
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Marlene Kienberger | + 2788 word(s) | 2788 | 2021-05-12 11:17:02 | | | |
| 2 | Rita Xu | Meta information modification | 2788 | 2021-05-12 11:57:06 | | |
Video Upload Options
After cellulose, lignin is the second most abundant biopolymer worldwide. Lignins are non-toxic and renewable, and hence may play an essential role during the change-over from a fossil-based to a bio-based economy. Lignin isolation from Kraft cooking is state of the art, the three state of the art processes are summarized and discussed, further the concept of sequerntial liquid lignin recovery is introduced.
1. Introduction
After cellulose, lignin is the second most abundant biopolymer worldwide. Lignins are non-toxic and renewable, and hence may play an essential role during the change-over from a fossil-based to a bio-based economy. The value-added application of technical lignin not only protects the environment, but also makes use of the natural resource wood, and hence promotes its utilization. Technical lignins can widely be used, e.g., for vanillin production [1][2][3], as precursor for carbon fiber production [4][5][6][7][8] or as fertilizer [9][10][11]. Further, the valorization in the formulation of wood adhesives is well a successful application [12][13][14][15].
The pulp and paper industry can serve as a backbone in the biorefinery concept and can provide the market with both products and energy, where lignin is used as raw material. In a state of the art integrated pulp and paper mill, the produced energy is first used for covering internal demands. If available, power surpluses are fed to the electricity grid and heat surpluses are used in district heating for the surroundings. However, worldwide is the pulp and paper sector the fourth largest energy user [16], 2008 the energy demand in the pulp and paper industry was 7 EJ [17].
The bottleneck in an integrated pulp and paper mill is often the recovery boiler. An increase in the pulp production leads to an increase of the heat load which cannot be handled by the installed recovery boilers. However, an increased production can be realized without the need for investing in a new boiler by implementing a lignin isolation from black or spent sulfite liquor to decrease the heat value thereof. In addition to the decrease of the heating value of the black liquor, economic considerations are undoubtedly a main driving force for lignin isolation [18]. While industrial applications for Kraft lignin are rare at present, the market for lignosulfonates is well established; however, most of the products and applications on the market are not high value products/applications.
Lignin isolation from spent or black liquor is a process that has been in operation since the beginning of the 20th century. Driven by climate change, which requires a transition to more sustainable production processes and products, an increasing interest in the use of lignin as a raw material for products, fuel and energy led to a distinct increase of research in this field during the past 20 years. The development of new lignin isolation processes from Kraft pulping is in the focus of the research community, which is mainly related to the incentive of a large market share reaching approximately 80% [19]. At present, three different isolation processes are used on an industrial scale for the isolation of lignin from Kraft pulping: the WestVaco process, the LignoBoost process, and the LignoForce SystemTM. Rising interest in lignin valorization leads to the necessity of an overview of processes, which are on the marked or at least ready for the market.
The isolation of lignosulfonates from spent sulfite liquor is widely implemented, but little research has been published dealing with process development for the isolation of lignosulfonates during recent decades.
The present paper gives an overview of isolation technologies for technical lignin. Solubility and appearance of technical lignin is highly influenced by the origin and the dissolving method used for lignin disintegration. While during Kraft and sulfite cooking lignin is already modified, and also lignin derived from organosolv processes appears not in its natural form, but is not that highly influenced by the cooking process.
The three state of the art processes for Kraft lignin separation will be summarized to provide a focus of the main part of the paper, and the similarities and differences, which make each process unique are discussed. Kraft lignin precipitation and separation can be understood by the principles of solubility and by the colloidal stability of precipitated lignin. Process conditions during precipitation like pH, temperature, black liquor composition, hydrodynamics and the acidifying agent used influence the resulting lignin. The operation of the acidification step at higher pressures and temperatures leads to a liquid remnant lignin, the Sequential Liquid-Lignin Recovery and Purification process (SLRP), proposes and claims such operation conditions. In this review, the three new processes for Kraft lignin isolation, LignoBoost, LignoForce SystemTM, and SLRP are compared based on the product quality, i.e., the ash content which corresponds to the content of inorganic impurities. Based on literature data the lignin quality was calculated assuming a constant wash water demand.
