
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Valeria Maselli | + 3121 word(s) | 3121 | 2021-05-11 11:29:41 | | | |
| 2 | Vivi Li | Meta information modification | 3121 | 2021-05-13 03:35:29 | | |
Video Upload Options
Microorganism resistance to conventional antibiotics represents one of the major global health concerns. This entry focuses on a peptide (OctoPartenopin) extracted from suckers of Octopus vulgaris; bioassay-guided chromatographic fractionation was used to identify this sequence, which holds significant antibacterial activity against Gram-positive and Gram-negative bacteria. OctoPartenopin is encrypted within the calponin sequence and was associated with the high levels of proteolytic activity already reported in octopus arm suckers.
1. Introduction
Octopus is a highly versatile and opportunistic predator [1][2][3]; it hunts for food by ‘speculative pounce’ [4] and ‘groping’, and it explores the surfaces of rocks or the sea-bed with their arms, webs, and suckers [5]. The common octopus, Octopus vulgaris (Cuvier, 1797) is a cosmopolitan merobenthonic cephalopod with a short life cycle [6], living on rocky, sandy, and muddy bottoms from the coastline to the edge of the continental shelf, and it is very common in the Mediterranean Sea and eastern Atlantic Ocean [7]. Octopus and all cephalopods, as a result of evolutionary selective pressure, have developed a winning strategy for surviving in different environments [8][9]. O. vulgaris is an economically important seafood species [10], and a model for studying complex behavioral, neuronal, and genomic plasticity [11][12]. Moreover, it is well known for its intelligence linked to adult neurogenesis mechanisms, curiosity, and adaptability; recently, transcriptomic and genomic tools were developed to investigate these issues on this species and the congeneric O. bimaculoides [8][13][14][15]. Most octopus neurons are found in the arms, which can independently taste and touch, and control basic motions without input from the brain [16][17][18]. Each arm is made of a dozen of suckers and is packed with hundreds of sensors [19][20][21][22][23].
Interestingly, the octopus female leaves its eggs to fend for themselves, and eggs are resistant to infections. In particular, it is believed that cephalopod antimicrobial peptides (AMPs) are trapped in the egg capsule and mainly expressed by female accessory sex glands, conferring on them efficient protection of organs against microorganisms [24]. In this context, studies reporting the identification of AMPs in cephalopods were conducted on squids and cuttlefish [25][26]. Nevertheless, there is still a scarcity of knowledge regarding the defense mechanisms involved in O. vulgaris immune response, even if more researchers are focusing their attention on this topic [13][27][28][29][30][31]. As a matter of fact, octopus lacks an adaptive immune system [32][33], but has an efficient innate immune system comprised of cellular and humoral components that act as the first line of defense against a broad spectrum of pathogens [13][34]. Although the marine environment presents various and high levels of exposure to a considerable number of pathogens, only granulocytes were observed in the hemolymph of three species of Octopoda [35][36], and they were characterized and cultured in O. vulgaris [31][37]. At the same time, O. vulgaris presents a hemagglutination activity [38][39] and an anti-protease activity associated with α-macroglobulin in the hemolymph [40]. In order to fight against pathogens, it is likely that octopus exploits secreted AMPs as part of an innate defense mechanism, similarly to other aquatic animals [41][42][43][44][45][46].
The growing problem of resistance to conventional antibiotics and the need for novel drugs has stimulated interest in the development of antimicrobial peptides as human therapeutics (ADP, http://aps.unmc.edu/AP/main.php). Recently, much attention has been directed towards marine-derived bioactive peptides due to their special living environment, composition, and properties as well as to their antiviral, antitumor, antidiabetic, and antihypertensive activities and their role in the food industry for preservation and elongation of shelf-lives [47]. Since marine organisms live in close contact with microbes, they have proved to be a rich source of AMPs [48][49] with novel chemistry and diverse biological properties [47][50][51][52][53][54].
For example, AMPs were described in many mollusks as bivalves [55]; cysteine-rich peptides were identified in mussels [56]; defensins and proline-rich peptides were found in oysters [57] and gastropods [58][59]. Proteins with antimicrobial activity, such as egg case proteins Sep-ECPs [60] or hemocyanin [59][61], were identified in gastropods. Moreover, several other AMPs were identified encrypted within the sequence of proteins and could be derived from the cleavage of bulky proteins; interestingly, several AMPs from marine organisms used as food sources are released in the corresponding body fluids/tissues following the action of specific proteases or hydrolytic treatments [62], similarly to what has been observed in other animal products [63][64]. Due to their natural origin and antimicrobial activity, the latter molecules were proposed as additives in food industry for the preservation of edible products and the elongation of corresponding shelf-life [53], but also as active ingredients for the preparation of packaging materials.
The pharmaceutical company interest in AMPs is linked to their ability to disrupt bacterial membranes and, following cell internalization, to target components, such as nucleic acids, preventing microorganisms from developing resistance. Their main molecular features are a predominant cationic nature and a high percentage of hydrophobic residues, which enable them to assume an amphipathic primary or secondary structure. The low propensity to induce resistance, coupled with their low toxicity and the possibility of improving their activity through the rational design of peptidomimetics—together with high production through genetically engineered bacteria, bioreactor, or green-synthesis alternatives—make AMPs highly attractive for commercial purposes. On the other hand, AMPs from natural sources have also been widely used in the preservation of foods and to increase the product shelf-life [65].
