
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Agnieszka Dettlaff-Pokora | + 1531 word(s) | 1531 | 2021-05-08 12:10:09 | | | |
| 2 | Rita Xu | Meta information modification | 1531 | 2021-05-13 05:51:45 | | |
Video Upload Options
SARS-CoV-2 impairs the renin-angiotensin-aledosterone system via binding ACE2 enzyme. ACE2 plays a key role in the biosynthesis of angiotensin (1-7), catalyzing the conversion of angiotensin 2 into angiotensin (1-7) and the reaction of angiotensin synthesis (1-9), from which angiotensin is (1-7) produced under the influence of ACE (Angiotensin-Converting Enzyme).
1. Introduction
SARS-CoV-2 impairs the renin-angiotensin-aledosterone system via binding ACE2 enzyme. ACE2 (Angiotensin-Converting Enzyme 2) is an enzyme that exists in two forms. The dominant form is the long form of the enzyme, which is found on the cell membranes of cells in many organs. Low levels of cell free ACE2 activity was also found in the blood, urine and human cerebrospinal fluid. This form of the enzyme is called the short or soluble form. It is formed from the long outer membrane in a process catalyzed by enzymes from the group of metalloproteinases. ACE2 plays a key role in the biosynthesis of angiotensin (1-7), catalyzing the conversion of angiotensin 2 into angiotensin (1-7) and the reaction of angiotensin synthesis (1-9), from which angiotensin (1-7) is produced under the influence of ACE (Angiotensin-Converting Enzyme). ACE2, unlike ACE, is not inhibited by ACE inhibitors at the doses used in humans during the treatment of arterial hypertension. Membrane ACE2 is one of the receptors that allows SARS-CoV-2 (Severe Acute Respiratory Syndrome Coronavirus 2) to enter the host cells, and consequently the multiplication of the virus and the progression of COVID-19. Binding of SARS-CoV-2 to membrane ACE2 causes internalization and degradation of the enzyme and, consequently, reduction of its activity. This may increase the concentration of angiotensin 2 and decrease the concentration of angiotensin (1-7). This, in turn, can increase the damage to the lungs (and other organs). The soluble form of ACE2 present in the blood also binds SARS-CoV-2. Theoretically, this could prevent the virus from entering the cells and thus prevent it from multiplying. The presence of ACE2 on the cell membranes of cells in organs suggests that these organs may potentially be vulnerable to damage during SARS-CoV-2 infection. Few, sometimes controversial, results from experimental animals and clinical observations suggest that ACE inhibitors and AT1R blockers [1][2][3][4] may induce ACE2 in some organs and, consequently, facilitate virus entry into cells and its multiplication [5][6][7][8]. On this basis, it has been hypothesized that the use of ACE inhibitors and AT1R blockers in the treatment of arterial hypertension during the COVID-19 pandemic may make the patient more susceptible to SARS-CoV-2 infection, and the course of COVID-19 may be more severe. To date, however, there is no scientific evidence that ACE inhibitors or AT1R blockers increase the risk of infection and adversely affect the course of the disease. In conclusion, the research results available so far indicate that SARS-CoV-2 infection is one of the factors that may lead to dysregulation of the RAA system. The clinical consequences of this process require further clinical observations and experimental studies.
The classical system of renin-angiotensin-aldosterone (RAA) is widely recognized as the main regulator of blood pressure and the water and electrolyte balance of the human body. Renin plays an essential role in the activation of this system. Renin, also called angiotensingenase (EC 3.4.23.15), is synthesized as preprorenin which is transformed post-translational into prorenin. This, in turn, is converted to biologically active renin and is secreted by the glomerular cells of the glomerular apparatus. The process of synthesis and secretion of renin is very precisely regulated [9]. Renin catalyzes the hydrolytic detachment of the N-terminus of angiotensinogen (a protein formed mainly in the liver, composed of about 400 amino acids), a decapeptide called angiotensin 1. Then, after the hydrolytic detachment of two amino acids (-histidine-leucine-COOH) from the C-terminus of angiotensin 1, angiotensin 2 is formed-an oligopeptide composed of 8 amino acids (octapeptide), with a strong pressor effect. The process of angiotensin 2 synthesis is catalyzed by the Angiotensin-Converting Enzyme (ACE; EC 3.4.15.1). This enzyme is found in the cell membranes of the vascular endothelium, renal tubular cells (mainly the proximal tubules) and neuroepithelial cells [9].