While Kraft lignin is mainly used internally, lignosulfonates do have a market already. From the annually 50–70 M tons produced lignin, just 1–2% are used for the production of added-value products. Lignosulfonates have more sulfur groups than Kraft lignin and thus are water soluble, which lead to a broad variety of products for lignosulfonates, like animal feed, dust controlling agent, pesticides and more [20].
2. State of the Art Isolation Processes for Kraft Lignin
The annual pulp production from the Kraft process worldwide amounts to ~180 M tons, thereby ~55 M tons of lignin are dissolved into the black liquor [21]. The chemical recovery cycle uses lignin as fuel for the recovery boiler, which makes it the most important bio-fuel for the process. The generated heat is first used on site for steam production, which is used, e.g., in the fiber line, and heat surpluses are used for electricity generation. Lignin derived during Kraft cooking is soluble at pH > 10, by decreasing the pH lignin precipitates. The isolation of lignin from black liquor can hence be easily done by acidification of the highly alkaline black liquor with any acidifying agent and is most commonly used in industrial lignin isolation [15]. In literature, H2SO4 and CO2 are mainly discussed as acidifying agents because these two acids do not change the matrix of the black liquor [22].
Lignin molecules in the black liquor are assumed to be dissolved in a colloid-like form and the phenolic OH groups are weakly acidic. Hence, their dissociation can be described in dependence on the pH. At high pH, the negatively charged lignin molecules are associated with sodium as the counter ion. When the pH drops to the range of the pKa of the phenolic OH groups (pKa—10), their degree of protonation increases, while at the same time hydrophobic forces, such as the van der Waals forces, increase and the lignin molecules partially precipitate [23][24]. In general, isolated lignin is acidified well below the pKa of lignin. A pH below 2 ensures complete protonation of hydroxyl and carboxyl groups to form sodium free hydrogen lignate. It is worth bearing in mind that acidified and dried lignin is no longer soluble in aqueous solutions; hence, the process is irreversible [1]. In addition to precipitation, ultrafiltration and electrochemical methods can also be used to separate lignin from black liquor, but they are at present not of industrial relevance [13].
Figure 1 gives an overview of the lignin isolation processes on industrial and pilot scales. Blue indicates the longest running process, which is further called the WestVaco process. The two processes in green are the LignoBoost process and the LignoForce SystemTM, which both inserted one additional process step to enhance the filterability of precipitated lignin and the processing to pure lignin is claimed too. Those two processes are also operated on industrial scale. The fourth process is the SLRP process, where at present no industrial size application is known.
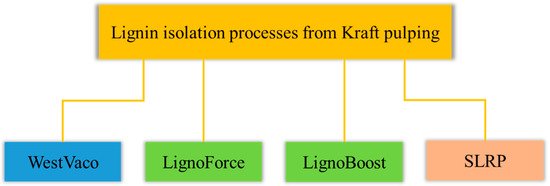
Figure 1. Overview of the lignin isolation processes on industrial and pilot scale.
All four processes use a mean black liquor with a dry substance content of 20–40% as a feed stream for the lignin precipitation. Furthermore, CO2 is used as acidifying agent in a first acidification step. The key advantage of using CO2 as acidifying agent is that the Na- and S-balance during chemical recovery is not disturbed [25]. The goal of a second acidifying step is to exchange sodium, bound to the lignin, by hydrogen leading to a high sodium recovery rate and to a lignin having a low ash content. SLRP, LignoBoost and LignoForce use sulfuric acid for this secondary acidification. The first patent for the WestVaco process does not make the washing procedure claim. In consecutive patents different procedures are named.
