
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Antonio Rodriguez-Sinovas | + 1529 word(s) | 1529 | 2021-05-06 09:43:30 | | | |
| 2 | Vicky Zhou | -2 word(s) | 1527 | 2021-05-13 09:40:20 | | |
Video Upload Options
Connexins are a family of transmembrane proteins that play a key role in cardiac physiology. Gap junctional channels put into contact the cytoplasms of connected cardiomyocytes, allowing the existence of electrical coupling. However, in addition to this fundamental role, connexins are also involved in cardiomyocyte death and survival. Thus, chemical coupling through gap junctions plays a key role in the spreading of injury between connected cells. Moreover, in addition to their involvement in cell-to-cell communication, mounting evidence indicates that connexins have additional gap junction-independent functions. Opening of unopposed hemichannels, located at the lateral surface of cardiomyocytes, may compromise cell homeostasis and may be involved in ischemia/reperfusion injury. In addition, connexins located at non-canonical cell structures, including mitochondria and the nucleus, have been demonstrated to be involved in cardioprotection and in regulation of cell growth and differentiation.
1. Introduction
Connexins are a large family of highly homologous transmembrane proteins, comprising 20 and 21 different isoforms in mice and humans, respectively [1][2][3]. All these isoforms are named according to their expected molecular weight in kDa and have distinct biophysical properties. However, and despite their electrophysiological differences, all connexins share a similar structure. They are oriented so that the amino- and carboxyterminal (CT) tails of the protein are located within the cell cytoplasm, and they include four transmembrane-spanning α-helix domains linked by two extracellular segments (E1 and E2) and one cytoplasmic loop (Figure 1) [4]. Differences between connexin isoforms are mostly due to variations in the amino acid sequence at the CT domain, although also, to some extent, at the cytoplasmic loop [5]. The CT domain has multiple serine, threonine and tyrosine residues susceptible to phosphorylation, which is a very important phenomenon regulating connexin trafficking, assembly and function [6][7][8][9], and is largely responsible for the appearance of multiple bands in Western blots. Connexin 43 (Cx43) is, by far, the best-known and most ubiquitously expressed isoform, and it is encoded by the GJA1 gene, located, in humans, in chromosome 6 [10]. The gene nomenclature for the two other most common human cardiac connexin isoforms, Cx40 and Cx45, is GJA5 and GJC1, respectively [2].
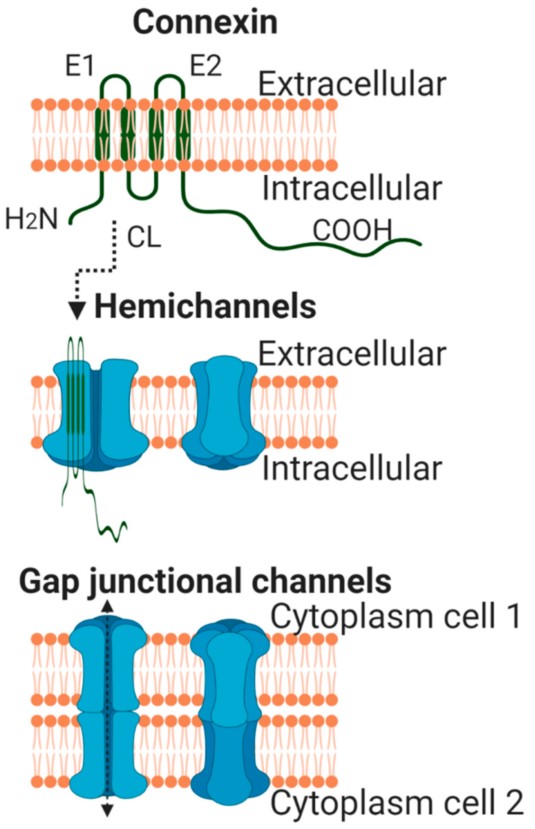
Figure 1. Schematic view of a single connexin molecule located at the plasma membrane (upper panel). Lower panels depict hemichannels and intercellular or gap junctional channels. Created with Biorender.com (accessed on 15 April 2021). Modified from [11][12].
