
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Karla Helbig | + 3708 word(s) | 3708 | 2021-02-10 04:43:42 | | | |
| 2 | Peter Tang | Meta information modification | 3708 | 2021-05-14 04:24:20 | | |
Video Upload Options
Staphylococcus pseudintermedius is a pathogenic bacterium of concern within the veterinary sector and is involved in numerous infections in canines, including topical infections such as canine pyoderma and otitis externa, as well as systemic infections within the urinary, respiratory and reproductive tract. The high prevalence of methicillin-resistant Staphylococcus pseudintermedius (MRSP) within such infections is a growing concern.
1. Introduction
Over the last decade, Staphylococcus pseudintermedius has been identified as a bacterial species of concern within the veterinary sector. S. pseudintermedius is an opportunistic pathogen frequently isolated from healthy canines and, more importantly, associated with numerous infections in animals [1]. Dogs are the most common animal species infected with S. pseudintermedius, with 84.7% of all S. pseudintermedius isolates originating from canine diseases including skin, ear and urinary tract infections [2]. It has been reported that up to 97.8% of methicillin-resistant S. pseudintermedius (MRSP) isolates show multidrug resistance (MDR) to three or more antibiotics routinely used in veterinary medicine [2][3][4].
S. pseudintermedius was first isolated in 1976; however, it was formerly identified as Staphylococcus intermedius due to the morphological similarities between the two species [5]. In 2005, using a DNA–DNA hybridisation technique on S. intermedius isolates collected from animals, S. pseudintermedius was revealed as a novel species [6].
It is now known that S. pseudintermedius belongs to a collective known as the Staphylococcus intermedius group (SIG) which encompasses three distinct species, S. pseudintermedius, S. intermedius and S. delphini [7]. While all members within the SIG group have been shown to colonise numerous animal species, S. pseudintermedius is said to be the most common SIG species associated with animals—particularly, the most prevalent commensal bacterium in dogs [7][8][9][10][11]. Therefore, for the purpose of this review, all literature describing canine isolates formerly identified as S. intermedius will be referred to as S. pseudintermedius, unless otherwise shown by genomic investigation.
2. Staphylococcus pseudintermedius: A Pathogenic Bacterium of Veterinary Concern
Although S. pseudintermedius is primarily known for its pathogenic potential in canine infections, it is important to understand that S. pseudintermedius is also a significant member of the normal flora in canines [12][13][14]. Several studies have isolated S. pseudintermedius from 46–92% of healthy dogs, with the highest prevalence at the perineum (the skin between the anus and vulva/scrotum), followed by either the nasal or oral mucosa [12][13][14]. One study found that 0–4.5% of healthy dogs are colonised with MRSP as part of their normal flora [15]. Methicillin resistance in Staphylococcal spp. is known to alter the affinity to all β-lactam antibiotics. MRSP is a growing concern, with a recent study finding that 63% of S. pseudintermedius strains isolated from sick dogs were methicillin-resistant, with 78% of these isolates also described as MDR, being resistant to three or more antibiotic classes [16]. Additionally, MRSP can be transferred from sick dogs to otherwise healthy canines via direct transmission or indirect environmental transmission [17]. S. pseudintermedius transmission and subsequent colonisation may be associated with numerous infections, with skin infections being the most common (see Figure 1). However, S. pseudintermedius is also present as a pathogen in multiple other canine disease pathologies [17][18][19][20]. This highlights the fact that antibiotic resistance is a real concern moving forward in the veterinary space, especially for the treatment of S. pseudintermedius in canines, and adds perspective about whether antibiotics are a viable treatment option for the future of veterinary medicine.
2.1. Canine Pyoderma
Canine pyoderma is one of the most common bacterial skin infections diagnosed in small veterinary medicine and is associated with redness, lesions, pain and inflammation [21]. Canine pyoderma can vary from moderate infections to severe infections and is triggered by underlying factors such as allergic skin disease, ectoparasites and endocrinopathies. This initiates the colonisation of pathogenic S. pseudintermedius, which is the most common pathogen associated with cutaneous infections, isolated as the predominant pathogen in up to 92% of canine pyoderma cases (see Figure 1) [22][23][24][25]. While it is evident that S. pseudintermedius is the predominant pathogen associated with canine pyoderma, it is also the most common commensal species in dogs, and there is currently a lack of clear evidence as to whether commensal species cause infection or if external isolates initiate infection.
To assess this knowledge gap, multiple studies have compared the sequence diversity between commensal and pathogenic S. pseudintermedius isolates from the same canines; however, the collective evidence from these studies has not been able to conclusively answer this question. A handful of studies have shown no distinguishable differences between S. pseudintermedius isolates from healthy or atopic dogs using molecular techniques, therefore indicating that no specific strains or clusters of strains are associated with canine pyoderma [26][27][28]. More specifically, S. pseudintermedius isolates collected from the mucosa, a colonisation site for commensal species, and lesion sites from infected dogs in one study were either indistinguishable or closely related, perhaps indicating that the commensal S. pseudintermedius isolates may also be the causative agent in these pyoderma cases [26]. In contrast, isolates from canine pyoderma lesions have also been shown to be completely unrelated to mucosal isolates, suggesting either the commensal species mutate to become pathogenic or external isolates of S. pseudintermedius colonise to cause infection [26]. It is clear that longitudinal studies are required to truly assess genetic relatedness between commensal isolates and those involved in pyoderma cases, despite the fact that these studies will undoubtedly be difficult to perform given the inability to predict the onset of canine pyoderma, which would in turn require very large cohorts of animals to give meaningful study outcomes.
