.A. Multifaceted polymersome platforms: Spanning from self-assembly to drug delivery and protocells. Prog. Polym. Sci. 2016, 60, 51–85.
- Bordes, P.; Pollet, E.; Averous, L. Nano-biocomposites: Biodegradable polyester/nanoclay systems. Prog. Polym. Sci. 2009, 34, 125–155.
- Xu, W.; Wu, X.; Sun, W. Review on polymer/layered silicates nanocomposites. J. Chin. Ceram. Soc. 2016.
- Bedell, M.L.; Navara, A.M.; Du, Y.; Zhang, S.; Mikos, A.G. Polymeric Systems for Bioprinting. Chem. Rev. 2020.
- Strobel, L.A.; Rath, S.N.; Maier, A.K.; Beier, J.P.; Arkudas, A.; Greil, P.; Horch, R.E.; Kneser, U. Induction of bone formation in biphasic calcium phosphate scaffolds by bone morphogenetic protein-2 and primary osteoblasts. J. Tissue Eng. Regen. Med. 2014, 8, 176–185.
- Le Guéhennec, L.; Van Hede, D.; Plougonven, E.; Nolens, G.; Verlée, B.; De Pauw, M.C.; Lambert, F. In vitro and in vivo biocompatibility of calcium-phosphate scaffolds three-dimensional printed by stereolithography for bone regeneration. J. Biomed. Mater. Res. Part A 2020, 108, 412–425.
- Wang, Y.; Wang, K.; Li, X.; Wei, Q.; Chai, W.; Wang, S.; Che, Y.; Lu, T.; Zhang, B. 3D fabrication and characterization of phosphoric acid scaffold with a HA/β-TCP weight ratio of 60:40 for bone TE applications. PLoS ONE 2017, 12, e0174870.
- Antonetti, C.; Ciorba, S.; Licursi, D.; Coccia, V.; Cotana, F.; Galletti, A.M.R. Production of levulinic acid and n-butyl levulinate from the waste biomasses grape pomace and Cynara cardunculus L. In Proceedings of the 1st International Electronic Conference on Catalysts Science, Online, 10–30 November 2020; p. 30.
- Bouler, J.-M.; Pilet, P.; Gauthier, O.; Verron, E. Biphasic calcium phosphate ceramics for bone reconstruction: A review of biological response. Acta Biomater. 2017, 53, 1–12.
- Liu, F.; Liu, Y.; Li, X.Y.; Wang, X.H.; Li, D.N.; Chung, S.; Chen, C.; Lee, I.S. Osteogenesis of 3D printed macro-pore size biphasic calcium phosphate scaffold in rabbit calvaria. J. Biomater. Appl. 2019, 33, 1168–1177.
- Huang, W.; Zhang, X.; Wu, Q.; Wu, B. Fabrication of HA/β-TCP scaffolds based on micro-syringe extrusion system. Rapid Prototyp. J. 2013, 19, 319–326.
- Franchin, G.; Wahl, L.; Colombo, P. Direct ink writing of ceramic matrix composite structures. J. Am. Ceram. Soc. 2017, 100, 4397–4401.
- Siemens, A.G. Manufacturing of SiO2-Coated β-TCP Structures by 3D Printing using a Preceramic Polymer as Printing Binder and Silica Source. J. Ceram. Sci. Technol. 2017, 9, 37–42.
- Sachs, E.; Cima, M.; Williams, P.; Brancazio, D.; Cornie, J. Three dimensional printing: Rapid tooling and prototypes directly from a CAD model. J. Eng. Ind. 1992, 114, 481–488.
- Zocca, A.; Elsayed, H.; Bernardo, E.; Gomes, C.; Lopez-Heredia, M.; Knabe, C.; Colombo, P.; Günster, J.J.B. 3D-printed silicate porous bioceramics using a non-sacrificial preceramic polymer binder. Biofabrication 2015, 7, 025008.
- Musskaya, O.N.; Krut’ko, V.K.; Kulak, A.I.; Filatov, S.A.; Batyrev, E.V.; Safronova, T.V. Calcium Phosphate Compositions with Polyvinyl Alcohol for 3D Printing. Inorg. Mater. Appl. Res. 2020, 11, 192–197.
- Tang, H.-H.; Chiu, M.-L.; Yen, H.-C. Slurry-based selective laser sintering of polymer-coated ceramic powders to fabricate high strength alumina parts. J. Eur. Ceram. Soc. 2011, 31, 1383–1388.