All connexin isoforms are integral components of plasma membranes where they form channels around a central pore. These channels are formed by the oligomerization of six individual connexin molecules and are known as hemichannels or connexons (Figure 1) [4][13]. Hundreds to thousands of these hemichannels gather in plaques, termed gap junctions, where they dock with opposing connexons from adjacent cells, forming intercellular channels (Figure 1) [4][13]. In cardiomyocytes, gap junctions are mainly located at the cell poles (Figure 2), perpendicular to the long axis of the cell, within the intercalated discs, which are complex structures in which plasma membranes of neighboring cells are in close contact and that also include adherens junctions, hemichannels and ion channels [14][15]. Three cysteine residues, located in each extracellular loop of each connexin molecule, are important in the docking process. Disulfide bonds between these cysteines are needed to create the β-sheet conformation required for the interaction between the two opposing hemichannels [4][5][16]. In fact, Cx43 lacking these cysteines is not able to form gap junctional intercellular channels [17]. Connexons can be formed by a unique connexin isoform or by several connexin isoforms (giving rise to homomeric or heteromeric connexons, respectively), although not all are compatible [16]. In turn, intercellular channels can be homotypic (formed by connexons with the same composition) or heterotypic (when each connexon has a different composition) [18][19]. Such mixed conformations modify channel electrophysiological properties and alter gap junctional conductance [20][21].
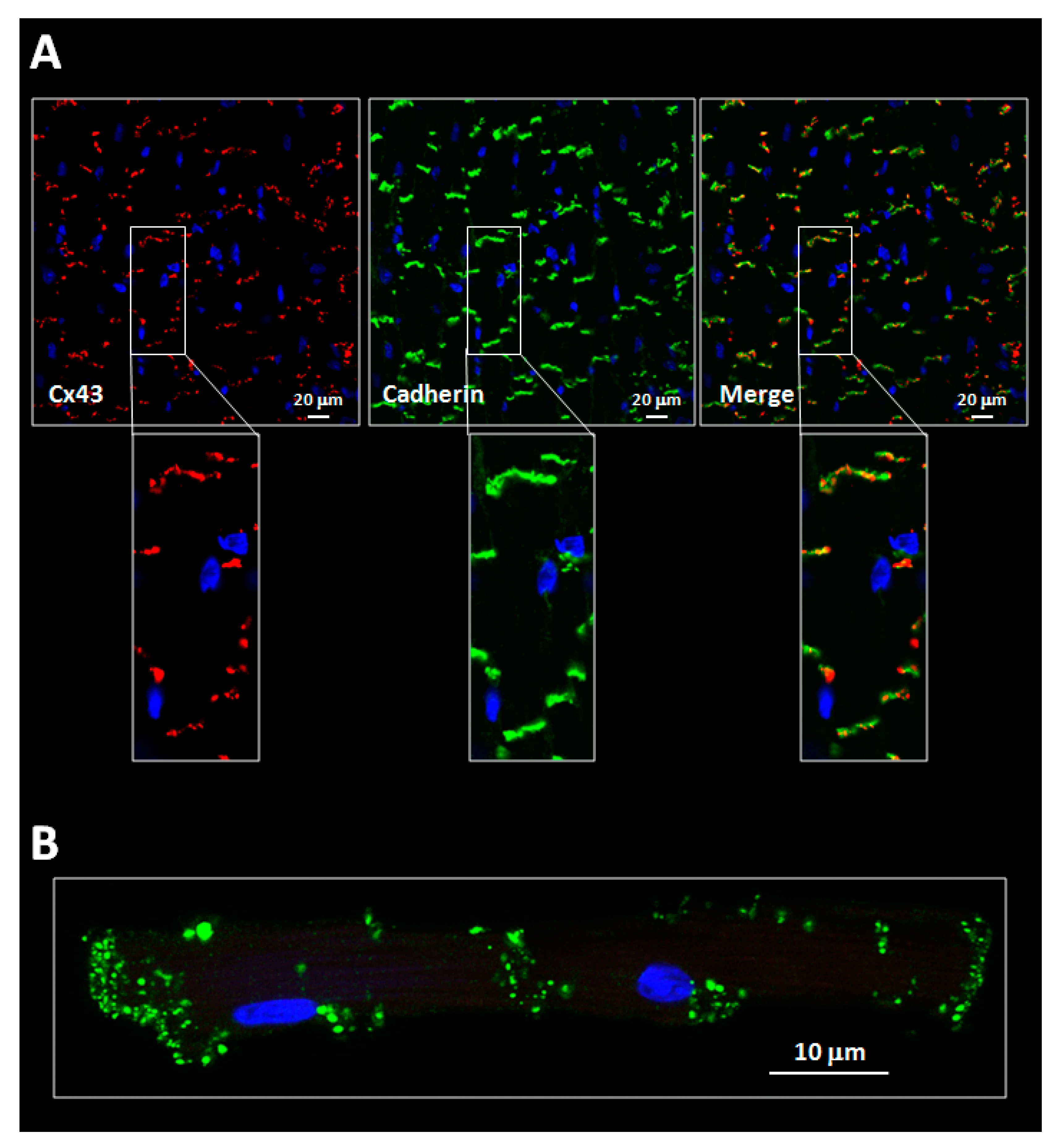
Figure 2. (A) Confocal images showing expression of connexin 43 (Cx43, red) in cardiac slices obtained from wild-type mice hearts. Cx43 is mostly expressed at the cell poles, within the intercalated discs, where it colocalizes with pan-cadherin (green) (see magnified images). (B) Confocal image of a pair of end-to-end connected mice cardiomyocytes showing Cx43 expression (green) at gap junctions. Nuclei were stained with Hoechst 33642 (blue).
