
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Marcela Káňová | + 1991 word(s) | 1991 | 2021-05-12 09:59:39 | | | |
| 2 | Vicky Zhou | Meta information modification | 1991 | 2021-05-14 05:25:32 | | |
Video Upload Options
Serotonin (5-hydroxytryptamine, 5-HT) plays two important roles in humans—one central and the other peripheral—depending on the location of the 5-HT pools of on either side of the blood-brain barrier. In the central nervous system it acts as a neurotransmitter, controlling such brain functions as autonomic neural activity, stress response, body temperature, sleep, mood and appetite. This role is very important in intensive care, as in critically ill patients multiple serotoninergic agents like opioids, antiemetics and antidepressants are frequently used. High serotonin levels lead to altered mental status, deliria, rigidity and myoclonus, together recognized as serotonin syndrome. In its role as a peripheral hormone, serotonin is unique in controlling the functions of several organs. In the gastrointestinal tract it is important for regulating motor and secretory functions. Apart from intestinal motility, energy metabolism is regulated by both central and peripheral serotonin signaling. It also has fundamental effects on hemostasis, vascular tone, heart rate, respiratory drive, cell growth and immunity. Serotonin regulates almost all immune cells in response to inflammation, following the activation of platelets.
1. Introduction
Serotonin carries out a number of immune functions as a neurotransmitter and as a peripheral hormone. It is critical for the inflammatory response, possibly influencing the development of the systemic inflammatory response syndrome (SIRS). Further, it affects cardiovascular and respiratory functions, controls platelet function and hemostasis. As an important gastrointestinal signaling molecule, serotonin has a number of diverse functions in metabolism, including influencing motor and sensory functions through its effects on the microbiome, as well as in controlling energy balance.
2. The Role of Serotonin
2.1. Serotonin Synthesis
Serotonin (5-hydroxytryptamine, 5-HT) is located mainly in the serotoninergic neural network of the central nervous system, in the gastrointestinal (GI) tract and in platelets, where 5-HT is stored. Serotonin acts as both a neurotransmitter and as a peripheral hormone. However, most 5-HT production occurs in the enterochromaffin (EC) cells of the intestinal mucosa. The gut is the largest endocrine organ in human body, and it produces almost 95% of all the serotonin. This is triggered in response to acetylcholine, neuronal stimulation, increase in intraluminal gut pressure or low gut luminal pH. Serotonin is released into the lamina propria where dendritic cells, T lymphocytes and other immune cells are located, as well as into the intestinal lumen (Figure 1).