The rise in MRSP may explain the discordance between S. pseudintermedius species isolated from canine pyoderma. MRSP is isolated in up to 59% of canine pyoderma cases (see Figure 1), perhaps indicating that S. pseudintermedius isolates involved in infections may acquire genes required for methicillin resistance and appear unrelated to the commensal species [22][23][24][25][29]. Additionally, the acquisition of external strains that may lead to disease may be supported by two independent studies which have shown that MRSP from infected dogs can transmit to healthy contact dogs and the environment (e.g., sleeping and eating areas) [17][19]. In the majority of cases, the healthy dogs were only MRSP-positive when the infected dog was MRSP-positive, inferring contamination rather than colonisation in healthy dogs [17]. However, there was one case where the healthy dog remained MRSP-positive, resulting in an ear infection, even after the infected dog recovered [17]. This implies that S. pseudintermedius isolates recovered from canine infections that are unrelated to the dog’s commensal species may be caused by external species acquired from contact with infected dogs. However, this still leaves room for research into the factors that may contribute to the difference between acquired infection versus no infection upon contact of a healthy dog with an MRSP-infected dog showing disease pathology. However, these findings suggest that MRSP may have the ability to spread further within the dog community, which may affect the availability of treatment options to treat MRSP infections [17][19]. Additionally, MRSP isolates are often also multidrug-resistant, demonstrating high levels of antibiotic resistance against multiple antibiotic classes [30]. One study showed that the number and variety of antibacterial drug classes previously prescribed to dogs resulted in higher cases of MRSP, particularly those that received beta-lactam drugs and concurrent immunomodulatory therapy [30][31]. The evidence of high antibacterial resistance in pyoderma isolates is concerning from a treatment perspective, especially as S. pseudintermedius causes various other diseases throughout dogs.
2.2. Otitis Externa
Otitis externa (OE), or inflammation of the outer ear, is a disease routinely diagnosed in small veterinary practices [32][33][34][35]. Primary causes of OE are factors that initiate inflammation, including foreign bodies, such as grass awns, endocrinopathies including hypothyroidism as well as the presence of parasites [35][36][37]. However, the most frequent primary cause of OE highlighted across multiple studies is allergies, including adverse food reactions or atopic dermatitis [35][36][37], with studies showing that up to 75% of those diagnosed with OE were also diagnosed with atopic dermatitis [35][36][37][38]. Bacteria and yeasts, particularly S. pseudintermedius and Malassezia spp., respectively, two commensal species of the skin, are listed as secondary causes of otitis externa [34]. S. pseudintermedius is a predominant pathogen associated with OE, isolated from 20–94.3% of OE cases in canines (see Figure 1) [32][34][36][38][39][40][41][42][43]. The variation surrounding the prevalence of S. pseudintermedius in OE is still under investigation; however, geographical location has been suggested as a potential strong contributor, but with no influence on seasonal trend [40].
While dog breeds such as spaniels, German Shepherd and Shar-Pei are represented significantly more in OE cases [36][37][40], the breed and the age of the dog may also influence the type of pathogen present [36]. Interestingly, breed as a predisposing factor to OE may be explained by ear confirmation, particularly in spaniel breeds, with two independent studies identifying a significant increase in diagnosis frequency in dogs with pendulous ears, likely due to the moist, warm conditions facilitating secondary bacterial and fungal growth [37][40]. This is not surprising as the outer ear has a similar structure to the epidermis of the skin and, therefore, species that affect the skin, such as S. pseudintermedius, can also affect the external ear canal [35]. This is important for veterinarians to consider, as dogs with canine pyoderma are therefore at a higher risk of developing a secondary infection of S. pseudintermedius within the ear canal, which should be considered when prescribing treatment options for OE. Importantly, MRSP strains have been isolated in 10–48.1% of canine OE cases (see Figure 1) [2][39][41][42], with one study showing that all MRSP isolates were also multidrug-resistant, being resistant to two or more antibiotic classes [39]. This study also found that recent administration of beta-lactam antimicrobials significantly increased the frequency of methicillin and fluoroquinolone resistance [39], with the number and duration of prior exposures significantly increasing resistance to particular antimicrobial classes and the prevalence of methicillin resistance, respectively [39]. These studies highlight that antibiotic treatment of OE moving forward is likely to be plagued with difficulties and that novel treatment options to reduce antimicrobial resistance should be considered.
2.3. Urinary Tract Infections (UTI)
Urinary tract infections (UTIs), particularly those caused by bacteria, are another common diagnosis within small veterinary practices, with approximately 14% of dogs contracting a UTI within their lifetime [44][45]. Numerous bacteria species have been isolated previously from canine UTI cases, including Enterococcus spp., Proteus spp., Staphylococcus spp. and Streptococcus spp., with Escherichia coli identified as the most common uropathogen, isolated from up to 51% of canine UTIs [44][45][46][47][48][49]. More recently, S. pseudintermedius has been shown as the most common Staphylococcal spp. present in canine UTIs, with studies reporting a variable frequency of S. pseudintermedius isolation in 6.3–94.7% of UTIs in canines (see Figure 1) [45][46][48][49][50][51][52][53]. The large variation in S. pseudintermedius isolation has in part been explained in a recent multicentre study over a 6-year period across 14 European countries [53]. This study found that the bacterial species isolated from canine UTI cases varied based on geographical location, as did the antimicrobial resistance of the respective bacteria isolated [53]. Within this study, S. pseudintermedius was the most frequently isolated pathogen from UTI cases in most countries; however, this varied from 0% S. pseudintermedius isolation rate in Spain to as high as 94.7% in Italy; similarly, the isolation of MRSP varied from as low as 1.15% in Sweden to as high as 50% in Italy [53]. Within this study, the variation in antimicrobial resistance, particularly MRSP, was potentially attributed to countries, such as Sweden, following tighter regulations in regard to antimicrobial regulation and use, therefore resulting in lower resistance rates. However, differences in methods used to identify antimicrobial resistance were also reported across countries, which may have impacted the prevalence rates. In addition, the differences in methods used for urine sampling and bacterial isolation may have also affected the S. pseudintermedius prevalence rates [45]. This highlights the importance of unifying the methods of isolation and antimicrobial resistance characterisation for more accurate representations of S. pseudintermedius prevalence and resistance. Alarmingly, despite the variation in sampling and detection methods across the sector, multiple studies have reported high rates of MRSP and MDR S. pseudintermedius from UTIs in canines [45][46][52][54], with a significant increase in methicillin and gentamicin resistance in S. pseudintermedius isolates over a 16-year period, and a significant increase in fluoroquinolone resistance over the last 7-year period [45][46][52][54]. Additionally, there has been a temporal increase in MDR resistance in MRSP isolates, with all MRSP isolates in the study by Marques and colleagues displaying resistance to all antibiotics tested [52]. This is concerning as S. pseudintermedius has been isolated in up to 33% of recurrent UTI in canines [55], therefore alluding to the fact that antimicrobial resistance in S. pseudintermedius is impacting the resolution of UTI, resulting in major therapeutic limitations.