Intercellular channels put into contact the cytoplasms of connected cells, allowing the transfer of ions and small intracellular molecules between them, in a process known as gap junctional intercellular communication (GJIC) [16]. GJIC is a conserved phenomena in many phyla, from invertebrates to chordates [3][16]. This fact gives an idea of the critical importance of GJIC to coordinate cell responses and function in multicellular organisms. This is especially important in the heart, where gap junctions and GJIC are responsible for maintaining a coordinated cardiac contraction.
In addition to their location at the plasma membrane, some connexins have been described in other less conventional cell structures, including the nucleus [22] and the mitochondria [23][24][25].
2. Cardiac Connexins
GJIC is particularly relevant in the mammalian heart. It was in this organelle where the existence of direct intercellular communication between adjacent cells was first proposed, in 1925 [26]. The earliest images of vertebrate gap junctions, appearing as pentalaminar structures in cross-sectional images, were obtained from mouse and guinea pig hearts and were published in the late 1950s [27]. The name “gap junction” was coined in 1967 by Revel and Karnovsky, based on observations made in ventricular cardiac tissues demonstrating that, in these areas, the plasma membranes of two adjacent cells were in close contact (20–30 Å), but not fused, and that heavy metals such as lanthanum could be infused into the space or “gap” between both membranes [28]. Later on, freeze-fracture electron microscopy studies demonstrated that gap junctions contain packed arrays of intercellular channels, which are arranged in a lattice of hexagonal structures [28][29].