- Ji, S.H.; Kim, D.S.; Park, M.S.; Yun, J.S.J.N. Sintering Process Optimization for 3YSZ Ceramic 3D-Printed Objects Manufactured by Stereolithography. Nanomaterials 2021, 11, 192.
- Bagheri, A.; Jin, J. Photopolymerization in 3D Printing. ACS Appl. Polym. Mater. 2019, 1, 593–611.
- Hutmacher, D.W. Scaffold design and fabrication technologies for engineering tissues--state of the art and future perspectives. J. Biomater. Sci. Polym. Ed. 2001, 12, 107–124.
- Golubevas, R.; Stankeviciute, Z.; Zarkov, A.; Golubevas, R.; Hansson, L.; Raudonis, R.; Kareiva, A.; Garskaite, E. Acrylate–gelatin–carbonated hydroxyapatite (cHAP) composites for dental bone-tissue applications. Mater. Adv. 2020, 1, 1675–1684.
- Szczes, A.; Holysz, L.; Chibowski, E. Synthesis of hydroxyapatite for biomedical applications. Adv. Colloid Interfaces 2017, 249, 321–330.
- Yuan, H.F.; Zheng, X.Y.; Liu, W.; Zhang, H.; Shao, J.J.; Yao, J.X.; Mao, C.Y.; Hui, J.F.; Fan, D.D. A novel bovine serum albumin and sodium alginate hydrogel scaffold doped with hydroxyapatite nanowires for cartilage defects repair. Colloid Surf. B 2020, 192.
- Mondal, S.; Pal, U. 3D hydroxyapatite scaffold for bone regeneration and local drug delivery applications. J. Drug Deliv. Sci. Technol. 2019, 53.
- Chaudhari, A.A.; Vig, K.; Baganizi, D.R.; Sahu, R.; Dixit, S.; Dennis, V.; Singh, S.R.; Pillai, S.R. Future prospects for scaffolding methods and biomaterials in skin tissue engineering: A review. Int. J. Mol. Sci. 2016, 17, 1974.
- Ramakrishna, S.; Jose, R.; Archana, P.S.; Nair, A.S.; Balamurugan, R.; Venugopal, J.; Teo, W.E. Science and engineering of electrospun nanofibers for advances in clean energy, water filtration, and regenerative medicine. J. Mater. Sci. 2010, 45, 6283–6312.
- Yan, Q.; Dong, H.; Su, J.; Han, J.; Song, B.; Wei, Q.; Shi, Y. A Review of 3D Printing Technology for Medical Applications. Engineering 2018, 4, 729–742.
- Jazayeri, H.E.; Rodriguez-Romero, M.; Razavi, M.; Tahriri, M.; Ganjawalla, K.; Rasoulianboroujeni, M.; Malekoshoaraie, M.H.; Khoshroo, K.; Tayebi, L. The cross-disciplinary emergence of 3D printed bioceramic scaffolds in orthopedic bioengineering. Ceram. Int. 2018, 44, 1–9.
- Svehla, M.; Morberg, P.; Zicat, B.; Bruce, W.; Sonnabend, D.; Walsh, W.R. Morphometric and mechanical evaluation of titanium implant integration: Comparison of five surface structures. J. Biomed. Mater. Res. Part A 2000, 51, 15–22.
- Shao, H.; He, J.; Lin, T.; Zhang, Z.; Zhang, Y.; Liu, S. 3D gel-printing of hydroxyapatite scaffold for bone tissue engineering. Ceram. Int. 2019, 45, 1163–1170.
- Pei, X.; Ma, L.; Zhang, B.Q.; Sun, J.X.; Sun, Y.; Fan, Y.J.; Gou, Z.R.; Zhou, C.C.; Zhang, X.D. Creating hierarchical porosity hydroxyapatite scaffolds with osteoinduction by three-dimensional printing and microwave sintering. Biofabrication 2017, 9.
- Song, X.L.; Tetik, H.; Jirakittsonthon, T.; Parandoush, P.; Yang, G.; Lee, D.; Ryu, S.; Lei, S.T.; Weiss, M.L.; Lin, D. Biomimetic 3D Printing of Hierarchical and Interconnected Porous Hydroxyapatite Structures with High Mechanical Strength for Bone Cell Culture. Adv. Eng. Mater. 2019, 21.