In this context, octopus species and their relative transcriptomes were recently screened in order to identify novel AMPs [66][67]. The present study describes the identification of a novel AMP (OctoPartenopin) extracted from suckers of O. vulgaris that is active against bacteria and yeast species. The amino acid sequence identified is part of a repeated motif in calponin-like proteins, involved in muscular contraction. OctoPartenopin was used as lead molecule for the rational design of novel compounds, thus allowing the synthesis of four analogues with improved antimicrobial and antibiofilm activity. We also suggest a possible role of OctoPartenopin in maternal care of fertilized eggs.
2. Antimicrobial Activity of the Sucker Extract and Purified Fraction
Octopus suckers aqueous extract (SE) (1 mg/mL) was assessed for its antimicrobial activity by evaluating the diameter of the clear zone of growth inhibition of Gram positive bacterium S. aureus, Gram-negative bacterium P. aeruginosa, and a yeast C. albicans. A clear inhibition zone was observed in the case of S. aureus and P. aeruginosa, but no activity was found towards C. albicans (Table 1).
| Strain | SE | HPLC Fraction | Positive Control | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| A | B | C | D | E | F | AMP | G | AMPH-B | ||
| Mean ± SD (Ø mm) | ||||||||||
| Gram-positive | ||||||||||
| S. aureus ATCC 6538 | 11.0 ± 1.2 | 12.0 ± 2.0 | - | 8.0 ± 1.5 | - | 6.0 ± 2.8 | - | S | S | NT |
| Gram-negative | ||||||||||
| P. aeruginosa ATCC 9027 | 8.0 ± 2.3 | 10.0 ± 3.1 | - | 6.0 ± 3.0 | - | - | - | R | S | NT |
| Yeast | ||||||||||
| C. albicans ATCC 90028 | - | 7.0 ± 1.8 | - | - | - | - | - | NT | NT | S |
In order to isolate and identify molecular species responsible for the observed antimicrobial activity, SE was further subjected to a chromatographic fractionation on a semi-preparative reverse-phase C18 column (Figure 1a). Eluted fractions were collected on a time-based mode, to limit the number of fractions to analyze, and eluate was collected to finally obtain six fractions (A–F) (Figure 1a); aliquots (same volume) of each fraction were further subjected to the above-mentioned tests for antimicrobial activity. These assays evidenced that fraction A was the most active against the tested microorganisms (Table 1); thus, we concentrated our attention on it.
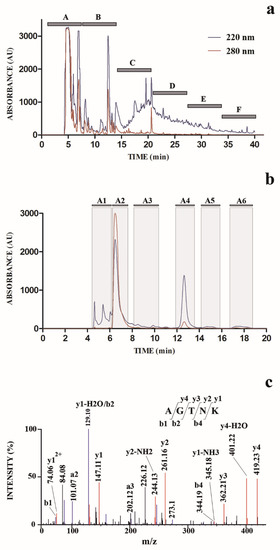
The chromatographic profile shown in Figure 1a suggested the presence in fraction A of a number of abundant hydrophilic compounds with similar retention times (eluting between 4 and 8 min); thus, an optimized chromatographic experiment was performed to separate and identify these species. Accordingly, SE fraction A was subjected to a dedicated semi-preparative chromatography in which the elution gradient as well as the flow rate (in this case lowered) were chosen with the aim to fractionate hydrophilic compounds. We collected six fractions (A1–A6) (Figure 1b) according to the recorded chromatographic profile; aliquots (same volume) of these fractions were further tested to determine the corresponding antimicrobial activity.
In vitro antimicrobial action of fractions A1–A6 was then evaluated by a broth microdilution method. Corresponding antimicrobial activity was expressed as minimum inhibitory concentration (MIC), results are reported in Table 2. All purified fractions were active against Gram-positive bacteria, Gram-negative bacteria, and fungi. Among the tested strains, the A1–A6 fractions showed the lowest activity against C. albicans, with MIC >300 µg/mL. Interestingly, another octopus peptide (octominin) exhibited a significant activity against this yeast, inducing cell wall damage and causing loss of cell membrane integrity [66][67].
| Strain | MIC80 (µg/mL) HPLC Fraction | |||||
|---|---|---|---|---|---|---|
| A1 | A2 | A3 | A4 | A5 | A6 | |
| S. aureus ATCC 6538 | 150 ± 1 | 120 ± 5 | 180 ± 4 | 120 ± 2 | 120 ± 5 | 200 ± 6 |
| P. aeruginosa ATCC 9027 | >300 | 200 ± 2 | >300 | 180 ± 5 | >300 | 200 ± 5 |
| C. albicans ATCC 90028 | >300 | >300 | >300 | 300 ± 2 | >300 | >300 |
All fractions showed a similar effect on P. aeruginosa and S. aureus even though the activity against S. aureus was more evident for fractions A2, A4, and A5 with a MIC of 120 µg/mL. In general, lower MIC values were obtained for the Gram-positive bacteria in comparison to the Gram-negative one (Table 2). Fraction A4 seemed to have the best antimicrobial activity against all the examined microorganisms, with MIC values of 120, 180, and 300 µg/mL for S. aureus, P. aeruginosa, and C. albicans, respectively. Accordingly, fraction A4 was selected for subsequent characterization.