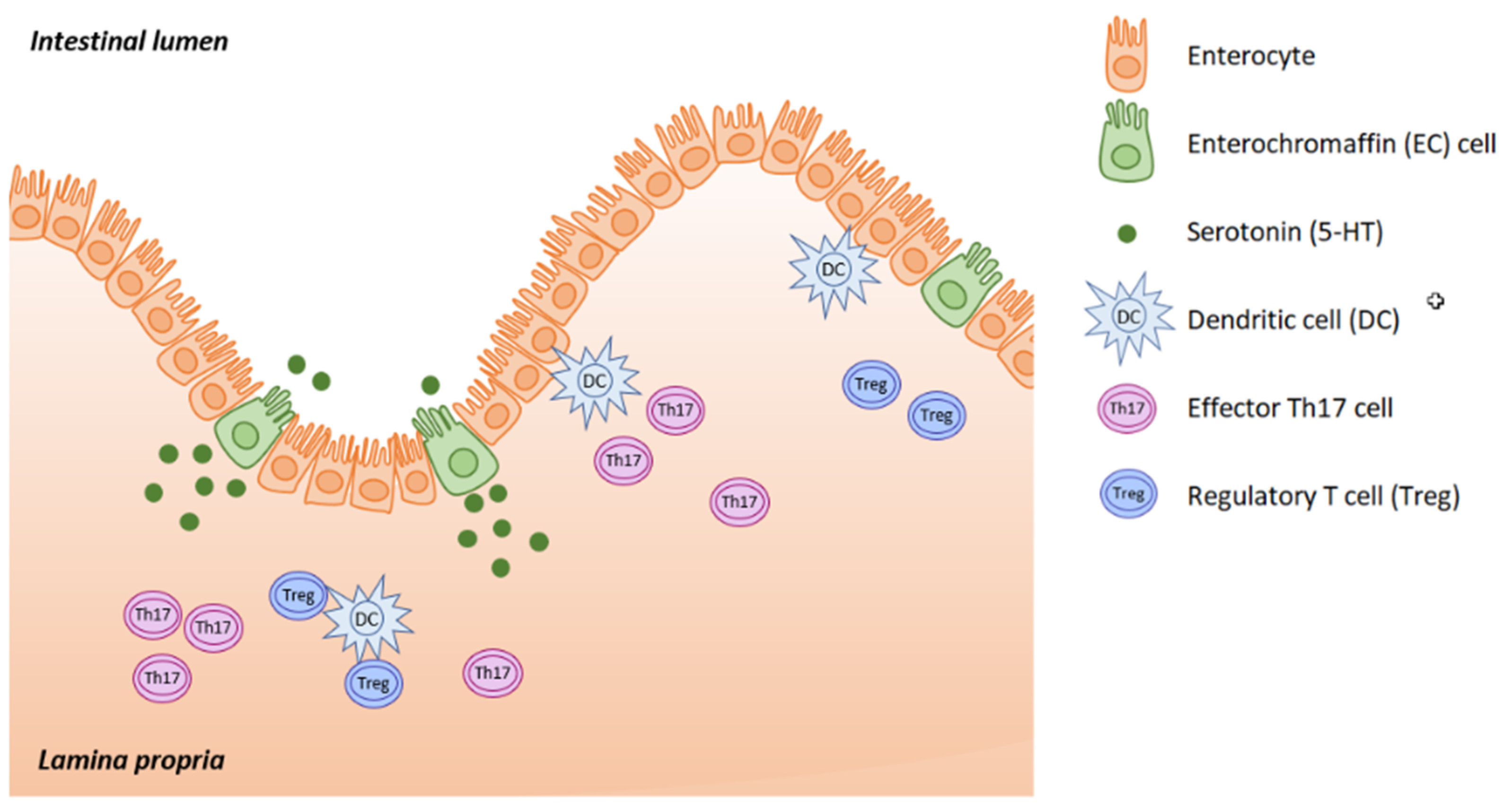
Figure 1. Serotonin is synthesized in EC cells and the majority is released into the lamina propria, with smaller amounts being released into the gut lumen. (Adapted from [1]).
A wide range of functions is made possible by the diversity of serotonin receptors. Fifteen different serotonin receptors belonging to seven receptor families (5-HTR1-7) are encoded by 18 genes (Table 1), and different cells express different serotonin receptors (Table 2). Almost all 5-HTR, except the 5HT3 group, are G- protein coupled receptors. The signaling pathway involves GTPase triggering a second messenger cascade, leading to protein modification (a process called serotonylation). Activation of 5-HTR1,5 reduces c-ATP, while 5-HTR4,6,7 activation increases c-ATP activity. The only exception is 5-HTR3, which is a nonselective cation channel [2].
Table 1. Serotoninergic receptors and their distribution.
| 5-HTR Family | Distribution |
|---|---|
| 5-HTR1 | CNS, cardiovascular system |
| 5-HTR2 | CNS, PNS, GI tract, platelets, cardiovascular system |
| 5-HTR3 | CNS, PNS, GI tract |
| 5-HTR4 | CNS, PNS, GI tract, cardiovascular system |
| 5-HTR5 | CNS |
| 5-HTR6 | CNS |
| 5-HTR7 | CNS, PNS, GI tract, cardiovascular system |
Table 2. Serotoninergic receptors in immune cells.
| Monocytes/macrophages | 5-HTR 1A, 1E, 2A, 3A, 4, 7 |
| Microglia | 5-HTR 2B, 5A, 7 |
| Dendritic cells | 5-HTR 1B, 1E, 2A, 2B, 4, 7 |
| Neutrophils | 5-HTR 1A, 1B, 2 |
| Basophil, Mast cells | 5-HTR 1A |
| Eosinophils | 5-HTR 1A, 1B, 1E, 2A, 2B, 6 |
| B cells | 5-HTR 1A, 2A, 3, 7 |
| T cells | 5-HTR 1A, 1B, 2A, 2C, 3A, 7 |
| Platelets | 5-HTR 2A, 3 |
| NK cells | |
| Endothelial cells | 5-HTR 1B, 2A, 2B, 4 |
| Vascular smooth muscle cells | 5-HTR 1D, 2A, 2B, 7 |
2.2. Immune Response
Peripheral serotonin is important for a proper immune response, especially in the fight against infection and sepsis in critically ill patients. 5-HT also impacts various inflammatory diseases, e.g., inflammatory conditions of the gut (inflammatory bowel disease), rheumatoid arthritis or allergic airway dispositions. Serotonin is a potent modulator of both the innate and the adaptive immune system through its binding to immune-cell 5-HT receptors [1].