Most cell types within the myocardium, including cardiomyocytes, fibroblasts, leukocytes and endothelial and smooth muscle cells, express connexins [30][31]. However, here, we are going to refer mainly to those expressed in cardiomyocytes. Although they represent less than a third of the total cell number in the heart [32][33][34], cardiomyocytes occupy 70–85% of the total cardiac volume and are responsible for maintaining the cardiac beat. Cardiomyocytes express three main connexin isoforms, which are essential to sustain a coordinated cardiac contraction: Cx43, Cx40 and Cx45 [31][35][36][37]. However, Cx43 is, by far, the most abundant connexin isoform expressed in the heart [38]. Although its specific location depends on the animal species, in general, it can be considered that Cx43 is widely expressed both in the ventricular myocardium and in the atria, and in some parts of the conducting system [31][36][38]. Initial studies conducted in rat hearts demonstrated that Cx43 expression is increased during embryonic development, which correlates with an enhancement in conduction velocity [38]. On the other hand, Cx40 can be found in the atria, the atrioventricular node and in the His–Purkinje system [31][35][36]. Interestingly, the distribution patterns of Cx40 and Cx43 are, to a large extent, comparable in rat, guinea pig, porcine, bovine and human hearts, both in neonates and adults [5][35][36][37][39]. Regarding Cx45, its expression is mostly restricted to the pacemaker and conducting system [5][31][35][40], representing only about 0.3% of the total connexin content in the ventricular myocardium [41]. An additional connexin isoform, Cx30.2, is also present in the mouse sinoatrial node and other parts of the conducting system, although this might be a special rodent feature [35][42]. In fact, Cx31.9, the human orthologue of mouse Cx30.2, is not detectable in the human cardiac conduction system [43]. Finally, Cx46 has been reported to also be expressed in the murine conducting system [44].
As it has been discussed previously, connexins are predominantly expressed at both cardiomyocyte ends, forming plaques of intercellular channels that put into contact the cytoplasms of adjacent cells (Figure 2) [13]. These plaques, the gap junctions, are one of the components of the intercalated disc, a complex cell structure in which plasma membranes of neighbouring cells are in close contact, and that also includes adherens junctions, desmosomes and other ion channels [14][15]. In the heart, Cx43 located at gap junctions is predominantly found, under normal conditions, in the two slower-migrating forms, P2 and P1 [23][24][45][46][47][48][49][50][51]. In contrast, the amount of the faster-migrating, non-phosphorylated Cx43 species (P0) is often markedly lower or even below the detection limit [23][24][45][46][47][48][49][50][51].
3. Concluding Remarks
Gap junction-dependent functions of cardiac connexins are key determinants of cardiac pathophysiology. GJIC mediates electrical coupling, allowing impulse propagation between neighboring cardiomyocytes, and its disruption may lead to atrial or ventricular arrhythmias. In addition, GJIC is also involved in chemical coupling, which allows the transfer of cytosolic signals between connected cells, and may be important in propagation of ischemia/reperfusion injury during myocardial infarction. However, in addition to the role connexins play in cell-to-cell communication, mounting evidence demonstrates that connexins have multiple additional functions. Connexins modulate cell growth and differentiation, both in a gap junction-dependent and in a gap junction-independent manner, with the last involving regulation of transcription at the cell nucleus, either by the full-length protein or by N-terminally truncated fragments derived from the CT domain. Furthermore, the non-canonical roles of connexins include those of unopposed hemichannels, whose opening might be involved in paracrine signaling, but also in loss of cell homeostasis and intracellular edema in some pathologies, including myocardial infarction. Mitochondrial connexins may control some steps of mitochondrial respiration and ROS production and may constitute a key player in preconditioning protection and chemotherapy cardiotoxicity. Further studies are required, in any case, to fully unveil the importance of these emerging processes in cardiac physiology and disease.
References
- Dobrowolski, R.; Willecke, K. Connexin-caused genetic diseases and corresponding mouse models. Antioxid. Redox Signal. 2009, 11, 283–295.
- Sohl, G.; Willecke, K. An update on connexin genes and their nomenclature in mouse and man. Cell Commun. Adhes. 2003, 10, 173–180.
- Beyer, E.C.; Berthoud, V.M. Gap junction gene and protein families: Connexins, innexins, and pannexins. Biochim. Biophys. Acta 2018, 1860, 5–8.
- Sosinsky, G.E.; Nicholson, B.J. Structural organization of gap junction channels. Biochim. Biophys. Acta 2005, 1711, 99–125.
- Van Veen, A.A.; van Rijen, H.V.; Opthof, T. Cardiac gap junction channels: Modulation of expression and channel properties. Cardiovasc. Res. 2001, 51, 217–229.