Maturing of the precipitated lignin promotes coagulation what improves its filterability and is implemented in all processes except the SLRP process. In the LignoBoost and LignoForce process, the maturing is followed by a filtration step using filter press equipment. The WestVaco process does not give any information about the filter equipment installed.
Kraft pulping uses NaOH and Na2S as cooking chemicals, which leads to a high sulfur load in the black liquor. One topic to be addressed when dealing with the lignin isolation from Kraft pulping by acidification is the release of toxic H2S and other odorous sulfur components, such as mercaptans, dimethyldisulfide, or dimethylsulfide.
2.1. The WestVaco Process—Indulin
Indulin lignin is the by-product of linerboard pulp. Kraft lignin was already derived by acidification of soap-free mean black liquor for the precipitation since the late 1940s. The process was patented by the West Virginia Pulp and Paper Company in 1949 and is still in use. Figure 2 shows the flow chart of the main patent of the WestVaco process [26].
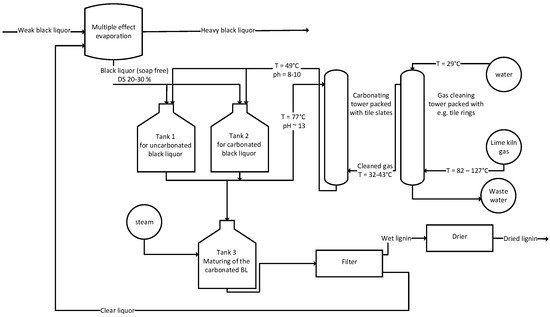
Figure 2. Schematic drawing of the WestVaco process.
Black liquor is extracted from the evaporation train with a solids content of 20–30 wt%. Tank 1 and 2 are operated alternatingly, either being used as tanks for the un-carbonated or for the already carbonated black liquor. A tank is needed for the acidification, which is performed with plant derived CO2 and more than one passing of the carbonating column is needed to obtain a pH of 9. The carbonating column is set up as packed column. To increase the filterability of the precipitated lignin, maturing is performed in tank 3 and the temperature of the carbonated liquor is increased to 71–93 °C. The preferred temperature mainly depends on the source of black liquor and the rate of heating. After heating, the black liquor is allowed to cool down to improve the filterability once again. The treated black liquor is then filtered and either dried or further processed [27]. Further processing of the lignin can involve acid hydrolysis to remove both, the hemicellulose and the sodium. The covalently bound sulfur originating from the digestion process remains on the lignin [28]. In 1960, Keilen et al. claimed a process for improved coagulation of the precipitated lignin and the transformation from the sodium-lignate into “free lignin”, which describes an ash-free lignin. The intention of this development is to improve the filtration properties and the recovery of sodium [29]. The further processing of either just precipitated or also cleaned lignin by spray drying is the last step in the production process of lignates and “free lignin” [30].
In a consecutive patent Ball and Vardell claimed a continuous process using sulfuric acid as acidifying agent in combination with a black liquor having a dry solids content of ~50 wt%. The process is operated at elevated temperature and pressure to be able to process the viscous black liquor. The main advantages of this development are the high yield and the fast coagulation of precipitated lignin [31].
In the 1950s and 1960s, the West Virginia Pulp and Paper Company built a large patent family claiming the whole process chain starting from black liquor to a washed, ash free lignin. The corresponding products were developed and claimed at the same time.
2.2. LignoBoost
The LignoBoost process was jointly developed during a project involving the Chalmers University of Technology and the Swedish research institute Innventia, which was launched in 1996. Today, the Valmet Corporation of Finland commercializes the process. The first full scale industrial LignoBoost plant was commissioned at Domtar’s pulp mill in North Carolina with an annual capacity of 25,000 tons of isolated Kraft lignin. The second LignoBoost plant was installed in the Stora Enso Sunila mill in 2015 in Finland with an annual capacity of ~50,000 tons of isolated lignin [24].