3. Peptide Identification by Mass Spectrometry
The fraction A4 was selected for further structural characterization, which was accomplished by nanoLC-ESI-Q-Orbitrap MS/MS analysis. The use of a high-resolution mass spectrometer allowed us to measure the molecular mass values of the components present in this fraction with a high accuracy, together with those of the fragments originated during the MS/MS fragmentation analysis, thus allowing the identification of the corresponding molecular structure. Measured mass of mono- and multiply charged ions, corresponding isotopic distribution, and recorded mass values of resulting fragment ions strongly suggested the peptide nature of the molecules present in fraction A4. Thus, nanoLC-ESI-Q-Orbitrap MS/MS raw data were investigated by automated de novo peptide sequencing; results are reported in Supplementary Table S1. In order to guarantee the accuracy of the resulting data output, results were filtered to selectively maintain peptide sequence entries having a local confidence score for each amino acidic site >90%. Thus, pentapeptide AGTNK was identified as the molecular species with the largest area of ions extracted from the nanoLC-ESI-MS profile, whose sequence showed the highest average local confidence (ALC) score (>95%) (Supplementary Table S1). The other two identified amino acid sequences showed both lower areas of ions extracted from the nanoLC-ESI-MS profile, as well as were identified with lower ALC scores.
Identification of peptide AGTNK was confirmed by independent database searching of the nanoLC-ESI-Q-Orbitrap MS/MS raw data against the protein sequences of O. bimaculoides, which were retrieved from NCBI. In this case, database searching again identified the inner peptide AGTNK present in calponin-2-like isoform X1 (gi:961140629/NCBI reference sequence XP_014789315) as the molecular species showing the highest identification score. Several additional peptide sequences were also identified (Supplementary Table S1). Nevertheless, peptide AGTNK was largely more abundant than the other peptides, as demonstrated by the number of spectra (#Spec) corresponding to its sequence recorded during the nanoLC-ESI-Q-Orbitrap MS/MS analysis (Supplementary Table S1). The fragmentation MS/MS spectrum of the pentapeptide AGTNK is reported in Figure 1c; this compound was named OctoPartenopin.
4. Discussion
Octopus has developed a successful strategy for surviving in different hostile environments; octopus suckers continuously provide a great inspiration to biologists, engineers, and movie special effect supervisors for the development of novel bioinspired artificial devices [68][69][70][71]. Octopus arm suckers are specialized chemo-tactile organs with high sensitivity, equipped with millions of distributed sensory receptors allowing the animal to process in parallel massive amounts of mechanical and chemical information resulting from its densely innervation [16][21][22][72][73][74]. In fact, the octopus uses suckers for a variety of tasks, such as anchoring to the substratum, catching prey, locomotion, clean maneuvers, recognition by chemoreception, behavioral displays, and as a manipulating tool for collecting objects [75][76]. Octopus suckers are made of a tightly packed three-dimensional array of (radial, circular, and meridional) muscles with different fiber orientations [77][78]. They also have fibrous connective tissue layers and crossed connective tissue fibers, fixed in the musculature.
Calponin is a key protein involved in octopus muscular contraction and a large number of molecular isoforms were identified in O. bimaculoides (XP_014789315). Proteolytic modification of calponin seems to play a crucial role during the inflammatory response, but is also associated with rapid growth in octopus and other cephalopods, as result of an enhanced proteolytic activity present in animal fibers [79][80]. The maximum autolytic activity in octopus (O. vulgaris) arm muscle is 15-fold higher than in Pacific whiting, a fish well known to contain high levels of endogenous proteases [81], in particular cysteine and aspartic-proteinases, as cathepsin B [82]. Thus, it is believed that this proteolytic activity may be responsible of the release of antimicrobial peptides, which are exploited to enable the octopus to survive the harsh marine environment. Thus, it is reasonable to speculate that different proteolytic enzymes should be constitutively active in octopus fibers of suckers, generating protein fragments with antimicrobial activity (as observed in this study), similarly to what is observed in other animals in which actin-binding protein degradation products were demonstrated to play a role in various biological functions [83]. It is worth mentioning recent data regarding squid and cuttlefish demonstrating that AMPs trapped in the egg capsule confer efficient protection against microorganisms; those peptides are derived from female accessory sex glands, but also from the partial degradation of corresponding tissue proteins [24].
In this study, we isolated the pentapeptide AGTNK from O. vulgaris suckers and we proved that this molecule has a significant antimicrobial activity against S. aureus and P. aeruginosa. Sequence analysis demonstrated that this peptide is encrypted within the sequence of calponin-2-like isoform X1 and occurs therein multiple times (together with some variant sequences), yielding several bioactive peptide molecules from each parental protein, as result of the action of still-unknown proteases.