2.4. Respiratory Infections
Respiratory tract infections (RTI) in canines are relatively common and encompass various diseases including bacterial pneumonia, canine infectious respiratory disease complex (CIRDC) and viral infections; additionally, they are readily passed between dogs in social settings such as dog parks and boarding kennels [56]. There are numerous bacterial and viral pathogens that cause RTI in dogs, resulting in clinical symptoms such as coughing, sneezing or excess discharge [57]. Based on these symptoms, in addition to the medical history and physical examination of the patient, a presumptive diagnosis is generally made. However, to identify the causative agents or perform antibiotic susceptibility testing, samples of the airway lavage are generally used to culture the bacterial species [58][59]. As a result, studies have reported several bacterial species associated with RTI, particularly Staphylococcus spp., including S. pseudintermedius, which is isolated in 9.3–60% of RTI in canines (see Figure 1) [50][56][57][58][59][60][61][62][63][64].
There are many factors influencing the prevalence of S. pseudintermedius in canine RTI, including the type of RTI diagnosed, which has been shown to affect the bacterial species isolated [58]. Interestingly, Staphylococcus spp. is more likely to be isolated from canines with aspiration pneumonia, usually caused by fluid from the stomach or mouth entering the lungs, compared to dogs that have community-acquired pneumonia [58]. The heightened prevalence of S. pseudintermedius in cases of aspiration pneumonia is likely due to the presence of S. pseudintermedius in the mouths of healthy canines, therefore entering the lungs and causing infection. This variation in dominant species present in different RTI was confirmed by a recent study which also found differences in bacterial communities between community-acquired pneumonia and secondary-bacterial pneumonia [59]. In particular, dogs with community-acquired pneumonia showed a loss of bacterial diversity and a dominant taxon [59]. Meanwhile, dogs with secondary-bacterial pneumonia also had a dominant species; however, usually those derived from the upper respiratory tract—for example, S. pseudintermedius from the mouth—thus indicating that bacterial symbiosis is a common phenomenon in canine bacterial pneumonia [59].
In addition to the type of RTI influencing the prevalence of S. pseudintermedius isolation, studies aiming to monitor the antimicrobial resistance patterns of canine isolates throughout Europe found a higher proportion of S. pseudintermedius isolates from RTI in Poland from 2008 to 2014, therefore suggesting that geographical location may contribute to variation in S. pseudintermedius isolation from canine RTI [56][60]. This variation due to geographical location was in agreement with a recent study which additionally found a potential association between RTI and the season or the age of the dog, albeit not statistically significant [62].
As mentioned, both bacterial and viral pathogens are associated with canine RTI; therefore, previous research has explored the potential interaction between bacterial and viral pathogens involved in respiratory infections. Preliminary results performed in a mouse model showed that mice co-infected with S. pseudintermedius and canine influenza virus (CIV) showed significant increases in bacterial and viral load in various organs compared to mice infected with S. pseudintermedius or CIV alone and, subsequently, a significant increase in lesion scores in the tissues of co-infected mice [61]. This indicates that the control or treatment of viral infections in addition to S. pseudintermedius infections in canine RTI are equally as important for a successful outcome [61]. However, this phenomenon would need to be looked at in dogs to see if these data are translatable to actual clinical data.
In regard to treatment, bacterial RTI in canines are currently prescribed antibiotics such as trimethoprim, amoxicillin-clavulanic acid or enrofloxacin, either as monotherapy or dual therapy, depending on the severity of the infection [64][65]. A recent study found that 99.4% of all isolates recovered from canine RTI were resistant to at least one antibiotic, with 64.7% of isolates listed as MDR, with Staphylococcus spp. making up 7.1% of the MDR isolates [62]. Importantly, there is a significant association between the sex of the dog or the geographical season and the presence of MDR isolates, which is important to take into account when prescribing treatment options [62]. This high rate of resistance was confirmed in a similar study, as an alarming 57.4% of dogs had a bacterial isolate that was resistant to the antibiotics that were previously or currently prescribed to that dog [58]. Therefore, confirming previous antibiotic administration can increase the resistance profiles of respiratory bacterial isolates [58]. With increasing trends of antibiotic resistance in respiratory isolates, studies suggest minimising this using broad-spectrum antibiotic use and to avoid using previously prescribed antibiotics, which may limit the treatment availability for bacterial RTI in canines in the future [56].
2.5. Reproductive Tract Infections
Previous research has identified an increase in the frequency of S. pseudintermedius in healthy dams isolated from vaginal samples, the placenta as well as colostrum and milk samples around the time of parturition [66][67][68][69][70]. However, the presence of S. pseudintermedius within the reproductive tract, such as the uterus and the mammary glands, has been associated with diseases in canines including pyometra and mastitis, respectively, which may result in complications including neonatal mortality [66][69][70][71][72][73]. Canine pyometra is an infection within the uterus of breeding female dogs, with E. coli and Staphylococcus spp., predominantly isolated from pyometra cases [74]. To date, two studies have isolated S. pseudintermedius in 10.5–18% of pyometra cases; however, these two studies had relatively small sample sizes and therefore may not accurately represent the prevalence of S. pseudintermedius in canine pyometra cases (see Figure 1) [73][75]. There have been no additional studies to explain the role of S. pseudintermedius in pyometra cases; therefore, further work is required to determine the pathogenicity of S. pseudintermedius in canine pyometra cases.