For the isolation of lignin, mean black liquor with a total solids content of 30–45% is extracted from the evaporation train and cooled to the precipitation temperature of 60–80 °C [32]. The pH of the liquor is then lowered from ~13.5 to ~10 by addition of CO2; the amount of CO2 needed is reported as being 150–300 kg CO2 per ton of isolated lignin. The major part of lignin precipitates and the generated slurry is subsequently matured at 50 °C for 30 min. Maturing increases the filterability of precipitated lignin because of particle growth [33]. After maturing, the slurry is filtered in a chamber filter press and the filtrate, now termed lignin lean liquor, is returned to the liquor cycle, where it is reintroduced into the evaporation train at the stage after the point where the mean black liquor has been removed for precipitation. This implementation point ensures that the lignin lean liquor cannot be repeatedly cycled to the precipitation step, which would decrease the precipitation yield. The filter cake, consisting of precipitated lignin, residual lignin lean liquor, inorganic salts, and hemicellulose, is then re-dispersed in a sulfuric acid solution at a pH of 2–4. Re-dispersion prevents blocking of the filter, which is a result of acid washing of the filter cake due to further precipitation of lignin from the remaining lignin lean liquor. By the ion exchange of sodium and hydrogen, an ash content of 0.2–1.4% is obtained and at the same time the ion exchange ensures the recovery of sodium [34][35]. The slurry is then filtered again in a filter press and washed with a diluted sulfuric acid solution. Pressing and blowing of the filter cake removes as much liquid as possible before it is discharged and further processed depending on the final application [36][37][38]. Figure 3 shows a schematic drawing of the LignoBoost process and the most important process parameters.
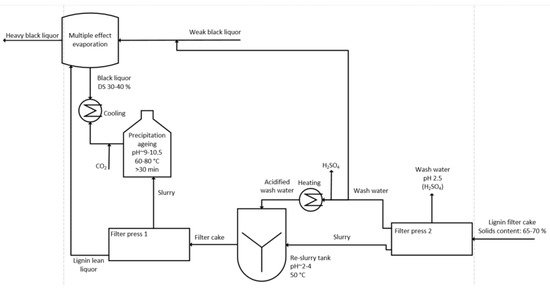
Figure 3. Schematic drawing of the LignoBoost process.
Closed loops are a need in today‘s manufacturing processes, and hence the filtrate and the wash water of the second filtration needs to be implemented in the water cycle. Moreover the use of unbleached or oxygen de-lignified pulp is reported for washing of the filter cake [39].
2.3. LignoForce
The LignoForce SystemTM was invented by FPInnovations and was commercialized by NORAM engineering. The first commercial scale-up of the LignoForce SystemTM was started up in 2016 in the company West Fraser in Canada [40]. In comparison to the LignoBoost process, a filtration step and an oxidation step of the black liquor are introduced prior to lignin precipitation. To decrease the totally reduced sulfur compounds (TRS) like hydrogen sulfide, methyl mercaptan, dimethylsulfide, and dimethyldisulfides, during the further processing, the oxidation thereof is performed using O2. Pure oxygen is thus added to the filtered black liquor until a sulfide content of 0–0.5 g/L is reached. In addition to the sulfur components, hemicellulose and lignin are also oxidized [41]. Oxidation of the hemicellulose leads to the formation of the corresponding isosaccharinic acids or shorter organic acids, like acetic acid or lactic acid, which leads to a decrease in the pH. Alongside with the oxidation of the hemicellulose, lignin is oxidized to carboxylated lignin, which has a much higher solubility in water. The overall lignin yield of this process is hence expected to be lower. However, by implementing the oxidation step better filtration properties are reported and acid washing is realized already after the first acidification with CO2 to ensure low ash content [42]. The CO2 demand for the acidification to a pH-value of ~9.5 corresponds to 300–400 kg CO2 per ton isolated lignin [43]. Figure 4 shows the flowchart including the most important process parameters for the LignoForce SystemTM.