Starting from this preliminary data on the natural peptide OctoPatenopin, we designed and synthetized the C-terminal amidated homologue (P0) and four analogues with the aim of improving its antimicrobial performance. Synthetic peptides were analyzed for their antimicrobial activity against Gram-positive, Gram-negative bacteria, and yeast. The results clearly showed that the addition of one residue at the N-terminus did not induce any enhancement of activity. Conversely, peptide elongation at the C- and N-termini with short sequences present in the conserved, repeated motif of calponin-2-like isoform X1 (yielding peptides P2 and P3) was associated with a significant increase of corresponding antimicrobial activity. Interestingly, the whole repeated motif of calponin, as present in peptide P4, did not induce a significant enhancement of antimicrobial properties. All synthetic peptides were overall more active compared to the natural compound, pointing out the need of additional structure–activity functional studies to decipher the structural elements essential for activity. Further studies are also necessary to identify the most active sequence, which will probably comprise modifications both at the C- and N-terminus of the native sequence P0.
We also performed an in vitro experiment to assess peptide antibiofilm activity, analyzing both inhibitory effects on biofilm formation and dissolution on mature biofilm. To this purpose, we tested the peptides under or at MIC concentrations. Our results showed a peptide concentration-dependent inhibition of biofilm formation and a good eradication capacity for all microorganisms tested, suggesting a cell disaggregation and disruption mechanism [84][85][86][87]. In particular peptides P1, P2, and P4 seemed to have the best inhibition activity for all microorganisms tested, whereas significant eradication of dose-dependent clearance of biofilm biomass was observed for peptides P0, P2, and P3. In conclusion, preliminary antibiofilm experiments showed that peptide P2 seemed to have the best activity in both inhibition and eradication of biofilm of all three microorganisms tested.
Although peptides with moderate MIC values (between 50 and 200 μM) are not suitable candidates for use as single potent antibiotics in the pharmaceutical industry; nonetheless, they could find applications in synergy with conventional antibiotics, further favoring the entry of other drugs by destabilizing the microorganism membrane. At the same time, due to their natural origin and their presence in edible material, they may find promising applications in the food industry to increase shelf-life of food products through dedicated treatments and/or as active ingredients for packaging, as they do not cause harmful or undesirable side effects [65][88].
Tandem repeats in proteins is not a new phenomenon but is widely reported in literature. An interesting example is the presence in many organisms of pattern recognition receptors (known as PRRRs), which are part of the innate immune system, and are deputed to recognition and binding of conserved pathogen associated molecular patterns (known as PAMPs). These tandem repeats often present antibacterial activity when used as peptides [89]. It is likely that in the octopus suckers, tandem repeats play several roles and their eventual release into the medium may be critical for immunity similarly to what has been found for other AMPs present as tandem repeats in host proteins [90][91][92]. Clearly, we cannot exclude that the presence of tandem repeats in the suckers will also favor the formation of particular secondary structure motifs, which likely play a key role in the activity of the protein.
As a matter of fact, our results could be speculatively claimed to interpret octopus maternal care behavior, in which animal female broods and tends fertilized eggs until they hatch [93]. The chorion tissue of eggs allows the exchange of oxygen; thus, maternal care and mother movements were interpreted as essential for preventing embryo fouling and suffocation [94][95]. Based on the results presented in this study, female arm movements and egg touching through suckers should allow animal oxygenation and cleaning of fertilized eggs, but also corresponding protection from microorganism-driven infections [24]. Likely, octopus females may release antimicrobial peptides, such as OctoPartenopin, directly on the eggs to protect them from the attack of pathogens. In this context, it was previously demonstrated that when females abandon their eggs, the latter die [96]. Further studies are requested in this context to prove the functional role of OctoPartenopin and female animal suckers in egg protection from pathogens.
References
- Ambrose, R.F. Food preferences, prey availability, and the diet of Octopus bimaculatus Verrill. J. Exp. Mar. Biol. Ecol. 1984, 77, 29–44.
- Mather, J. Navigation by spatial memory and use of visual landmarks in octopuses. J. Comp. Physiol. A 1991, 168, 491–497.
- Nixon, M.; Young, J.Z. The Brain and Lives of Cephalopods; Oxford University Press: Oxford, UK, 2003.
- Yarnall, J.L. Aspects of the behaviour of Octopus cyanea gray. Anim. Behav. 1969, 17, 747–754.
- Forsythe, J.W.; Hanlon, R.T. Foraging and associated behavior by Octopus cyanea Gray, 1849 on a coral atoll, French Polynesia. J. Exp. Mar. Biol. Ecol. 1997, 209, 15–31.
- Katsanevakis, S.; Verriopoulos, G. Modelling the effect of temperature on hatching and settlement patterns of meroplanktonic organisms: The case of octopus. Sci. Mar. 2006, 70, 699–708.
- Balguerias, E.; Hernandez–Gonzalez, C.; Perales–Raya, C. On the identity of Octopus vulgaris Cuvier, 1797 stocks in the Saharan Bank (Northwest Africa) and their spatio-temporal variations in abundance in relation to some environmental factors. Bull. Mar. Sci. 2002, 71, 147–163.
- Liscovitch-Brauer, N.; Alon, S.; Porath, H.T.; Elstein, B.; Unger, R.; Ziv, T.; Admon, A.; Levanon, E.Y.; Rosenthal, J.J.; Eisenberg, E. Trade-off between Transcriptome Plasticity and Genome Evolution in Cephalopods. Cell 2017, 169, 191–202.