Additionally, S. pseudintermedius has also been isolated from canines with clinical mastitis; however, the prevalence of S. pseudintermedius in mastitis cases has not been well researched [66][69][70][71][72]. Despite the lack of research into prevalence, it has been shown that all dogs experimentally inoculated with S. pseudintermedius develop clinical mastitis, resulting in symptoms including painful, hot and inflamed mammary glands [72], thus indicating that S. pseudintermedius can be pathogenic in the mammary gland and may be responsible for many canine mastitis cases [72]. It has been shown that in limited cases, S. pseudintermedius is the causative agent of mastitis in cows; therefore, further research is required to determine how often S. pseudintermedius is present in canine mastitis and whether it is the causative agent in dogs also [76].
While the presence of S. pseudintermedius in the reproductive tract may cause infection in the female canines, it has also been shown that identical or closely related S. pseudintermedius strains have been isolated from the mother’s milk and vaginal tract and the puppies’ skin and placental samples, indicating that S. pseudintermedius may be transmitted by intrauterine or vertical transmission [67][77][78]. While, in many cases, such transmission results in the healthy colonisation of commensal S. pseudintermedius, in the puppies, it has been shown that the transmission of S. pseudintermedius, specifically MRSP, has been associated with premature death within the first 2–3 weeks of life, also known as neonatal mortality [66][79][80][81]. Multiple studies have reported outbreaks of neonatal mortality due to septicaemia, with S. pseudintermedius or MRSP isolated from the blood or organs of all deceased puppies [66][79][82]. Interestingly, it was found that S. pseudintermedius strains collected from the organs of puppies were found to be linked to isolates from the mother’s milk and vaginal samples, therefore indicating that the vertical transmission of pathogenic S. pseudintermedius can result in fatal sepsis in puppies [79][82]. However, the cause of neonatal mortality is multifactorial and, in addition to infections, congenital defects and low birth weight may also contribute to neonatal mortality [83]. Interestingly, puppies born with no detectable microbiota (including S. pseudintermedius) have a slower growth rate compared to those born with a microbiota within placental and meconium samples [67]. While this was not directly attributed to neonatal mortality, the lack of commensal species including S. pseudintermedius may be a contributing factor. Considering that the transmission of pathogenic S. pseudintermedius from the mother to her puppies may result in fatal sepsis, however, the lack of microbiota, including S. pseudintermedius may contribute to low birth weight and thus may lead to neonatal mortality. Therefore, there is a fine line between commensal S. pseudintermedius colonisation and pathogenic infection, with some studies suggesting that commensal colonisation of S. pseudintermedius may protect against pathogenic S. pseudintermedius, by bacterial interference [78]. Similarly to respiratory tract infections, it appears that symbiosis of commensal species is important and should be taken into consideration when developing treatment options.
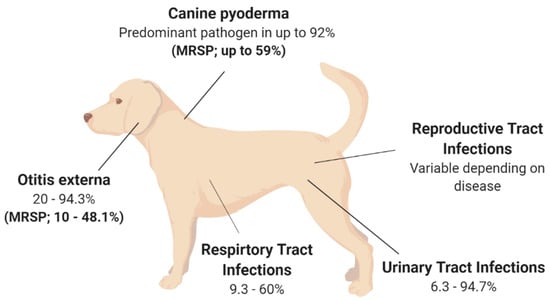
Figure 1. Isolation rates of S. pseudintermedius and MRSP from various disease states in canines. The isolation rates are presented as a range due to the variation described between studies; variation is described in detail in the main text. Canine pyoderma [22][23][24][25][29]; Otitis externa [2][32][34][36][38][39][40][41][42][43]. Urinary Tract Infections [45][46][48][49][50][51][52][53]; Respiratory Tract Infections [50][56][57][58][59][60][61][62][63][64]; Reproductive Tract Infections [66][69][70][71][72][73][76].
3. Current and Future Treatment Options for Staphylococcus pseudintermedius in Canines
S. pseudintermedius has the potential to cause or associate with a range of moderate to severe infections, of which, those left untreated, may have devastating outcomes. Therefore, future treatment options against such infections, especially in light of increasing antibiotic resistance, are a significant area of focus. It is important to note that the majority of studies on treatment options against S. pseudintermedius are in the context of canine pyoderma, as it is one of the primary reasons for antimicrobial prescription in the small animal veterinary sector [84]. Current guidelines created by diplomats of the American and European Colleges of Veterinary Dermatology state that canine pyoderma caused by S. pseudintermedius should be treated by topical and/or systemic antimicrobial therapy, as a gold standard [84]. A recent study found that 96.5% of dogs were prescribed antimicrobials for systemic and/or topical administration upon the diagnosis of canine pyoderma, with the majority of dogs receiving antibiotics including amoxicillin-clavulanate (55.7%), followed by cephalexin (43.9%) or clindamycin (10.0%) [85]. In addition to systemic antibiotics, 27.7% of canine pyoderma cases also received topical treatments, with active ingredients including fusidic acid, chlorhexidine and miconazole plus chlorhexidine shampoo [85]. Therefore, there is a significant need for alternative therapeutics. Two progressive areas of research for the treatment of canine pyoderma are vaccines and phage therapy, both of which have been successful in alternative animal diseases and offer promising alternative therapies for canine pyoderma and other S. pseudintermedius diseases moving forward.
References
- Bannoehr, J.; Guardabassi, L. Staphylococcus pseudintermedius in the dog: Taxonomy, diagnostics, ecology, epidemiology and pathogenicity. Vet. Dermatol. 2012, 23, 253–266,e51-2.
- Ruscher, C.; Lübke-Becker, A.; Wleklinski, C.-G.; Soba, A.; Wieler, L.H.; Walther, B. Prevalence of Methicillin-resistant Staphylococcus pseudintermedius isolated from clinical samples of companion animals and equidaes. Vet. Microbiol. 2009, 136, 197–201.
- Wegener, A.; Broens, E.M.; Zomer, A.; Spaninks, M.; Wagenaar, J.A.; Duim, B. Comparative genomics of phenotypic antimicrobial resistances in methicillin-resistant Staphylococcus pseudintermedius of canine origin. Vet. Microbiol. 2018, 225, 125–131.