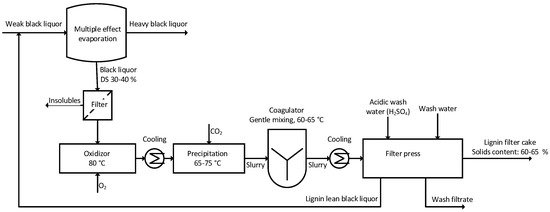
Figure 4. Schematic drawing of the LignoForce process.
2.4. Sequential Liquid-Lignin Recovery and Purification (SLRP)
The first provisional patent application was filed in 2009 by Michael Lake and John Blackburn. They founded the liquid lignin company, which is currently trying to find investors and industrial partners for commercialization of the SLRP process and the related products [44]. Unlike the WestVaco process, the LignoBoost process and the LignoForce SystemTM, the SLRP process operates at elevated pressure and temperature, which leads to a remaining liquid lignin. The comparably low CO2 demand of 170 kg of pure CO2 per ton lignin is mainly related to the highly efficient absorption of CO2 at high pressure [45]. Figure 5 shows the flowchart of the SLRP process and the most important process parameters. The mixture of liquid lignin and lignin lean liquor is separated after the carbonization step into the lighter lignin lean liquor, which is sent back to the evaporation train, and the heavier liquid lignin, which is further processed in a manner similar to the LignoBoost process and the LignoForce SystemTM.
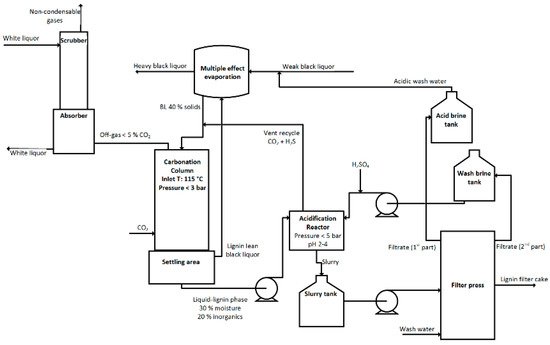
Figure 5. Schematic drawing of the SLRP process.
References
- Sandborn, L.T.; Richter, S.J.; Clemens, H.G. Process for Making Vanillin. U.S. Patent Application No. 663,180, 13 October 1936.
- Sande, W.E.; Sears, K.D. Method for Preparing Vanillin from Old Newsprint. U.S. Patent Application No. 350,835, 31 December 1996.
- Wang, Y.; Sun, S.; Li, F.; Cao, X.; Sun, R. Production of vanillin from lignin: The relationship between β-O-4 linkages and vanillin yield. Ind. Crop. Prod. 2018, 116, 116–121.
- Mainka, H.; Täger, O.; Körner, E.; Hilfert, L.; Busse, S.; Edelmann, F.T.; Herrmann, A.S. Lignin—An alternative precursor for sustainable and cost-effective automotive carbon fiber. J. Mater. Res. Technol. 2015, 4, 283–296.
- Baker, D.A.; Gallego, N.C.; Baker, F.S. On the characterization and spinning of an organic-purified lignin toward the manufacture of low-cost carbon fiber. J. Appl. Polym. Sci. 2012, 124, 227–234.
- Kadla, J.F.; Kubo, S.; Venditti, R.A.; Gilbert, R.D.; Compere, A.L.; Griffith, W. Lignin-based carbon fibers for composite fiber applications. Carbon 2002, 40, 2913–2920.
- Otani, S.; Fukuoka, Y.; Igarashi, I.; Sasaki, K. Method for Prqducing Carbonezed Lignin Fiber. U.S. Patent Application No. 492,878, 12 August 1969.
- Baker, D.A.; Rials, T.G. Recent advances in low-cost carbon fiber manufacture from lignin. J. Appl. Polym. Sci. 2013, 130, 713–728.
- Williams, C.H.; Rubin, D.H.; Hao, J.; Bar, B.; Frist, A. Composition Comprising Lignosulfonates for Crop Protection and Crop Improvement. U.S. Patent Application No. US 2006/0247130 A1, 02 November 2006.