On the other hand, serotonin plays an important role in adaptive immunity. This defense is already selective, the specificity being determined due to antigen-specific recognition by T and B lymphocytes. This response is slower, is responsible for immunological memory, and is highly specific (due to antibody specificity). Serotonin stimulates antigen--presenting cells (dendritic cells, macrophages), that activate adaptive immune reactions in which 5-HTR7 plays a major role. Serotonin can directly affect both T and B lymphocytes through their 5-HTR [1][2][3][4][5].
2.3. The Gut-Brain-Microbiome Axis
The connection between the gut and the brain allows their functions to be influenced by serotonin on both sides of the gut-brain axis. The importance of gut microbiota is increasingly being recognized, and 5-HT makes gut-brain-microbiome crosstalk possible through the microbial influence on tryptophan metabolism and serotonergic synthesis. The developing serotonergic system in early childhood may be vulnerable to microbial colonization prior to the formation of stable adult-like gut microbiota. In the elderly, the decreased number and diversity of gut bacteria can cause serotonin-related health problems. This may be explained by the ability of gut microbiota to control host tryptophan metabolism via the kynurenine pathway and to influence immune and stress responses of the host organism. Only a small portion of the 5-HT pool seems to be directly synthetized by gut bacteria such as E. coli, Corynebacterium spp. and Streptococcus spp. [6][7][8]. Although the gut microbiota is generally stable, it can be altered during enteric infections, stress response and antibiotic treatment. Neurodestructive processes that can lead to dementia and Alzheimer’s disease (AD) begin with gut dysbiosis, local and systemic inflammation and dysregulation of the gut-brain axis. Increased gut permeability results in invasion of different bacteria, viruses and their neuroactive products that support neuroinflammatory reactions in the brain [9].
The strong connection between activation of peripheral immune cells and CNS-located immune cells is responsible for the associations between inflammation, immune activation and neuropsychiatric disorders. That is why treating depressive disorders with selective serotonin reuptake inhibitors (SSRIs) can affect peripheral immune reactions [1]. SSRIs have an immunosuppressive effect by reducing peripheral immune cell proliferation, cytokine production and apoptosis modulation [10][11]. SSRIs can be used to treat not only mood disruption, but also autoimmune disorders such as inflammatory bowel disease (IBD). These drugs affect the role of peripheral serotonin in T-cell mediated gut inflammation. IBD is an autoimmune disease with excessive Th1 and Th17 responses. There is mounting evidence of a link between 5-HT and T-lymphocytes, suggesting that serotonin modulation may be useful for therapy [12][13].
2.4. Metabolism
The blood-brain barrier separates the central and the peripheral serotonin pools. Almost 95% of serotonin is synthetized by the EC with a minority coming from the serotoninergic neuron network. EC cells contain tryptophan hydroxylase-1 (TPH-1), whereas serotoninergic neurons contain TPH-2. The presence of food (resulting in mechanical pressure and acid) in the intestinal lumen stimulates the cholinergic VN in the enteric submucosa that subsequently activates serotonin release from EC cells. GI epithelium cells and EC cells are constantly being renewed, and so EC cells produce 5-HT in excess and release it into the connective tissue. There it acts locally in a paracrine fashion and can stimulate both extrinsic and intrinsic afferent neurons (submucosal and myenteric plexus; Figure 2). Serotonin action is terminated by reuptake from the place of action into enterocytes, where 5-HT is catabolized by intracellular monoamine oxidase (MAO).