- Worthing, K.A.; Abraham, S.; Coombs, G.W.; Pang, S.; Saputra, S.; Jordan, D.; Trott, D.J.; Norris, J.M. Clonal diversity and geographic distribution of methicillin-resistant Staphylococcus pseudintermedius from Australian animals: Discovery of novel sequence types. Vet. Microbiol. 2018, 213, 58–65.
- Hajek, V. Staphylococcus intermedius, a new species isolated from animals. Int. J. Syst. Evol. Microbiol. 1976, 26, 401–408.
- Devriese, L.A.; Vancanneyt, M.; Baele, M.; Vaneechoutte, M.; De Graef, E.; Snauwaert, C.; Cleenwerck, I.; Dawyndt, P.; Swings, J.; Decostere, A. Staphylococcus pseudintermedius sp. nov., a coagulase-positive species from animals. Int. J. Syst. Evol. Microbiol. 2005, 55, 1569–1573.
- Sasaki, T.; Kikuchi, K.; Tanaka, Y.; Takahashi, N.; Kamata, S.; Hiramatsu, K. Reclassification of phenotypically identified Staphylococcus intermedius strains. J. Clin. Microbiol. 2007, 45, 2770–2778.
- Mališová, L.; Šafránková, R.; Kekláková, J.; Petráš, P.; Žemličková, H.; Jakubů, V. Correct species identification (reclassification in CNCTC) of strains of Staphylococcus intermedius-group can improve an insight into their evolutionary history. Folia Microbiol. 2019, 64, 231–236.
- Bannoehr, J.; Franco, A.; Iurescia, M.; Battisti, A.; Fitzgerald, J.R. Molecular diagnostic identification of Staphylococcus pseudintermedius. J. Clin. Microbiol. 2009, 47, 469–471.
- Sasaki, T.; Tsubakishita, S.; Tanaka, Y.; Sakusabe, A.; Ohtsuka, M.; Hirotaki, S.; Kawakami, T.; Fukata, T.; Hiramatsu, K. Multiplex-PCR method for species identification of coagulase-positive Staphylococci. J. Clin. Microbiol. 2010, 48, 765–769.
- Chrobak, D.; Kizerwetter-Świda, M.; Rzewuska, M.; Moodley, A.; Guardabassi, L.; Binek, M. Molecular characterization of Staphylococcus pseudintermedius strains isolated from clinical samples of animal origin. Folia Microbiol. 2011, 56, 415–422.
- Ma, G.C.; Worthing, K.A.; Ward, M.P.; Norris, J.M. Commensal Staphylococci Including Methicillin-Resistant Staphylococcus aureus from Dogs and Cats in Remote New South Wales, Australia. Microb. Ecol. 2020, 79, 164–174.
- Paul, N.C.; Bärgman, S.C.; Moodley, A.; Nielsen, S.S.; Guardabassi, L. Staphylococcus pseudintermedius colonization patterns and strain diversity in healthy dogs: A cross-sectional and longitudinal study. Vet. Microbiol. 2012, 160, 420–427.
- Iverson, S.A.; Brazil, A.M.; Ferguson, J.M.; Nelson, K.; Lautenbach, E.; Rankin, S.C.; Morris, D.O.; Davis, M.F. Anatomical patterns of colonization of pets with Staphylococcal species in homes of people with methicillin-resistant Staphylococcus aureus (MRSA) skin or soft tissue infection (SSTI). Vet. Microbiol. 2015, 176, 202–208.
- van Duijkeren, E.; Catry, B.; Greko, C.; Moreno, M.A.; Pomba, M.C.; Pyörälä, S.; Ruzauskas, M.; Sanders, P.; Threlfall, E.J.; Torren-Edo, J.; et al. Review on methicillin-resistant Staphylococcus pseudintermedius. J. Antimicrob. Chemother. 2011, 66, 2705–2714.
- Hartantyo, S.H.P.; Chau, M.L.; Fillon, L.; Ariff, A.Z.B.M.; Kang, J.S.L.; Aung, K.T.; Gutiérrez, R.A. Sick pets as potential reservoirs of antibiotic-resistant bacteria in Singapore. Antimicrob. Resist. Infect. Control 2018, 7, 106.
- Laarhoven, L.M.; de Heus, P.; van Luijn, J.; Duim, B.; Wagenaar, J.A.; van Duijkeren, E. Longitudinal study on methicillin-resistant Staphylococcus pseudintermedius in households. PLoS ONE 2011, 6, e27788.
- van Duijkeren, E.; Kamphuis, M.; van der Mije, I.C.; Laarhoven, L.M.; Duim, B.; Wagenaar, J.A.; Houwers, D.J. Transmission of methicillin-resistant Staphylococcus pseudintermedius between infected dogs and cats and contact pets, humans and the environment in households and veterinary clinics. Vet. Microbiol. 2011, 150, 338–343.
- Windahl, U.; Gren, J.; Holst, B.S.; Börjesson, S. Colonization with methicillin-resistant Staphylococcus pseudintermedius in multi-dog households: A longitudinal study using whole genome sequencing. Vet. Microbiol. 2016, 189, 8–14.
- Feßler, A.T.; Schuenemann, R.; Kadlec, K.; Hensel, V.; Brombach, J.; Murugaiyan, J.; Oechtering, G.; Burgener, I.A.; Schwarz, S. Methicillin-resistant Staphylococcus aureus (MRSA) and methicillin-resistant Staphylococcus pseudintermedius (MRSP) among employees and in the environment of a small animal hospital. Vet. Microbiol. 2018, 221, 153–158.
- Loeffler, A.; Lloyd, D.H. What has changed in canine pyoderma? A narrative review. Vet. J. 2018, 235, 73–82.
- Griffeth, G.C.; Morris, D.O.; Abraham, J.L.; Shofer, F.S.; Rankin, S.C. Screening for skin carriage of methicillin-resistant coagulase-positive Staphylococci and Staphylococcus schleiferi in dogs with healthy and inflamed skin. Vet. Dermatol. 2008, 19, 142–149.