- Garrett, S.; Rosenthal, J.J. RNA editing underlies temperature adaptation in K+ channels from polar octopuses. Science 2012, 335, 848–851.
- Vaz–Pires, P.; Seixas, P.; Barbosa, A. Aquaculture potential of the common octopus (Octopus vulgaris Cuvier, 1797): A review. Aquaculture 2004, 238, 221–238.
- Di Cosmo, A.; Bertapelle, C.; Porcellini, A.; Polese, G. Magnitude Assessment of Adult Neurogenesis in the Octopus vulgaris Brain Using a Flow Cytometry–Based Technique. Front. Physiol. 2018, 9, 1050.
- Di Cosmo, A.; Maselli, V.; Polese, G. Octopus vulgaris: An Alternative in Evolution. Results Probl. Cell Differ. 2018, 65, 585–598.
- Castellanos–Martinez, S.; Arteta, D.; Catarino, S.; Gestal, C. De novo transcriptome sequencing of the Octopus vulgaris hemocytes using Illumina RNA–Seq technology: Response to the infection by the gastrointestinal parasite Aggregata octopiana. PLoS ONE 2014, 9, e107873.
- Albertin, C.B.; Simakov, O.; Mitros, T.; Wang, Z.Y.; Pungor, J.R.; Edsinger–Gonzales, E.; Brenner, S.; Ragsdale, C.W.; Rokhsar, D.S. The octopus genome and the evolution of cephalopod neural and morphological novelties. Nature 2015, 524, 220–224.
- Zhang, X.; Mao, Y.; Huang, Z.; Qu, M.; Chen, J.; Ding, S.; Hong, J.; Sun, T. Transcriptome analysis of the Octopus vulgaris central nervous system. PLoS ONE 2012, 7, e40320.
- Young, J.Z. The Anatomy of the Nervous System of Octopus vulgaris; Oxford University Press: New York, NY, USA, 1971; p. 690.
- Young, J.Z. The organization of a cephalopod ganglion. Phil. Trans. R. Soc. Lond. B 1972, 263.
- Young, J.Z. Brain, behaviour and evolution of cephalopods. Symp. Zool. Soc. Land. 1977, 38, 377–434.
- Zullo, L.; Eichenstein, H.; Maiole, F.; Hochner, B. Motor control pathways in the nervous system of Octopus vulgaris arm. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 2019, 205, 271–279.
- Graziadei, P. Receptors in the Suckers of Octopus. Nature 1962, 195, 57–59.
- Graziadei, P. Sensory receptor cells and related neurons in cephalopods. Cold Spring Harb. Symp. Quant. Biol. 1965, 30, 45–57.
- Graziadei, P.P.C.; Gagne, H.T. Sensory innervation in the rim of the octopus sucker. J. Morphol. 1976, 150, 639–680.
- Levy, G.; Hochner, B. Embodied Organization of Octopus vulgaris Morphology, Vision, and Locomotion. Front. Physiol. 2017, 8, 164.
- Laurencin, M.; Legrand, B.; Duval, E.; Henry, J.; Baudy-Floc’h, M.; Zatylny-Gaudin, C.; Bondon, A. From a Marine Neuropeptide to Antimicrobial Pseudopeptides Containing Aza–β3–Amino Acids: Structure and Activity. J. Med. Chem. 2012, 55, 2025–2034.
- Houyvet, B.; Zanuttini, B.; Corre, E.; Le Corguille, G.; Henry, J.; Zatylny-Gaudin, C. Design of antimicrobial peptides from a cuttlefish database. Amino Acids 2018, 50, 1573–1582.
- Destoumieux–Garzón, D.; Rosa, R.D.; Schmitt, P.; Barreto, C.; Vidal–Dupiol, J.; Mitta, G.; Gueguen, Y.; Bachère, E. Antimicrobial peptides in marine invertebrate health and disease. Philos. Trans. R Soc. Lond. B Biol. Sci. 2016, 371.
- Di Cosmo, A.; Polese, G. Neuroendocrine-Immune Systems Response to Environmental Stressors in the Cephalopod Octopus vulgaris. Front. Physiol. 2016, 7, 434.
- Gestal, C.; Castellanos-Martinez, S. Understanding the cephalopod immune system based on functional and molecular evidence. Fish. Shellfish Immunol. 2015, 46, 120–130.
- Rodríguez, J.P.; Beard, T.D.; Bennett, E.M.; Cumming, G.S.; Cork, S.J.; Agard, J.; Dobson, A.P.; Peterson, G.D. Trade-offs across space, time, and ecosystem services. Ecol. Soc. 2006, 11, 28.
- Castillo, M.G.; Salazar, K.A.; Joffe, N.R. The immune response of cephalopods from head to foot. Fish. Shellfish Immunol. 2015, 46, 145–160.
- Troncone, L.; De Lisa, E.; Bertapelle, C.; Porcellini, A.; Laccetti, P.; Polese, G.; Di Cosmo, A. Morphofunctional characterization and antibacterial activity of haemocytes from Octopus vulgaris. J. Nat. Hist. 2015, 49, 1457–1475.
- Warr, G.W. Immunity in invertebrates. J. Invertebr. Pathol. 1981, 38, 311–314.