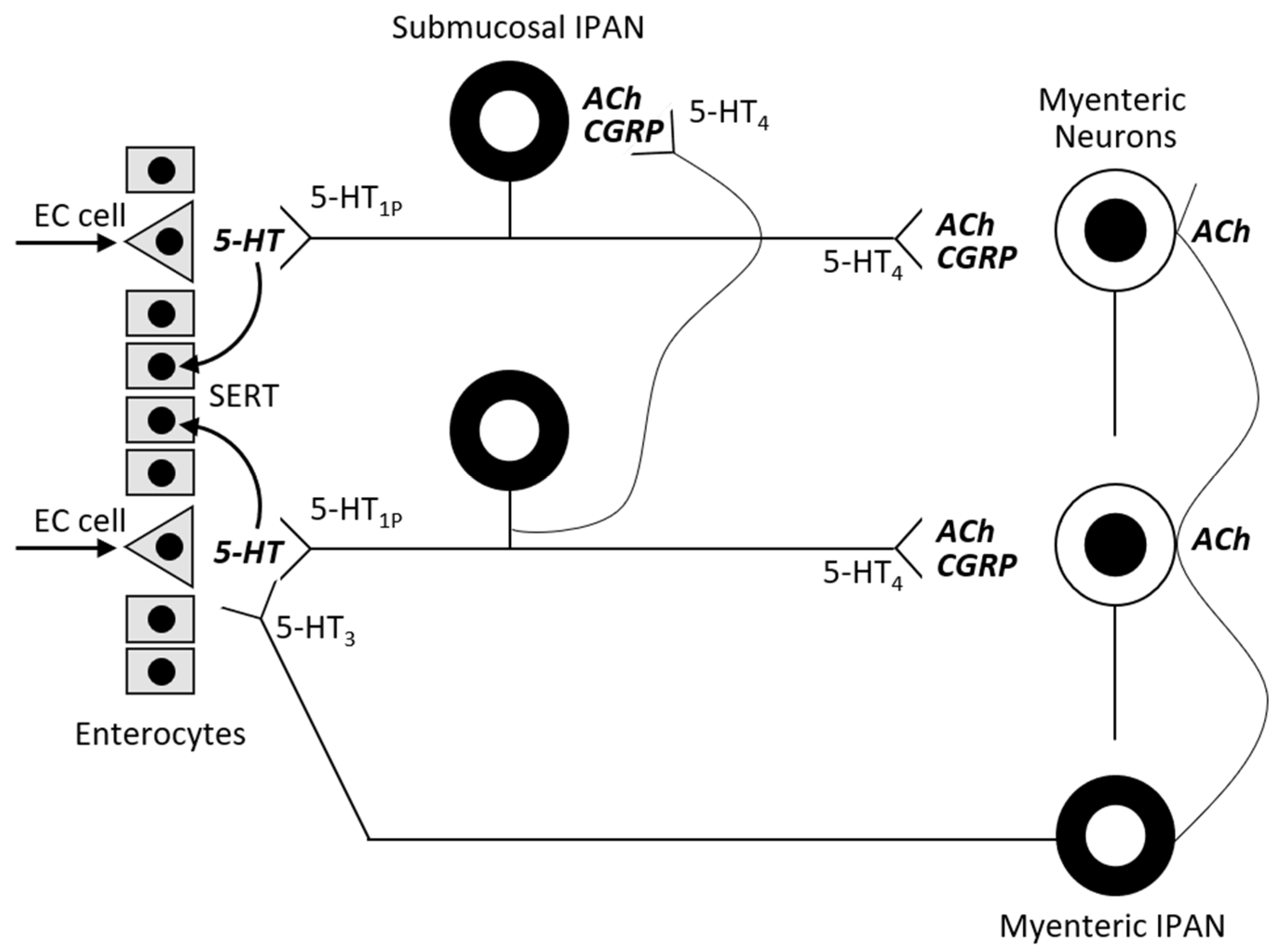
Figure 2. Actions of serotonin in the bowel wall (adapted from [14]).
2.5. Bolus vs. Continual Enteral Feeding
Metabolic changes in critically ill patients leads to the development of hypercatabolism, increased energy requirement, insulin resistance and a massive stimulation of proteolysis. Thus, the critically ill lose muscle mass as a consequence of these metabolic changes that ensure survival. Polyneuromyopathy in the critically ill is thus a major problem that exacerbates mortality and morbidity. These patients are mainly fed continuously as they tolerate it better, and it is easier to correct glycaemia by continuously giving insulin. But with regard to stimulating proteosynthesis (rapamycin m-TOR system) and suppressing proteolysis (proteasome-ubiquitin system), this method appears to be nonphysiological. Feeding boluses stimulate natural enterohormonal pathways with the release of bioactive intestinal polypeptides (cholecystokinin, peptide YY, glucagon-like peptide). The release of insulin and activation of the rapamycin system leads to the activation of proteosynthesis. On the other hand, the presence of energy-dense food in the small intestine activates the ileal brake. Critically ill patients experience frequent dietary intolerance due to this upregulation of enterogastric feedback. As pointed out before, once food enters the GI tract, intestinal serotonin regulates pancreatic secretion and peristaltic waves. Drugs targeting 5-HTR3 and 5-HTR4 have been used to treat irritable bowel syndrome. However, using their prokinetic and insulin stimulating potential to treat GI disorders in the critically ill (e.g., paralytic ileus after abdominal trauma or surgery), remains questionable [15][16][17].