ACE also catalyzes reactions in which the substrates are other oligopeptides involved directly or indirectly in the regulation of blood pressure, such as bradykinin and kallidin. Bradykinin is a nine amino acid oligopeptide that dilates blood vessels, including the coronary vessels. Kallidin is an oligopeptide of ten amino acids that is proteolyzed into bradykinin and lysine. The effect of ACE on bradykinin is its inactivation, e.g., loss of the ability to dilate blood vessels [10][11]. It follows that the higher the ACE activity, the greater the angiotensin 2 level leading to decreased bradykinin and vessels don’t dilate.
Substrates for ACE are also other oligopeptides, such as angiotensin (1-9) or angiotensin (1-7), oligopeptides related directly or indirectly to the regulation of blood pressure and other important physiological processes.
Angiotensin 2 produced by an ACE-catalyzed reaction binds to the angiotensin 2 receptor (AT1R), which is present mainly in the cell membranes of cells of the cardiovascular system, kidneys, adrenal cortex and the sympathetic nervous system. Binding of angiotensin 2 to AT1R present in the cell membranes of the cardiovascular system causes vasoconstriction. Binding to the AT1R receptor present in renal tubular cells causes an increase in sodium reabsorption in nephrons, which also leads to an increase in blood pressure and blood volume. The binding of angiotensin 2 with AT1R present in the cells of the glomerular layer of the adrenal cortex leads to the stimulation of the activity of aldosterone synthase, which catalyzes the synthesis of aldosterone from deoxycorticosterone according to a simplified scheme: deoxycorticosterone → corticosterone → 18-hydroxycorticosterone → aldosterone. Aldosterone is a major water-electrolyte balance controlling hormone controlling sodium reabsorption in distal nephron. The processes discussed above are presented in Figure 1.
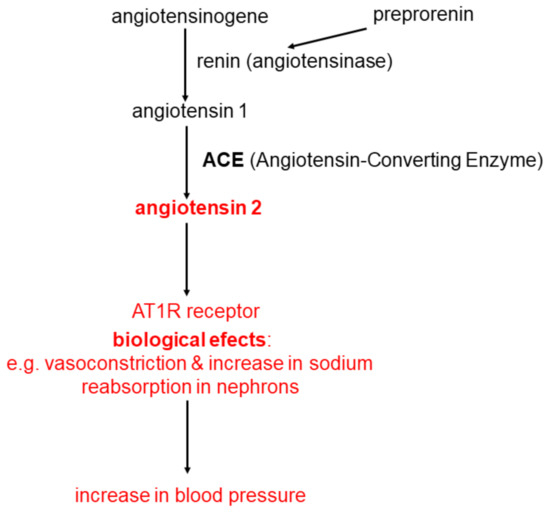
Figure 1. The classical system of renin-angiotensin-aldosterone (RAA).
2. Angiotensin Metabolism
In the last dozen or so years, the classic RAA system has been expanded with new elements, of which the most important from a clinical point of view seems to be the Angiotensin-Converting Enzyme 2 (ACE2). ACE2 owes its name to the high degree of structural homology with ACE. The structure of ACE2 is approximately 40% identical and approximately 60% similar to ACE [12]. ACE2 has an intrinsic enzymatic function and the function of a receptor that binds SARS-CoV and SARS-CoV-2. It occurs mainly on the cell membrane of cells in organs, such as: heart, blood vessel endothelial cells, renal tubular cells, intestine, testes, oral and nasal mucosa, cornea, oesophagus, stomach, liver, fat tissue and lungs (type 2 pneumocytes and macrophages), however, the expression level of ACE2 varies widely [13][14][15][16]. This suggests that many organs have the potential to be potentially damaged during SARS-CoV-2 infection. This is in line with clinical observations that multiple organs may be damaged in patients infected with the SARS-CoV-2 virus.
ACE2 is a carboxypeptidase that catalyzes the hydrolysis of a wide variety of oligopeptides. It seems that in the human body the most important reactions catalyzed by ACE2 [17][18] are the transformation of:
-
angiotensin 2 to angiotensin (1-7) according to the Equation:
angiotensin 2 + H2O → angiotensin (1-7) + phenylalanine -
angiotenisin 1 to angiotensin (1-9) according to the Equation:
angiotensin 1 + H2O → angiotensin (1-9) + leucine
Formed in the reaction angiotensin (1-9), whose biological function has not yet been elucidated, may be further converted to angiotensin (1-7) by ACE. From the above data it appears that ACE 2 plays an important role in the synthesis of angiotensin (1-7) catalyzing: (a) mainly the conversion of angiotensin 2 to angiotensin (1-7) and (b) the conversion of angiotensin 1 to angiotensin (1-9), which is converted into angiotensin (1-7) by ACE.