- Marchalonis, J.J. Immunity in Evolution; Harvard University Press: Cambridge, MA, USA, 1977.
- Iwanaga, S.; Lee, B.L. Recent advances in the innate immunity of invertebrate animals. J. Biochem. Mol. Biol. 2005, 38, 128–150.
- Kondo, M.; Tomonaga, S. Morphology of Octopus haemocytes. J. Nat. Fish. Univ. 2003, 51, 157–164.
- Bidder, A.; Boletzky, M.; Boletzky, S.; Hochberg, F.; Mangold, K.; Marthy, H.-J.; Portmann, A.; Teichert, C.; Worms, J. Cephalopodes; Masson: Paris, France, 1989; Vol. V.
- Novoa, B.; Tafalla, C.; Guerra, A.; Figueras, A. Cellular immunological parameters of the octopus, Octopus vulgaris. J. Shellfish Res. 2002, 21, 243–248.
- Fisher, W.; Dinuzzo, A. Agglutination of bacteria and erythrocytes by serum from 6 species of marine mollusks. J. Invertebr. Pathol. 1991, 57, 380–394.
- Rogener, W.; Renwrantz, L.; Uhlenbruck, G. Isolation and characterization of a lectin from the hemolymph of the cephalopod Octopus vulgaris (Lam.) inhibited by alpha-D–lactose and N–acetyl–lactosamine. Dev. Comp. Immunol. 1985, 9, 605–616.
- Thøgersen, I.B.; Salvesen, G.; Brucato, F.H.; Pizzo, S.V.; Enghild, J.J. Purification and characterization of an alpha–macroglobulin proteinase inhibitor from the mollusc Octopus vulgaris. Biochem. J. 1992, 285, 521–527.
- Rajanbabu, V.; Chen, J.Y. Applications of antimicrobial peptides from fish and perspectives for the future. Peptides 2011, 32, 415–420.
- Ismail, M.; Riad, R. Screening the Antimicrobial Activity of Different Sepia officinalis (Cephalopoda: Sepioidea) Parts Collected from Alexandria Mediterranean Waters, Egypt Against Some Human Pathogens. Singap. J. Sci. Res. 2018, 8, 1–7.
- Derby, C.D. Cephalopod ink: Production, chemistry, functions and applications. Mar. Drugs 2014, 12, 2700–2730.
- Besednova, N.N.; Kovalev, N.N.; Zaporozhets, T.S.; Kuznetsova, T.A.; Gazha, A.K. Cephalopods as a Source of New Antimicrobial Substances. Antibiot. Khimioter. 2016, 61, 32–42.
- Besednova, N.N.; Zaporozhets, T.S.; Kovalev, N.N.; Makarenkova, I.D.; Yakovlev, Y.M. Cephalopods: The potential for their use in medicine. Russ. J. Mar. Biol. 2017, 43, 101–110.
- Monolisha, S.; Aswathi, E.; Patterson, J.; Patterson, J. Molecular characterization and antimicrobial activity of Octopus aegina and Octopus dolfusii in Gulf of Mannar Coast. Int. J. Pharm. Sci. Res. 2013, 4, 3582–3587.
- Gogineni, V.; Hamann, M.T. Marine natural product peptides with therapeutic potential: Chemistry, biosynthesis, and pharmacology. Biochim. Biophys. Acta Gen. Subj. 2018, 1862, 81–196.
- Falanga, A.; Lombardi, L.; Franci, G.; Vitiello, M.; Iovene, M.R.; Morelli, G.; Galdiero, M.; Galdiero, S. Marine Antimicrobial Peptides: Nature Provides Templates for the Design of Novel Compounds against Pathogenic Bacteria. Int. J. Mol. Sci. 2016, 17, 785.
- Challinor, V.L.; Bode, H.B. Bioactive natural products from novel microbial sources. Ann. N. Y. Acad. Sci. 2015, 1354, 82–97.
- Cheung, R.C.; Ng, T.B.; Wong, J.H. Marine Peptides: Bioactivities and Applications. Mar. Drugs 2015, 13, 4006–4043.
- Wittine, K.; Saftić, L.; Peršurić, Ž.; Kraljević Pavelić, S. Novel Antiretroviral Structures from Marine Organisms. Molecules 2019, 24, 3486.
- Prasasty, V.; Radifar, M.; Istyastono, E. Natural Peptides in Drug Discovery Targeting Acetylcholinesterase. Molecules 2018, 23, 2344.
- Phyo, Y.Z.; Ribeiro, J.; Fernandes, C.; Kijjoa, A.; Pinto, M.M.M. Marine Natural Peptides: Determination of Absolute Configuration Using Liquid Chromatography Methods and Evaluation of Bioactivities. Molecules 2018, 23, 306.
- Semreen, M.H.; El-Gamal, M.I.; Abdin, S.; Alkhazraji, H.; Kamal, L.; Hammad, S.; El-Awady, F.; Waleed, D.; Kourbaj, L. Recent updates of marine antimicrobial peptides. Saudi Pharm. J. 2018, 26, 396–409.
- Zannella, C.; Mosca, F.; Mariani, F.; Franci, G.; Folliero, V.; Galdiero, M.; Tiscar, P.G.; Galdiero, M. Microbial diseases of bivalve mollusks: Infections, immunology and antimicrobial defense. Mar. drugs 2017, 15, 182.