- Meier, J.N.; Fyles, J.W.; MacKenzie, A.F.; O’Halloran, I.P. Effects of lignosulfonate-fertilizer applications on soil respiration and nitrogen dynamics. Can. J. Soil Sci. 1993, 73, 233–242.
- Detroit, W.J. Lignosulfonate Treatment Fertilizer Particles. U.S. Patent Application No. 24,044, 11 July 1989.
- Alonso, M.V.; Oliet, M.; Rodriguez, F.; Astarloa, G.; Echeverría, J.M. Use of a methylolated softwood ammonium lignosulfonate as partial substitute of phenol in resol resins manufacture. J. Appl. Polym. Sci. 2004, 94, 643–650.
- Hemmilä, V.; Adamopoulos, S.; Karlsson, O.; Kumar, A. Development of sustainable bio-adhesives for engineered wood panels – A Review. RSC Adv. 2017, 7, 38604–38630.
- Hemmilä, V.; Adamopoulos, S.; Hosseinpourpia, R.; Ahmed, S.A. Ammonium Lignosulfonate Adhesives for Particleboards with pMDI and Furfuryl Alcohol as Crosslinkers. Polymers 2019, 11, 1633.
- Antov, P.; Savov, V.; Krišťák, Ľ.; Réh, R.; Mantanis, G.I. Eco-Friendly, High-Density Fiberboards Bonded with Urea-Formaldehyde and Ammonium Lignosulfonate. Polymers 2021, 13, 220.
- Laurijssen, J.; Faaij, A.; Worrell, E. Benchmarking energy use in the paper industry: A benchmarking study on process unit level. Energy Effic. 2013, 6, 49–63.
- Levi, P.; Vass, T.; Mandová, H.; Gouy, A.; Schröder, A. International Engery Agency (IEA). Available online: (accessed on 19 April 2021).
- Vakkilainen, E.; Välimäki, E. Effect of Lignin Separation to Black Liquor and Recovery Boiler Operation. In Proceedings of the TAPPI Press-TAPPI Engineering, Pulping and Environmental Conference 2009-Innovations in Energy, Fiber and Compliance, Memphis, TN, USA, 11–14 October 2009; pp. 1515–1556.
- Hubbe, M.; Alén, R.; Paleologou, M.; Kannangara, M.; Kihlman, J. Lignin recovery from spent alkaline pulping liquors using acidification, membrane separation, and related processing steps: A review. Bioresource 2019, 14, 2300–2351.
- Aro, T.; Fatehi, P. Production and Application of Lignosulfonates and Sulfonated Lignin. ChemSusChem 2017, 10, 1861–1877.
- Confederation of European Paper Industries. Key Statistics 2017-European Pulp & Paper Industry; Confederation of European Paper Industries: Northhamptonshire, UK, 2017.
- Kihlman, J. The Sequential Liquid-Lignin Recovery and Purification process: Analysis of integration aspects for a kraft pulp mill. Nord. Pulp Pap. Res. J. 2016, 31, 573–582.
- Loutfi, H.; Blackwell, B.; Uloth, V. Lignin Recovery from Kraft Black Liquor: Preliminary Process Design. Tappi J. 1991, 74, 1–8.
- Zhu, W. Precipitation of Kraft Lignin Yield and Equilibrium; Chalmers University: Gothenburg, Sweden, 2015.
- Wallmo, H. Lignin Extraction from Black Liquor-Precipitation, Filtration and Washing; University of Götteborg: Gothenburg, Sweden, 2008.
- Brauns, F.E.; Brauns, D.A. The Chemistry of Lignin, Supplement Volume Covering the Literature for the Years 1949–1958; Academic Press: London, UK, 1960.
- Pollak, A.; Keilen, J.J.; Drum, L.F. Method of Producing Lignin from Black Liquor. U.S. Patent Application No. 8486224B2, 16 July 2013.