ACE2, converting angiotensin 2 to angiotensin (1-7), plays an important role in regulation of angiotensin 2 level (causes a decrease) and angiotensin (1-7) (causes an increase). Angiotensin (1-7) is predominantly degraded (inactivated) by ACE reaction:
It is evident that ACE and ACE2 play key role in angiotensin level regulation (key role of ACE in angiotensin 2 synthesis and ACE2 in degradation). Thanks to significant parts of ACE2 in angiotensin 2 degradation, ACE2 is called negative regulator od RAA system [19].
ACE is blocked by inhibitors used in hypertension treatment. In similar dosages ACE2 is not sensitive to these pharmaceuticals (ACE inhibitors do not inhibit ACE2) [20].
Molecular mechanism of angiotensin (1-7) action is connected with Mitochondrial assembly Receptor (MasR). On Figure 2 formation and action of angiotensin (1-7) was shown, together with comparison with formation and action of angiotensin 2. In general angiotensin 2 and angiotensin (1-7) actions are contrary: (a) angiotensin 2 is a vasoconstrictor, while angiotensin (1-7) is a vasodilator; (b) angiotensin 2 is proinflammatory, angiotensin (1-7) anti-inflammatory; (c) angiotensin 2 induces organ fibrosis, angiotensin (1-7) inhibits it; (d) angiotensin 2 is prooxidative, angiotensin (1-7) antioxidative; angiotensin 2 stimulates proliferation, angiotensin (1-7) inhibits it; angiotensin 2 is prothrombotic, angiotensin (1-7) anticoagulatory [21][22]. In general angiotensin (1-7) has a beneficial effect (is a protective for organs oligopeptide), while angiotensin 2 is in destructive one, having negative effect on the human body. The physiological state of equilibrium between ACE and ACE2 activities determines the correct level of angiotensin 2 and angiotensin (1-7). This, in turn, determines the proper functions of the human body. Any disturbance in the activity of these enzymes can lead to serious disturbances in the angiotensin 2/angiotensin (1-7) ratio in the human body, with consequent disturbances in the function of many organs.
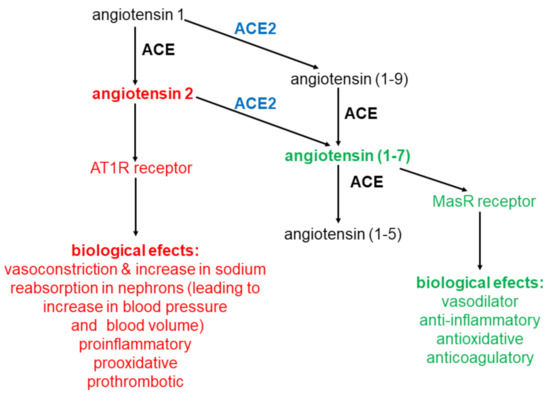
Figure 2. Angiotensins conversions. Metabolism of angiotensins is controlled by a pair of enzymes ACE and ACE2.
References
- Zimmerman, B.G. Monoclonal antibodies and nonpeptide antagonists to angiotensin II. Potential implications. Hypertension 1989, 14, 498–500.
- Kaur, H.; Bruno, J.G.; Kumar, A.; Sharma, T.K. Aptamers in the Therapeutics and Diagnostics Pipelines. Theranostics 2018, 8, 4016–4032.
- Schmitz, A.; Weber, A.; Bayin, M.; Breuers, S.; Fieberg, V.; Famulok, M.; Mayer, G. A SARS-CoV-2 Spike Binding DNA Aptamer that Inhibits Pseudovirus Infection by an RBD-Independent Mechanism. Angew. Chem. Int. Ed. 2021.
- Dhinan, A. Applications of Aptasensors in Health Care. Mater. Res. Found. 2019, 47, 1–50.
- Fang, L.; Karakiulakis, G.; Roth, M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir. Med. 2020, 8, e21.
- South, A.M.; Tomlinson, L.; Edmonston, D.; Hiremath, S.; Sparks, M.A. Controversies of renin–angiotensin system inhibition during the COVID-19 pandemic. Nat. Rev. Nephrol. 2020, 16, 305–307.