- Gerdol, M.; De Moro, G.; Manfrin, C.; Venier, P.; Pallavicini, A. Big defensins and mytimacins, new AMP families of the Mediterranean mussel Mytilus galloprovincialis. Dev. Comp. Immunol. 2012, 36, 390–399.
- Bachère, E.; Gueguen, Y.; Gonzalez, M.; de Lorgeril, J.; Garnier, J.; Romestand, B. Insights into the anti- microbial defense of marine invertebrates: The penaeid shrimps and the oyster Crassostrea gigas. Immunol. Rev. 2004, 198, 149–168.
- De Zoysa, M.; Whang, I.; Lee, Y.; Lee, S.; Lee, J.-S.; Lee, J. Defensin from disk abalone Haliotis discus discus: Molecular cloning, sequence characterization and immune response against bacterial infection. Fish. Shellfish Immunol. 2010, 28, 261–266.
- Dolashka, P.; Moshtanska, V.; Borisova, V.; Dolashki, A.; Stevanovic, S.; Dimanov, T.; Voelter, W. Antimicrobial proline-rich peptides from the hemolymph of marine snail Rapana venosa. Peptides 2011, 32, 1477–1483.
- Cornet, V.; Henry, J.; Goux, D.; Duval, E.; Bernay, B.; Le Corguillé, G.; Corre, E.; Zatylny–Gaudin, C. How Egg Case Proteins Can Protect Cuttlefish Offspring? PLoS ONE 2015, 10, e0132836.
- Kremer, N.; Schwartzman, J.; Augustin, R.; Zhou, L.; Ruby, E.; Hourdez, S.; McFall-Ngai, M. The dual nature of haemocyanin in the establishment and persistence of the squid—vibrio symbiosis the dual nature of haemocyanin in the establishment and persistence of the squid—vibrio symbiosis. Proc. R Soc. Biol. Sci. 2014, 281.
- Kang, H.K.; Lee, H.H.; Seo, C.H.; Park, Y. Antimicrobial and Immunomodulatory Properties and Applications of Marine-Derived Proteins and Peptides. Mar. Drugs 2019, 17, 350.
- Arena, S.; Renzone, G.; Scaloni, A. A multi–approach peptidomic analysis of hen egg white reveals novel putative bioactive molecules. J. Proteom. 2020, 215.
- Arena, S.; Scaloni, A. An Extensive Description of the Peptidomic Repertoire of the Hen Egg Yolk Plasma. J. Agric. Food Chem. 2018, 66, 3239–3255.
- Rai, M.; Pandit, R.; Gaikwad, S.; Kövics, G. Antimicrobial peptides as natural bio–preservative to enhance the shelf–life of food. J. Food Sci. Technol. 2016, 53, 3381–3394.
- Nikapitiya, C.; Dananjaya, S.H.S.; Chandrarathna, H.; De Zoysa, M.; Whang, I. Octominin: A Novel Synthetic Anticandidal Peptide Derived from Defense Protein of Octopus minor. Mar. Drugs 2020, 18, 56.
- Sanjeewa, K.K.A.; Nagahawatta, D.P.; Yang, H.-W.; Oh, J.Y.; Jayawardena, T.U.; Jeon, Y.-J.; De Zoysa, M.; Whang, I.; Ryu, B. Octominin Inhibits LPS-Induced Chemokine and Pro–inflammatory Cytokine Secretion from RAW 264.7 Macrophages via Blocking TLRs/NF-κB Signal Transduction. Biomolecules 2020, 10, 511.
- Hu, M.Y.; Yan, H.Y.; Chung, W.S.; Shiao, J.C.; Hwang, P.P. Acoustically evoked potentials in two cephalopods inferred using the auditory brainstem response (ABR) approach. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2009, 153, 278–283.
- Baik, S.; Kim, D.W.; Park, Y.; Lee, T.J.; Ho Bhang, S.; Pang, C. A wet–tolerant adhesive patch inspired by protuberances in suction cups of octopi. Nature 2017, 546, 396–400.
- Cianchetti, M.; Calisti, M.; Margheri, L.; Kuba, M.; Laschi, C. Bioinspired locomotion and grasping in water: The soft eight–arm OCTOPUS robot. Bioinspiration Biomim. 2015, 10, 035003.
- Laschi, C.; Cianchetti, M.; Mazzolai, B.; Margheri, L.; Follador, M.; Dario, P. Soft Robot Arm Inspired by the Octopus. Adv. Robot. 2012, 26, 709–727.
- Yekutieli, Y.; Sagiv–Zohar, R.; Aharonov, R.; Engel, Y.; Hochner, B.; Flash, T. Dynamic model of the octopus arm. I. Biomechanics of the octopus reaching movement. J. Neurophysiol. 2005, 94, 1443–1458.
- Graziadei, P.; Young, J.Z. The nervous system of the arms. In The Anatomy of the Nervous System of Octopus Vulgaris; Clarendon Press: Oxford, UK, 1971.
- Graziadei, P.P.C.; Gagne, H.T. Neural components in octopus sucker. J. Cell Biol. 1973, 59, A21–A121.