Peripheral serotonin determines bowel movement—it is increased in diarrhea and decreased in constipation. Postprandial symptoms appear earlier and do not coincide with the peak of serotonin at about 2 to 3 h after meal. It is possible that the major actors are upper intestinal mediators such as gastrin, cholecystokinin, motilin, pancreatic polypeptide and secretin, or members of the vasoactive intestinal polypeptide family. There are also differences in platelet SERT function—reduced SERT causes higher circulating serotonin and diarrhea. The opposite is true for increased SERT in platelet membranes, resulting in higher reuptake and reduced serotonin levels in the bloodstream [18]. Altered motility causing hypoxia in patients with IBD was shown to induce serotonin synthesis by stimulating adenosine receptors, leading to diarrhea [19].
2.6. Serotonin and Anesthesia
The serotonin system seems to play an important role in modulating the sleep/wake cycle and can affect anesthesia in the same way, mainly inhalation anesthesia. Released 5-HT spreads through the ascending reticular formation, responsible for regulating sleep-wake transitions. Serotonin release is naturally reduced during slow wave sleep, and inhalation anesthetic agents suppress serotonin release. Thus, patients taking serotoninergic antidepressants may need higher doses of such anesthetics [20]. Serotonin also modulates pain control. It is released from peripheral nerve fibers in locally inflamed tissue, and nociceptive information is carried by nerve fibers to the CNS. Serotoninergic brainstem neurons first affect descending projections into the spinal cord and thereby modulate nociception. Moreover, raphe serotoninergic neurons have ascending projections into cortical and limbic areas, and thus affect the psychological perception of pain [21][22].
2.7. Serotonin Syndrome
Serotonin syndrome (SS) is a potentially life-threatening condition, which is due to excessive serotonin action on the central and peripheral nervous system. SS is an adverse drug reaction due to serotoninergic medication overdose, mostly via inadvertent interaction of several serotoninergic drugs. Especially from the point of view of intensive care specialists, this condition is not as rare as previously thought. SS occurs in the ICU most often because critically ill patients are given multiple serotoninergic agents. Many receive opioids, prokinetics and antidepressant medications. They are also routinely prescribed antiemetics, antibiotics and other drugs, where many pose a risk of serotonin release [23].
The main challenge in clinical practice is to recognize or to diagnose SS early on. Many critically ill patients already present an altered mental status—up to 80% of ICU patients are delirious (confusion assessment method, CAM-ICU positive) and show sympathetic hyperactivity (hyperthermia, hypertension, tachycardia, diaphoresis). Moreover, patients often have nausea or diarrhea or suffer from neuromuscular excitation. But these symptoms may be due to a range of diverse causes including hyperthermia, leukocytosis, or delirium in septic patients, tachycardia or hypertension caused by pain, or withdrawal syndrome. Nausea, vomiting, or diarrhea are also caused by enteral nutrition, paralytic ileus and so on.
3. Conclusions
Serotonin regulates a wide range of physiological and pathophysiological processes in most human organs and plays an important role in immunity and inflammation. New serotoninergic drugs open up the possibility of effectively managing a number of diseases. Intensive care specialists must take SS into account, as early diagnosis has the potential to significantly improve a patient’s prognosis.
References
- Wu, H.; Denna, T.H.; Storkersen, J.N.; Gerriets, V.A. Beyond a neurotransmitter: The role of serotonin in inflammation and immunity. Pharmacol. Res. 2019, 140, 100–114.
- Shajib, M.S.; Khan, I. The role of serotonin and its receptors in activation of immune responses and inflammation. Acta Physiol. 2014, 213, 561–574.
- Herr, N.; Bode, C.; Duerschmied, D. The effects of serotonin in immune cells. Front. Cardiovasc. Med. 2017, 4, 48.
- Ahern, G.P. 5-HT and the immune system. Curr. Opin. Pharmacol. 2011, 11, 29–33.