- Burrell, L.M.; Risvanis, J.; Kubota, E.; Dean, R.G.; Macdonald, P.S.; Lu, S.; Tikellis, C.; Grant, S.L.; Lew, R.A.; Smith, A.I.; et al. Myocardial infarction increases ACE2 expression in rat and humans. Eur. Heart J. 2005, 26, 369–375.
- Ramchand, J.; Patel, S.K.; Kearney, L.G.; Matalanis, G.; Farouque, O.; Srivastava, P.M.; Burrell, L.M. Plasma ACE2 Activity Predicts Mortality in Aortic Stenosis and Is Associated With Severe Myocardial Fibrosis. JACC Cardiovasc. Imaging 2020, 13, 655–664.
- Patel, S.; Rauf, A.; Khan, H.; Abu-Izneid, T. Renin-angiotensin-aldosterone (RAAS): The ubiquitous system for homeostasis and pathologies. Biomed. Pharmacother. 2017, 94, 317–325.
- Carey, R.M.; Siragy, H.M. Newly recognized components of the renin-angiotensin system: Potential roles in cardiovascular and renal regulation. Endocr. Rev. 2003, 24, 261–271.
- Atlas, S.A. The Renin-Angiotensin Aldosterone System: Pathophysiological Role and Pharmacologic Inhibition. J. Manag. Care Pharm. 2007, 13, 9–20.
- Tipnis, S.R.; Hooper, N.M.; Hyde, R.; Karran, E.; Christie, G.; Turner, A.J. A Human Homolog of Angiotensin-converting Enzyme cloning and functional expression as a captopril-insensitive carboxypeptidase. J. Biol. Chem. 2000, 275, 33238–33243.
- Serfozo, P.; Wysocki, J.; Gulua, G.; Schulze, A.; Ye, M.; Liu, P.; Jin, J.; Bader, M.; Myöhänen, T.; García-Horsman, J.A.; et al. Ang II (Angiotensin II) Conversion to Angiotensin-(1-7) in the Circulation Is POP (Prolyloligopeptidase)-Dependent and ACE2 (Angiotensin-Converting Enzyme 2)-Independent. Hypertension 2020, 75, 173–182.
- Sungnak, W.; Huang, N.; Bécavin, C.; Berg, M.; Queen, R.; Litvinukova, M.; Talavera-López, C.; Maatz, H.; Reichart, D.; Sampaziotis, F.; et al. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat. Med. 2020, 26, 681–687.
- Hamming, V.; Timens, W.; Bulthuis, M.L.C.; Lely, A.T.; Navis, G.J.; Van Goor, H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004, 203, 631–637.
- Lely, A.T.; Hamming, V.; Van Goor, H.; Navis, G.J. Renal ACE2 expression in human kidney disease. J. Pathol. 2004, 204, 587–593.
- Batlle, D.; Wysocki, J.; Soler, M.J.; Ranganath, K. Angiotensin-converting enzyme 2: Enhancing the degradation of angiotensin II as a potential therapy for diabetic nephropathy. Kidney Int. 2012, 81, 520–528.
- Ferrario, C.M.; Chappell, M.C.; Tallant, E.A.; Brosnihan, K.B.; Diz, D.I. Counterregulatory Actions of Angiotensin-(1-7). Hypertension 1997, 30, 535–541.
- Vickers, C.; Hales, P.; Kaushik, V.; Dick, L.; Gavin, J.; Tang, J.; Godbout, K.; Parsons, T.; Baronas, E.; Hsieh, F.; et al. Hydrolysis of Biological Peptides by Human Angiotensin-converting Enzyme-related Carboxypeptidase. J. Biol. Chem. 2002, 277, 14838–14843.
- Donoghue, M.; Hsieh, F.; Baronas, E.; Godbout, K.; Gosselin, M.; Stagliano, N.; Donovan, M.; Woolf, B.; Robison, K.; Jeyaseelan, R.; et al. A Novel Angiotensin-Converting Enzyme–Related Carboxypeptidase (ACE2) Converts Angiotensin I to Angiotensin 1-9. Circ. Res. 2000, 87, E1–E9.
- Ferrario, C.M. ACE2: More of Ang-(1–7) or less Ang II? Curr. Opin. Nephrol. Hypertens. 2011, 20, 1–6.
- Zhang, H.; Penninger, J.M.; Li, Y.; Zhong, N.; Slutsky, A.S. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: Molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020, 46, 586–590.