- Bryan, J.; Frank, L.A.; Rohrbach, B.W.; Burgette, L.J.; Cain, C.L.; Bemis, D.A. Treatment outcome of dogs with meticillin-resistant and meticillin-susceptible Staphylococcus pseudintermedius pyoderma. Vet. Dermatol. 2012, 23, 361-e65.
- Huerta, B.; Maldonado, A.; Ginel, P.J.; Tarradas, C.; Gómez-Gascón, L.; Astorga, R.J.; Luque, I. Risk factors associated with the antimicrobial resistance of Staphylococci in canine pyoderma. Vet. Microbiol. 2011, 150, 302–308.
- Yoo, J.-H.; Yoon, J.W.; Lee, S.-Y.; Park, H.-M. High prevalence of Fluoroquinolone- and Methicillin-resistant Staphylococcus pseudintermedius isolates from canine pyoderma and otitis externa in veterinary teaching hospital. J. Microbiol. Biotechnol. 2010, 20, 798–802.
- Fazakerley, J.; Williams, N.; Carter, S.; McEwan, N.; Nuttall, T. Heterogeneity of Staphylococcus pseudintermedius isolates from atopic and healthy dogs. Vet. Dermatol. 2010, 21, 578–585.
- Shimizu, A.; Berkhoff, H.A.; Kloos, W.E.; George, C.G.; Ballard, D.N. Genomic DNA fingerprinting, using pulsed-field gel electrophoresis, of Staphylococcus intermedius isolated from dogs. Am. J. Vet. Res. 1996, 57, 1458–1462.
- Hesselbarth, J.; Witte, W.; Cuny, C.; Rohde, R.; Amtsberg, G. Characterization of Staphylococcus intermedius from healthy dogs and cases of superficial pyoderma by DNA restriction endonuclease patterns. Vet. Microbiol. 1994, 41, 259–266.
- Wang, Y.; Yang, J.; Logue, C.M.; Liu, K.; Cao, X.; Zhang, W.; Shen, J.; Wu, C. Methicillin-resistant Staphylococcus pseudintermedius isolated from canine pyoderma in North China. J. Appl. Microbiol. 2012, 112, 623–630.
- Pires Dos Santos, T.; Damborg, P.; Moodley, A.; Guardabassi, L. Systematic Review on Global Epidemiology of Methicillin-Resistant Staphylococcus pseudintermedius: Inference of Population Structure from Multilocus Sequence Typing Data. Front. Microbiol. 2016, 7, 1599.
- Hensel, N.; Zabel, S.; Hensel, P. Prior antibacterial drug exposure in dogs with meticillin-resistant Staphylococcus pseudintermedius (MRSP) pyoderma. Vet. Dermatol. 2016, 27, 72-e20.
- Dziva, F.; Wint, C.; Auguste, T.; Heeraman, C.; Dacon, C.; Yu, P.; Koma, L.M. First identification of methicillin-resistant Staphylococcus pseudintermedius strains among coagulase-positive staphylococci isolated from dogs with otitis externa in Trinidad, West Indies. Infect. Ecol. Epidemiol. 2015, 5, 29170.
- Lyskova, P.; Vydrzalova, M.; Mazurova, J. Identification and antimicrobial susceptibility of bacteria and yeasts isolated from healthy dogs and dogs with otitis externa. J. Vet. Med. A Physiol. Pathol. Clin. Med. 2007, 54, 559–563.
- Bugden, D.L. Identification and antibiotic susceptibility of bacterial isolates from dogs with otitis externa in Australia. Aust. Vet. J. 2013, 91, 43–46.
- Paterson, S. Discovering the causes of otitis externa. Practice 2016, 38, 7–11.
- Zur, G.; Lifshitz, B.; Bdolah-Abram, T. The association between the signalment, common causes of canine otitis externa and pathogens. J. Small Anim. Pract. 2011, 52, 254–258.
- Saridomichelakis, M.N.; Farmaki, R.; Leontides, L.S.; Koutinas, A.F. Aetiology of canine otitis externa: A retrospective study of 100 cases. Vet. Dermatol. 2007, 18, 341–347.
- Ngo, J.; Taminiau, B.; Fall, P.A.; Daube, G.; Fontaine, J. Ear canal microbiota—A comparison between healthy dogs and atopic dogs without clinical signs of otitis externa. Vet. Dermatol. 2018, 29, 425-e140.
- Zur, G.; Gurevich, B.; Elad, D. Prior antimicrobial use as a risk factor for resistance in selected Staphylococcus pseudintermedius isolates from the skin and ears of dogs. Vet. Dermatol. 2016, 27, 468-e125.
- Perry, L.R.; MacLennan, B.; Korven, R.; Rawlings, T.A. Epidemiological study of dogs with otitis externa in Cape Breton, Nova Scotia. Can. Vet. J. 2017, 58, 168–174.
- Chan, W.Y.; Hickey, E.E.; Khazandi, M.; Page, S.W.; Trott, D.J.; Hill, P.B. In vitro antimicrobial activity of narasin against common clinical isolates associated with canine otitis externa. Vet. Dermatol. 2018, 29, 149-e57.
- Sim, J.X.F.; Khazandi, M.; Chan, W.Y.; Trott, D.J.; Deo, P. Antimicrobial activity of thyme oil, oregano oil, thymol and carvacrol against sensitive and resistant microbial isolates from dogs with otitis externa. Vet. Dermatol. 2019, 30, 524-e159.
- Petrov, V.; Zhelev, G.; Marutsov, P.; Koev, K.; Georgieva, S.; Toneva, I.; Urumova, V. Microbiological and antibacterial resistance profile in canine otitis externa—A comparative analysis. Bulg. J. Vet. Med. 2019, 22, 447–456.
- Roberts, M.; White, J.; Lam, A. Prevalence of bacteria and changes in trends in antimicrobial resistance of Escherichia coli isolated from positive canine urinary samples from an Australian referral hospital over a 5-year period (2013–2017). Vet. Rec. Open 2019, 6, e000345.