- Bas, O.; De-Juan-Pardo, E.M.; Meinert, C.; D’Angella, D.; Baldwin, J.G.; Bray, L.J.; Wellard, R.M.; Kollmannsberger, S.; Rank, E.; Werner, C.; et al. Biofabricated soft network composites for cartilage tissue engineering. Biofabrication 2017, 9.
- Wang, J.Q.; Zhang, F.J.; Tsang, W.P.; Wan, C.; Wu, C. Fabrication of injectable high strength hydrogel based on 4-arm star PEG for cartilage tissue engineering. Biomaterials 2017, 120, 11–21.
- Rajzer, I.; Kurowska, A.; Jablonski, A.; Jatteau, S.; Sliwka, M.; Ziabka, M.; Menaszek, E. Layered gelatin/PLLA scaffolds fabricated by electrospinning and 3D printing—For nasal cartilages and subchondral bone reconstruction. Mater. Des. 2018, 155, 297–306.
- Hsieh, Y.-H.; Shen, B.-Y.; Wang, Y.-H.; Lin, B.; Lee, H.-M.; Hsieh, M.-F. Healing of osteochondral defects implanted with biomimetic scaffolds of poly (ε-caprolactone)/hydroxyapatite and glycidyl-methacrylate-modified hyaluronic acid in a minipig. Int. J. Mol. Sci. 2018, 19, 1125.
- Oberoi, G.; Nitsch, S.; Edelmayer, M.; Janjić, K.; Müller, A.S.; Agis, H. 3D Printing—encompassing the facets of dentistry. Front. Bioeng. Biotechnol. 2018, 6, 172.
- Ucar, Y.; Meric, I.A.; Ekren, O. Layered Manufacturing of Dental Ceramics: Fracture Mechanics, Microstructure, and Elemental Composition of Lithography-Sintered Ceramic. J. Prosthodont. 2019, 28, E310–E318.
- Shah, P.; Chong, B.S. 3D imaging, 3D printing and 3D virtual planning in endodontics. Clin. Oral Investig. 2018, 22, 641–654.
- Lin, L.W.; Fang, Y.F.; Liao, Y.X.; Chen, G.; Gao, C.X.; Zhu, P.Z. 3D Printing and Digital Processing Techniques in Dentistry: A Review of Literature. Adv. Eng. Mater. 2019, 21.
- Venkatasubbu, G.D.; Ramasamy, S.; Ramakrishnan, V.; Kumar, J. Hydroxyapatite-alginate nanocomposite as drug delivery matrix for sustained release of ciprofloxacin. J. Biomed. Nanotechnol. 2011, 7, 759–767.
- Bi, Y.-G.; Lin, Z.-T.; Deng, S.-T. Fabrication and characterization of hydroxyapatite/sodium alginate/chitosan composite microspheres for drug delivery and bone tissue engineering. Mater. Sci. Eng. C 2019, 100, 576–583.
- Zhou, Q.J.; Wang, T.W.; Wang, C.; Wang, Z.; Yang, Y.A.; Li, P.; Cai, R.H.; Sun, M.; Yuan, H.Y.; Nie, L. Synthesis and characterization of silver nanoparticles-doped hydroxyapatite/alginate microparticles with promising cytocompatibility and antibacterial properties. Colloid Surf. A 2020, 585.
- Nie, L.; Deng, Y.; Zhang, Y.; Zhou, Q.; Shi, Q.; Zhong, S.; Sun, Y.; Yang, Z.; Sun, M.; Politis, C.; et al. Silver-doped biphasic calcium phosphate/alginate microclusters with antibacterial property and controlled doxorubicin delivery. J. Appl. Polym. Sci. 2021, 138, 50433.
- Kim, H.-W.; Kim, Y.-J. Fabrication of strontium-substituted hydroxyapatite scaffolds using 3D printing for enhanced bone regeneration. J. Mater. Sci. 2021, 56, 1673–1684.
- Rodzeń, K.; Sharma, P.K.; McIlhagger, A.; Mokhtari, M.; Dave, F.; Tormey, D.; Sherlock, R.; Meenan, B.J.; Boyd, A.J.P. The Direct 3D Printing of Functional PEEK/Hydroxyapatite Composites via a Fused Filament Fabrication Approach. Polymers 2021, 13, 545.
- Huang, J.; Huang, Z.; Liang, Y.; Yuan, W.; Bian, L.; Duan, L.; Rong, Z.; Xiong, J.; Wang, D.; Xia, J. 3D printed gelatin/hydroxyapatite scaffolds for stem cell chondrogenic differentiation and articular cartilage repair. Biomater. Sci. 2021.