- Laird, D.W. Connexin phosphorylation as a regulatory event linked to gap junction internalization and degradation. Biochim. Biophys. Acta 2005, 1711, 172–182.
- Moreno, A.P. Connexin phosphorylation as a regulatory event linked to channel gating. Biochim. Biophys. Acta 2005, 1711, 164–171.
- Leithe, E.; Mesnil, M.; Aasen, T. The connexin 43 C-terminus: A tail of many tales. Biochim. Biophys. Acta 2018, 1860, 48–64.
- Procida, K.; Jorgensen, L.; Schmitt, N.; Delmar, M.; Taffet, S.M.; Holstein-Rathlou, N.H.; Nielsen, M.S.; Braunstein, T.H. Phosphorylation of connexin43 on serine 306 regulates electrical coupling. Heart Rhythm 2009, 6, 1632–1638.
- Fishman, G.I.; Eddy, R.L.; Shows, T.B.; Rosenthal, L.; Leinwand, L.A. The human connexin gene family of gap junction proteins: Distinct chromosomal locations but similar structures. Genomics 1991, 10, 250–256.
- Axelsen, L.N.; Calloe, K.; Holstein-Rathlou, N.H.; Nielsen, M.S. Managing the complexity of communication: Regulation of gap junctions by post-translational modification. Front. Pharmacol. 2013, 4, 130.
- Aasen, T.; Leithe, E.; Graham, S.V.; Kameritsch, P.; Mayán, M.D.; Mesnil, M.; Pogoda, K.; Tabernero, A. Connexins in cancer: Bridging the gap to the clinic. Oncogene 2019, 38, 4429–4451.
- Nielsen, M.S.; Nygaard, A.L.; Sorgen, P.L.; Verma, V.; Delmar, M.; Holstein-Rathlou, N.H. Gap junctions. Compr. Physiol. 2012, 2, 1981–2035.
- Shaw, R.M.; Fay, A.J.; Puthenveedu, M.A.; von Zastrow, M.; Jan, Y.N.; Jan, L.Y. Microtubule plus-end-tracking proteins target gap junctions directly from the cell interior to adherens junctions. Cell 2007, 128, 547–560.
- Severs, N.J. The cardiac gap junction and intercalated disc. Int. J. Cardiol. 1990, 26, 137–173.
- Harris, A.L. Emerging issues of connexin channels: Biophysics fills the gap. Q. Rev. Biophys. 2001, 34, 325–472.
- Bao, X.; Chen, Y.; Reuss, L.; Altenberg, G.A. Functional expression in Xenopus oocytes of gap-junctional hemichannels formed by a cysteine-less connexin 43. J. Biol. Chem. 2004, 279, 9689–9692.
- Koval, M.; Molina, S.A.; Burt, J.M. Mix and match: Investigating heteromeric and heterotypic gap junction channels in model systems and native tissues. FEBS Lett. 2014, 588, 1193–1204.
- Beyer, E.C. Are these connexins compatible and does it matter? Channels 2015, 9, 63–64.
- Lin, X.; Xu, Q.; Veenstra, R.D. Functional formation of heterotypic gap junction channels by connexins-40 and -43. Channels 2014, 8, 433–443.
- Rackauskas, M.; Kreuzberg, M.M.; Pranevicius, M.; Willecke, K.; Verselis, V.K.; Bukauskas, F.F. Gating properties of heterotypic gap junction channels formed of connexins 40, 43, and 45. Biophys. J. 2007, 92, 1952–1965.
- Dang, X.; Doble, B.W.; Kardami, E. The carboxy-tail of connexin-43 localizes to the nucleus and inhibits cell growth. Mol. Cell Biochem. 2003, 242, 35–38.
- Boengler, K.; Dodoni, G.; Rodriguez-Sinovas, A.; Cabestrero, A.; Ruiz-Meana, M.; Gres, P.; Konietzka, I.; Lopez-Iglesias, C.; Garcia-Dorado, D.; Di Lisa, F.; et al. Connexin 43 in cardiomyocyte mitochondria and its increase by ischemic preconditioning. Cardiovasc. Res. 2005, 67, 234–244.