- Windahl, U.; Holst, B.S.; Nyman, A.; Grönlund, U.; Bengtsson, B. Characterisation of bacterial growth and antimicrobial susceptibility patterns in canine urinary tract infections. BMC Vet. Res. 2014, 10, 217.
- Penna, B.; Varges, R.; Martins, R.; Martins, G.; Lilenbaum, W. In vitro antimicrobial resistance of Staphylococci isolated from canine urinary tract infection. Can. Vet. J. 2010, 51, 738–742.
- Ling, G.V.; Norris, C.R.; Franti, C.E.; Eisele, P.H.; Johnson, D.L.; Ruby, A.L.; Jang, S.S. Interrelations of organism prevalence, specimen collection method, and host age, sex, and breed among 8,354 canine urinary tract infections (1969–1995). J. Vet. Intern. Med. 2001, 15, 341–347.
- Gatoria, I.S.; Saini, N.S.; Rai, T.S.; Dwivedi, P.N. Comparison of three techniques for the diagnosis of urinary tract infections in dogs with urolithiasis. J. Small Anim. Pract. 2006, 47, 727–732.
- Ball, K.R.; Rubin, J.E.; Chirino-Trejo, M.; Dowling, P.M. Antimicrobial resistance and prevalence of canine uropathogens at the Western College of Veterinary Medicine Veterinary Teaching Hospital, 2002–2007. Can. Vet. J. 2008, 49, 985–990.
- Hoekstra, K.A.; Paulton, R.J.L. Clinical prevalence and antimicrobial susceptibility of Staphylococcus aureus and Staph. intermedius in dogs. J. Appl. Microbiol. 2002, 93, 406–413.
- Waki, M.F.; Kogika, M.M.; Wirthl, V.A.B.F.; Oyafuso, M.K.; Prosser, C.S.; Monteiro, P.R.; Coelho, B.P.; Simões, D.M.N.; Kanayama, K.K. Association of urinary tract infection with urolithiasis in dogs. Clín. Vet. 2009, 14, 130–131.
- Marques, C.; Belas, A.; Franco, A.; Aboim, C.; Gama, L.T.; Pomba, C. Increase in antimicrobial resistance and emergence of major international high-risk clonal lineages in dogs and cats with urinary tract infection: 16 year retrospective study. J. Antimicrob. Chemother. 2018, 73, 377–384.
- Marques, C.; Gama, L.T.; Belas, A.; Bergström, K.; Beurlet, S.; Briend-Marchal, A.; Broens, E.M.; Costa, M.; Criel, D.; Damborg, P.; et al. European multicenter study on antimicrobial resistance in bacteria isolated from companion animal urinary tract infections. BMC Vet. Res. 2016, 12, 213.
- Rubin, J.E.; Gaunt, M.C. Urinary tract infection caused by methicillin-resistant Staphylococcus pseudintermedius in a dog. Can. Vet. J. 2011, 52, 162.
- Hutchins, R.G.; Vaden, S.L.; Jacob, M.E.; Harris, T.L.; Bowles, K.D.; Wood, M.W.; Bailey, C.S. Vaginal microbiota of spayed dogs with or without recurrent urinary tract infections. J. Vet. Intern. Med. 2014, 28, 300–304.
- Moyaert, H.; de Jong, A.; Simjee, S.; Rose, M.; Youala, M.; El Garch, F.; Vila, T.; Klein, U.; Rzewuska, M.; Morrissey, I. Survey of antimicrobial susceptibility of bacterial pathogens isolated from dogs and cats with respiratory tract infections in Europe: ComPath results. J. Appl. Microbiol. 2019, 127, 29–46.
- Kalhoro, D.H.; Gao, S.; Kalhoro, M.S. Staphylococcus pseudintermedius isolation from canine, bacterial colonization and clinical picture in balb/c mouse model. JAPS J. Anim. Plant Sci. 2017, 27, 422–429.
- Proulx, A.; Hume, D.Z.; Drobatz, K.J.; Reineke, E.L. In vitro bacterial isolate susceptibility to empirically selected antimicrobials in 111 dogs with bacterial pneumonia. J. Vet. Emerg. Crit. Care 2014, 24, 194–200.
- Vientós-Plotts, A.I.; Ericsson, A.C.; Rindt, H.; Reinero, C.R. Respiratory Dysbiosis in Canine Bacterial Pneumonia: Standard Culture vs. Microbiome Sequencing. Front. Vet. Sci. 2019, 6, 354.
- Morrissey, I.; Moyaert, H.; de Jong, A.; El Garch, F.; Klein, U.; Ludwig, C.; Thiry, J.; Youala, M. Antimicrobial susceptibility monitoring of bacterial pathogens isolated from respiratory tract infections in dogs and cats across Europe: ComPath results. Vet. Microbiol. 2016, 191, 44–51.
- Kalhoro, D.H.; Gao, S.; Xie, X.; Liang, S.; Luo, S.; Zhao, Y.; Liu, Y. Canine influenza virus coinfection with Staphylococcus pseudintermedius enhances bacterial colonization, virus load and clinical presentation in mice. BMC Vet. Res. 2016, 12, 87.
- Qekwana, D.N.; Naidoo, V.; Oguttu, J.W.; Odoi, A. Occurrence and Predictors of Bacterial Respiratory Tract Infections and Antimicrobial Resistance Among Isolates From Dogs Presented With Lower Respiratory Tract Infections at a Referral Veterinary Hospital in South Africa. Front. Vet. Sci. 2020, 7, 304.
- Rheinwald, M.; Hartmann, K.; Hähner, M.; Wolf, G.; Straubinger, R.K.; Schulz, B. Antibiotic susceptibility of bacterial isolates from 502 dogs with respiratory signs. Vet. Rec. 2015, 176, 357.
- Dear, J.D. Bacterial pneumonia in dogs and cats. Vet. Clin. N. Am. Small Anim. Pract. 2014, 44, 143–159.