- Packard, A. The skin of cephalopods (Coleoids): General and special adaptations. In The Mollusca, Form and Function; Trueman, E.R., Clarke, M.R., Eds.; Academic Press Inc.: Berkeley, CA, USA, 1988; Volume 11, pp. 37–67.
- Maselli, V.; Al–Soudy, A.S.; Buglione, M.; Aria, M.; Polese, G.; Di Cosmo, A. Sensorial Hierarchy in Octopus vulgaris’s Food Choice: Chemical vs. Visual. Animals 2020, 10, 457.
- Kier, W.M.; Stella, M.P. The arrangement and function of octopus arm musculature and connective tissue. J. Morphol. 2007, 268, 831–843.
- Kier, W.M.; Smith, A.M. The Structure and Adhesive Mechanism of Octopus Suckers1. Integr. Comp. Biol. 2002, 42, 1146–1153.
- Stanley, D.W.; Hultin, H.O. Protoelytic Activity in North American Squid and Its Relation to Quality. Can. Inst. Food Sci. Technol. J. 1984, 17, 163–167.
- Sakai, J.; Matsumoto, J.J. Proteolytic enzymes of squid mantle muscle. Comp. Biochem. Physiol. Part B Comp. Biochem. 1981, 68, 389–395.
- Hurtado, J.L.; Borderìas, J.; Montero, P.; An, H. Characterization of proteolytic activity in octopus (Octopus vulgaris) arm muscle. J. Food Biochem. 1999, 23, 469–483.
- Hurtado, J.L.; Montero, P.; Borderías, J.; An, H. Properties of Proteolytic Enzymes from Muscle of Octopus (Octopus vulgaris) and Effects of High Hydrostatic Pressure. J. Food Sci. 2002, 67, 2555–2564.
- Uribe, R.; Jay, D. A review of actin binding proteins: New perspectives. Mol. Biol. Rep. 2009, 36, 121–125.
- Galdiero, E.; de Alteriis, E.; De Natale, A.; D’Alterio, A.; Siciliano, A.; Guida, M.; Lombardi, L.; Falanga, A.; Galdiero, S. Eradication of Candida albicans persister cell biofilm by the membranotropic peptide gH625. Sci. Rep. 2020, 10, 5780.
- Park, S.C.; Lee, M.Y.; Kim, J.Y.; Kim, H.; Jung, M.; Shin, M.K.; Lee, W.K.; Cheong, G.W.; Lee, J.R.; Jang, M.K. Anti-Biofilm Effects of Synthetic Antimicrobial Peptides Against Drug–Resistant Pseudomonas aeruginosa and Staphylococcus aureus Planktonic Cells and Biofilm. Molecules 2019, 24, 4560.
- Roy, R.; Tiwari, M.; Donelli, G.; Tiwari, V. Strategies for combating bacterial biofilms: A focus on anti-biofilm agents and their mechanisms of action. Virulence 2018, 9, 522–554.
- Neundorf, I. Antimicrobial Peptides: Basics for Clinical Application; Springer: Singapore, 2019.
- Faustino, M.; Veiga, M.; Sousa, P.; Costa, E.M.; Silva, S.; Pintado, M. Agro–Food Byproducts as a New Source of Natural Food Additives. Molecules 2019, 24, 1056.
- Zhang, C.; Xue, Z.; Yu, Z.; Wang, H.; Liu, Y.; Li, H.; Wang, L.; Li, C.; Song, L. A tandem-repeat galectin–1 from Apostichopus japonicus with broad PAMP recognition pattern and antibacterial activity. Fish. Shellfish Immunol. 2020, 99, 167–175.
- Tazato, S.; Conlon, J.M.; Iwamuro, S. Cloning and expression of genes enocoding antimicrobial peptides and bradykinin from the skin and brain of Oki Tago’s brown frog, Rana tagoi okiensis. Peptides 2010, 31, 1480–1487.
- König, E.; Zhou, M.; Wang, L.; Chen, T.; Bininda-Emonds, O.R.; Shaw, C. Antimicrobial peptides and alytesin are co–secreted from the venom of the Midwife toad, Alytes maurus (Alytidae, Anura): Implications for the evolution of frog skin defensive secretions. Toxicon 2012, 60, 967–981.
- Rončević, T.; Gajski, G.; Ilić, N.; Goić-Barišić, I.; Tonkić, M.; Zoranić, L.; Simunić, J.; Benincasa, M.; Mijaković, M.; Tossi, A.; et al. PGLa-H tandem-repeat peptides active against multidrug resistant clinical bacterial isolates. Biochim. Biophys. Acta Biomembr. 2017, 1859, 228–237.
- Batham, E.J. Care of eggs by Octopus maorum. Trans. R. Soc. N. Z. 1957, 84, 629–638.
- Robison, B.; Seibel, B.; Drazen, J. Deep-Sea Octopus (Graneledone boreopacifica) Conducts the Longest-Known Egg-Brooding Period of Any Animal. PLoS ONE 2014, 9, e103437.
- Boletztky, S. Embryonic development of cephalopods at low temperatures. Antarct. Sci. 1994, 6, 139–142.
- Wodinsky, J. Hormonal Inhibition of Feeding and Death in Octopus: Control by Optic Gland Secretion. Science 1977, 198, 948.