- Beis, S.H.; Mukkamala, S.; Hill, N.; Joseph, J.; Baker, C.; Jensen, B.; Stemmler, E.A.; Wheeler, M.C.; Frederick, B.G.; van Heiningen, A.; et al. Fast Pyrolysis Lignins. BioResources 2010, 5, 1408–1424.
- Keilen, J.J.; Ball, F.J.; Gressang, R.W. Method of Coagulating Colloidal Lignates in Aqueous Dispersions. U.S. Patent Application No. US2623040, 23 December 1952.
- Ball, F.J.; Vardell, C.; Vardell, W.G. Decantation of Lignin. U.S. Patent Application No. 2997466, 22 August 1961.
- Ball, F.J.; Vardell, W.G. Continuous Acidulation and Coagulation of Lignin in Black Liquor. U.S. Patent Application No. 3048576, 7 August 1962.
- Öhman, F.; Wallmo, H.; Theliander, H. Precipitation and filtration of lignin from black liquor of different origin. Nord. Pulp Pap. Res. J. 2007, 22, 188–193.
- Kannangara, M.; Marinova, M.; Fradette, L.; Paris, J. Effect of mixing hydrodynamics on the particle and filtration properties of precipitated lignin. Chem. Eng. Res. Des. 2016, 105, 94–106.
- Öhman, F.; Wallmo, H.; Theliander, H. A novel method for washing lignin precipitated from kraft black liquor–Laboratory trials. Nord. Pulp Pap. Res. J. 2007, 22, 9–16.
- Ziesig, R.; Sedin, M.; Tomani, P.; Theliander, H. Production of a pure lignin product, Part 3: Distribution and removal of inorganics from softwood lignin. Nord. Pulp Pap. Res. J. 2015, 30, 199–206.
- Tomani, P. The Ligno Boost Process. Cellul. Chem. Technol. 2010, 44, 53–58.
- Tomani, P. Lignin Extraction from Black Liquor. In 43rd Pulp and Paper International Congress and Exhibition; Associacao Brasileira Tecnica de Celulose e Papel (ABTCP): Sao Paolo, Brazil, 2010.
- Tomani, P.; Axegård, P.; Berglin, N.; Lovell, A.; Nordgren, D.; Ab, I. Integration of Lignin Removal into a Kraft Pulp Mill and Use of Lignin as a Biofuel. Cellul. Chem. Technol. 2011, 2, 533–540.
- Zhu, W.; Hans, T. Precipitation of Lignin from Softwood Black Liquor: An Investigation of the Equilibrium and Molecular Properties of Lignin. BioResources 2015, 10, 1696–1714.
- Kouisni, L.; Holt-Hindle, P.; Maki, K.; Paleologou, M. The LignoForce System TM: A New Process for the Production of High-Quality Lignin from Black Liquor. J. Sci. Technol. For. Prod. Process. 2012, 2, 6–10.
- Kouisni, L.; Gagné, A.; Maki, K.; Holt-Hindle, P.; Paleologou, M. LignoForce System for the Recovery of Lignin from Black Liquor: Feedstock Options, Odor Profile, and Product Characterization. ACS Sustain. Chem. Eng. 2016, 4, 5152–5159.
- Kouisni, L.; Paleologou, M. Method for Separating Lignin from Black Liquor. U.S. Patent Application No. US2011/0297340A1, 16 July 2013.
- Wells, K.; Pors, D.; Foan, J.; Chan, C.; Maki, K.; Kouisni, L.; Paleologou, M. CO2 Impacts of Commercial Scale Lignin Extraction at Hinton Pulp Using the LignoForce Process & Lignin Substitution into Petroleum-Based Products. In Proceedings of the PACWEST Conference, Whistler, BC, Canada, 1 June 2015.
- Lake, M.A.; Blackburn, J.C.; Devon, S. LiquidLignin. Available online: (accessed on 28 June 2018).
- Lake, M.A.; Blackburn, J.C. P TM–An Innovative Lignin-Recovery Technology. Cellul. Chem. Technol. 2014, 48, 799–804.