- Rodriguez-Sinovas, A.; Boengler, K.; Cabestrero, A.; Gres, P.; Morente, M.; Ruiz-Meana, M.; Konietzka, I.; Miro, E.; Totzeck, A.; Heusch, G.; et al. Translocation of connexin 43 to the inner mitochondrial membrane of cardiomyocytes through the heat shock protein 90-dependent TOM pathway and its importance for cardioprotection. Circ. Res. 2006, 99, 93–101.
- Rodriguez-Sinovas, A.; Ruiz-Meana, M.; Denuc, A.; Garcia-Dorado, D. Mitochondrial Cx43, an important component of cardiac preconditioning. Biochim. Biophys. Acta 2018, 1860, 174–181.
- Schmidtmann, M. Über die intracellulaire Wasserstoffionenkonzentration unter physiologischen und einigen pathologischen Bedingungen. Z Gesamte Exp. Med. 1925, 45, 714–742.
- Sjostrand, F.S.; Andersson-Cedergren, E.; Dewey, M.M. The ultrastructure of the intercalated discs of frog, mouse and guinea pig cardiac muscle. J. Ultrastruct. Res. 1958, 1, 271–287.
- Revel, J.P.; Karnovsky, M.J. Hexagonal array of subunits in intercellular junctions of the mouse heart and liver. J. Cell Biol. 1967, 33, C7–C12.
- Gilula, N.B.; Reeves, O.R.; Steinbach, A. Metabolic coupling, ionic coupling and cell contacts. Nature 1972, 235, 262–265.
- Ongstad, E.; Kohl, P. Fibroblast-myocyte coupling in the heart: Potential relevance for therapeutic interventions. J. Mol. Cell Cardiol. 2016, 91, 238–246.
- Lambiase, P.D.; Tinker, A. Connexins in the heart. Cell Tissue Res. 2015, 360, 675–684.
- Zhou, P.; Pu, W.T. Recounting Cardiac Cellular Composition. Circ. Res. 2016, 118, 368–370.
- Pinto, A.R.; Ilinykh, A.; Ivey, M.J.; Kuwabara, J.T.; D’Antoni, M.L.; Debuque, R.; Chandran, A.; Wang, L.; Arora, K.; Rosenthal, N.A.; et al. Revisiting Cardiac Cellular Composition. Circ. Res. 2016, 118, 400–409.
- Bergmann, O.; Zdunek, S.; Felker, A.; Salehpour, M.; Alkass, K.; Bernard, S.; Sjostrom, S.L.; Szewczykowska, M.; Jackowska, T.; Dos, R.C.; et al. Dynamics of Cell Generation and Turnover in the Human Heart. Cell 2015, 161, 1566–1575.
- Jongbloed, M.R.; Mahtab, E.A.; Blom, N.A.; Schalij, M.J.; Gittenberger-de Groot, A.C. Development of the cardiac conduction system and the possible relation to predilection sites of arrhythmogenesis. Sci. World J. 2008, 8, 239–269.
- Van Kempen, M.J.; Velde, I.T.; Wessels, A.; Oosthoek, P.W.; Gros, D.; Jongsma, H.J.; Moorman, A.F.; Lamers, W.H. Differential connexin distribution accommodates cardiac function in different species. Microsc. Res. Tech. 1995, 31, 420–436.
- Gros, D.B.; Jongsma, H.J. Connexins in mammalian heart function. Bioessays 1996, 18, 719–730.
- Van Kempen, M.J.; Fromaget, C.; Gros, D.; Moorman, A.F.; Lamers, W.H. Spatial distribution of connexin43, the major cardiac gap junction protein, in the developing and adult rat heart. Circ. Res. 1991, 68, 1638–1651.
- Vozzi, C.; Dupont, E.; Coppen, S.R.; Yeh, H.I.; Severs, N.J. Chamber-related differences in connexin expression in the human heart. J. Mol. Cell Cardiol. 1999, 31, 991–1003.