- Lappin, M.R.; Blondeau, J.; Boothe, D.; Breitschwerdt, E.B.; Guardabassi, L.; Lloyd, D.H.; Papich, M.G.; Rankin, S.C.; Sykes, J.E.; Turnidge, J.; et al. Antimicrobial use Guidelines for Treatment of Respiratory Tract Disease in Dogs and Cats: Antimicrobial Guidelines Working Group of the International Society for Companion Animal Infectious Diseases. J. Vet. Intern. Med. 2017, 31, 279–294.
- Rota, A.; Milani, C.; Drigo, I.; Drigo, M.; Corrò, M. Isolation of methicillin-resistant Staphylococcus pseudintermedius from breeding dogs. Theriogenology 2011, 75, 115–121.
- Zakošek Pipan, M.; Kajdič, L.; Kalin, A.; Plavec, T.; Zdovc, I. Do newborn puppies have their own microbiota at birth? Influence of type of birth on newborn puppy microbiota. Theriogenology 2020, 152, 18–28.
- Saijonmaa-Koulumies, L.E.; Lloyd, D.H. Colonization of neonatal puppies by Staphylococcus intermedius. Vet. Dermatol. 2002, 13, 123–130.
- Rota, A.; Corrò, M.; Drigo, I.; Bortolami, A.; Börjesson, S. Isolation of coagulase-positive Staphylococci from bitches’ colostrum and milk and genetic typing of methicillin-resistant Staphylococcus pseudintermedius strains. BMC Vet. Res. 2015, 11, 160.
- Maluping, R.P.; Paul, N.C.; Moodley, A. Antimicrobial susceptibility of methicillin-resistant Staphylococcus pseudintermedius isolated from veterinary clinical cases in the UK. Br. J. Biomed. Sci. 2014, 71, 55–57.
- Vasiu, I.; Dąbrowski, R.; Martinez-Subiela, S.; Ceron, J.J.; Wdowiak, A.; Pop, R.A.; Brudaşcă, F.G.; Pastor, J.; Tvarijonaviciute, A. Milk C-reactive protein in canine mastitis. Vet. Immunol. Immunopathol. 2017, 186, 41–44.
- Ververidis, H.N.; Mavrogianni, V.S.; Fragkou, I.A.; Orfanou, D.C.; Gougoulis, D.A.; Tzivara, A.; Gouletsou, P.G.; Athanasiou, L.; Boscos, C.M.; Fthenakis, G.C. Experimental Staphylococcal mastitis in bitches: Clinical, bacteriological, cytological, haematological and pathological features. Vet. Microbiol. 2007, 124, 95–106.
- Ros, L.; Holst, B.S.; Hagman, R. A retrospective study of bitches with pyometra, medically treated with aglepristone. Theriogenology 2014, 82, 1281–1286.
- Hagman, R. Pyometra in Small Animals. Vet. Clin. N. Am. Small Anim. Pract. 2018, 48, 639–661.
- Robaj, A.; Sylejmani, I.; Hamidi, A. Occurrence and antimicrobial susceptibility of bacterial agents of canine pyometra. Indian J. Anim. Res. 2018, 52, 394–400.
- Pilla, R.; Bonura, C.; Malvisi, M.; Snel, G.G.M.; Piccinini, R. Methicillin-resistant Staphylococcus pseudintermedius as causative agent of dairy cow mastitis. Vet. Rec. 2013, 173, 19.
- Paul, N.C.; Damborg, P.; Guardabassi, L. Dam-to-offspring transmission and persistence of Staphylococcus pseudintermedius clones within dog families. Vet. Dermatol. 2014, 25, 3-e2.
- Saijonmaa-Koulumies, L.E.M.; Myllys, V.; Lloyd, D.H. Diversity and stability of the Staphylococcus intermedius flora in three bitches and their puppies. Epidemiol. Infect. 2003, 131, 931–937.
- Latronico, F.; Moodley, A.; D’Abramo, M.; Greco, M.F.; Corrente, M.; Guardabassi, L. Outbreak of methicillin-resistant Staphylococcus pseudintermedius in a litter of puppies: Evidence of vertical perinatal and horizontal transmission. In Proceedings of the ASM Conference on Methicillin-Resistant Staphylococci in Animals, London, UK, 22–25 September 2009; Volume 76.
- Milani, C.; Corrò, M.; Drigo, M.; Rota, A. Antimicrobial resistance in bacteria from breeding dogs housed in kennels with differing neonatal mortality and use of antibiotics. Theriogenology 2012, 78, 1321–1328.
- Meloni, T.; Martino, P.A.; Grieco, V.; Pisu, M.C.; Banco, B.; Rota, A.; Veronesi, M.C. A survey on bacterial involvement in neonatal mortality in dogs. Vet. Ital. 2014, 50, 293–299.
- Pipan, M.Z.; Švara, T.; Zdovc, I.; Papić, B.; Avberšek, J.; Kušar, D.; Mrkun, J. Staphylococcus pseudintermedius septicemia in puppies after elective cesarean section: Confirmed transmission via dam’s milk. BMC Vet. Res. 2019, 15, 41.
- Ogbu, K.I.; Ochai, S.O.; Danladi, M.M.A.; Abdullateef, M.H.; Agwu, E.O.; Gyengdeng, J.G. A review of Neonatal mortality in Dogs. Int. J. Life Sci. 2016, 4, 451–460.
- Hillier, A.; Lloyd, D.H.; Scott Weese, J.; Blondeau, J.M.; Boothe, D.; Breitschwerdt, E.; Guardabassi, L.; Papich, M.G.; Rankin, S.; Turnidge, J.D.; et al. Guidelines for the diagnosis and antimicrobial therapy of canine superficial bacterial folliculitis (Antimicrobial Guidelines Working Group of the International Society for Companion Animal Infectious Diseases). Vet. Dermatol. 2014, 25, 163-e43.
- Summers, J.F.; Hendricks, A.; Brodbelt, D.C. Prescribing practices of primary-care veterinary practitioners in dogs diagnosed with bacterial pyoderma. BMC Vet. Res. 2014, 10, 240.