- Baganz, N.L.; Blakely, R.D. A dialogue between the immune system and brain, spoken in the language of serotonin. ACS Chem. Neurosci. 2012, 4, 48–63.
- Williams, B.B.; Van Benschoten, A.H.; Cimermancic, P.; Donia, M.S.; Zimmermann, M.; Taketani, M.; Ishihara, A.; Kashyap, P.C.; Fraser, J.S.; Fischbach, M.A. Discovery and characterization of gut microbiota decarboxylases that can produce the neurotransmitter tryptamine. Cell Host Microbe 2014, 16, 495–503.
- Yano, J.M.; Yu, K.; Donaldson, G.P.; Shastri, G.G.; Ann, P.; Ma, L.; Nagler, C.R.; Ismagilov, R.F.; Mazmanian, S.K.; Hisao, Y.E. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 2015, 161, 264–276.
- O’Mahony, S.; Clarke, G.; Borre, Y.; Dinan, T.; Cryan, J. Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behav. Brain Res. 2015, 277, 32–48.
- Sochocka, M.; Donskow-Łysoniewska, K.; Diniz, B.S.; Kurpas, D.; Brzozowska, E.; Leszek, J. The Gut Microbiome Alterations and Inflammation-Driven Pathogenesis of Alzheimer’s Disease—A Critical Review. Mol. Neurobiol. 2019, 56, 1841–1851.
- Gobin, V.; Van Steendam, K.; Denys, D.; Deforce, D. Selective serotonin reuptake inhibitors as a novel class of immunosuppressants. Int. Immunopharmacol. 2014, 20, 148–156.
- Walker, F.R. A critical review of the mechanism of action for the selective serotonin reuptake inhibitors: Do these drugs possess anti-inflammatory properties and how relevant is this in the treatment of depression? Neuropharmacology 2013, 67, 304–317.
- Weaver, C.T.; Elson, C.O.; Fouser, L.A.; Kolls, J.K. The Th17 pathway and inflammatory diseases of the intestines, lungs, and skin. Ann. Rev. Pathol. 2013, 8, 477–512.
- Ghia, J.E.; Li, N.; Wang, H.; Collins, M.; Deng, Y.; El–Sharkawy, R.T.; Côté, F.; Mallet, J.; Khan, W.I. Serotonin has a key role in pathogenesis of experimental colitis. Gastroenterology 2009, 137, 1649–1660.
- Gershon, M.D. Serotonin receptors and transporters- roles in normal and abnormal gastrointestinal motility. Aliment. Pharmacol. Ther. 2004, 20, 3–14.
- Gershon, M.D.; Tack, J. The serotonin signalling system: From basic understanding to drug development for functional GI disorders. Gastroenterology 2007, 132, 397–414.
- Patel, J.; Rosenthal, M.D.; Heyland, D.K. Intermittent versus continuous feeding in critically ill adults. Curr. Opin. Clin. Nutr. Metab. Care 2018, 21, 116–120.
- Marik, P.E. Feeding critically ill patients the right “whey“: Thinking outside of the box, personal view. Ann. Intensive Care 2015, 5, 11.
- Camilleri, M. Serotonin in the gastrointestinal tract. Curr. Opin. Endocrinol. Diabetes Obes. 2009, 16, 53–59.
- Damen, R.; Haugen, M.; Svejda, B.; Alaimo, D.; Brenna, O.; Pfragner, R.; Gustafsson, B.I.; Kidd, M. The stimulatory adenosine receptors ADOR2AB regulates serotonin (5-HT) synthesis and release in oxygen-depleted EC cells in inflammatory bowel disease. PLoS ONE 2013, 8, e62607.
- Mukaida, K.; Shichino, T.; Koyanagi, S.; Himukashi, S.; Fukuda, K. Activity of the serotoninergic system during isoflurane anesthesia. Anesth. Analg. 2007, 104, 836–839.
- Jann, M.W.; Slade, J.H. Antidepressant agents for the treatment of chronic pain and depression. Pharmacotherapy 2007, 27, 1571–1587.
- Brazz, J.M.; Basbaum, A.I. Genetically expressed transneuronal tracer reveals direct and indirect serotoninergic descending control circuits. J. Comp. Neurol. 2008, 507, 1990–2003.
- Boyer, E.W.; Shannon, M. The serotonin syndrome. N. Engl. J. Med. 2005, 352, 112–1112.