- Coppen, S.R.; Dupont, E.; Rothery, S.; Severs, N.J. Connexin45 expression is preferentially associated with the ventricular conduction system in mouse and rat heart. Circ. Res. 1998, 82, 232–243.
- Bao, M.; Kanter, E.M.; Huang, R.Y.; Maxeiner, S.; Frank, M.; Zhang, Y.; Schuessler, R.B.; Smith, T.W.; Townsend, R.R.; Rohrs, H.W.; et al. Residual Cx45 and its relationship to Cx43 in murine ventricular myocardium. Channels 2011, 5, 489–499.
- Kreuzberg, M.M.; Sohl, G.; Kim, J.S.; Verselis, V.K.; Willecke, K.; Bukauskas, F.F. Functional properties of mouse connexin30.2 expressed in the conduction system of the heart. Circ. Res. 2005, 96, 1169–1177.
- Kreuzberg, M.M.; Liebermann, M.; Segschneider, S.; Dobrowolski, R.; Dobrzynski, H.; Kaba, R.; Rowlinson, G.; Dupont, E.; Severs, N.J.; Willecke, K. Human connexin31.9, unlike its orthologous protein connexin30.2 in the mouse, is not detectable in the human cardiac conduction system. J. Mol. Cell Cardiol. 2009, 46, 553–559.
- Chi, N.C.; Bussen, M.; Brand-Arzamendi, K.; Ding, C.; Olgin, J.E.; Shaw, R.M.; Martin, G.R.; Stainier, D.Y. Cardiac conduction is required to preserve cardiac chamber morphology. Proc. Natl. Acad. Sci. USA 2010, 107, 14662–14667.
- Solan, J.L.; Lampe, P.D. Spatio-temporal regulation of connexin43 phosphorylation and gap junction dynamics. Biochim. Biophys. Acta 2018, 1860, 83–90.
- Lampe, P.D.; Cooper, C.D.; King, T.J.; Burt, J.M. Analysis of Connexin43 phosphorylated at S325, S328 and S330 in normoxic and ischemic heart. J. Cell Sci. 2006, 119, 3435–3442.
- Sanchez, J.A.; Rodriguez-Sinovas, A.; Fernandez-Sanz, C.; Ruiz-Meana, M.; Garcia-Dorado, D. Effects of a reduction in the number of gap junction channels or in their conductance on ischemia-reperfusion arrhythmias in isolated mouse hearts. Am. J. Physiol. Heart Circ. Physiol. 2011, 301, H2442–H2453.
- Sanchez, J.A.; Rodriguez-Sinovas, A.; Barba, I.; Miro-Casas, E.; Fernandez-Sanz, C.; Ruiz-Meana, M.; Alburquerque-Bejar, J.J.; Garcia-Dorado, D. Activation of RISK and SAFE pathways is not involved in the effects of Cx43 deficiency on tolerance to ischemia-reperfusion injury and preconditioning protection. Basic Res. Cardiol. 2013, 108, 351.
- Remo, B.F.; Giovannone, S.; Fishman, G.I. Connexin43 cardiac gap junction remodeling: Lessons from genetically engineered murine models. J. Membr. Biol. 2012, 245, 275–281.
- Beardslee, M.A.; Lerner, D.L.; Tadros, P.N.; Laing, J.G.; Beyer, E.C.; Yamada, K.A.; Kleber, A.G.; Schuessler, R.B.; Saffitz, J.E. Dephosphorylation and intracellular redistribution of ventricular connexin43 during electrical uncoupling induced by ischemia. Circ. Res. 2000, 87, 656–662.
- Miura, T.; Ohnuma, Y.; Kuno, A.; Tanno, M.; Ichikawa, Y.; Nakamura, Y.; Yano, T.; Miki, T.; Sakamoto, J.; Shimamoto, K. Protective role of gap junctions in preconditioning against myocardial infarction. Am. J. Physiol. Heart Circ. Physiol. 2004, 286, H214–H221.





