
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Krasimir Vasilev | + 2952 word(s) | 2952 | 2020-05-29 13:14:36 | | | |
| 2 | Catherine Yang | Meta information modification | 2952 | 2020-06-03 04:38:46 | | | | |
| 3 | Catherine Yang | Meta information modification | 2952 | 2020-06-03 04:41:29 | | | | |
| 4 | Catherine Yang | -61 word(s) | 2891 | 2020-10-28 04:30:55 | | |
Video Upload Options
Over the last decades, there has been tremendous volume of research efforts focussed on engineering silver based (nano)materials. The interest in silver has been mostly driven by the element capacity to kill pathogenic bacteria. In this context the main area of application has been medical devices that are at significant risk of becoming colonised by bacteria and infected. However, silver nanomaterials have been incorporated in a number of other commercial products which may or may not benefit from antibacterial protection. The rapid expansion of the library and their use raises important questions about possible toxicity and generally human health.
1. Introduction
The physiochemical properties of engineered silver nanomaterials can contribute to both desired and undesired biological response. Understanding of the role that these properties play in modulating immune response can improve safety, which is particularly important for applications in healthcare. Several factors like size, shape, charge, functionalization, etc., can mediate the interaction of silver with immune cells [1]. The size of nanomaterials is a defining feature that determines their in vivo behavior and clearance [2]. In general, particles that are below 5 nm in size are easily cleared by the kidneys while those greater than 100 nm are recognized as foreign materials by immune cells [3][4]. With the decrease in size, the surface area to volume ratio increases enabling particle diffusion into cells [5]. For instance, cellular and nuclear internalization of 5 nm particles is found to be higher compared to 50 nm-sized AgNPs [6]. For example, Park et al. treated human macrophages (U-937) with AgNPs of sizes 4, 20, and 70 nm and found that the smallest particle (4 nm) had the greatest capacity to induce inflammation evident by an increased expression of pro-inflammatory markers [7].
The surface charge of nanomaterials also has an impact on cellular uptake. It was demonstrated that positively charged NPs are easily taken up by cells compared to their negative counterparts. This is because the cell membrane is negatively charged facilitating the uptake of oppositely charged particles. For instance, a recent study compared the cellular internalization of polyvinyl pyrrolidone (PVP)-stabilized AgNPs having a net negative charge of −20 mV and polyethyleneimine (PEI)-functionalized AgNPs having a net positive charge of +50 mV [8]. The results indicated that PEI-functionalized AgNPs penetrated the cell membrane easily due to their electropositive nature. Similarly, polyethylene glycol (PEG)-stabilized AgNP/DNA complexes with a zeta potential of 30.5 ± 2.5 mV showed enhanced uptake by immune cell compared to other complexes having a net negative charge [9]. These examples show that the functionalization of silver nanomaterials is an important aspect to be considered to reduce immunotoxicity. It is evident that when particles are functionalized by polyethylene glycol, they can escape the immune system and have a longer circulation time within the body [10].
The shape of a NP is another parameter that can influence uptake by immune cells. For instance, nanorods had a longer residence time in the gastrointestinal tract compared to spherical NPs, as nanorods had greater potential to overcome the rapid clearance by the reticuloendothelial system [11]. Nanorods also showed longer circulation time in blood [11]. Moreover, cellular uptake of PVP-stabilized silver nanoprisms and PVP-stabilized spherical AgNPs showed that human mesenchymal stem cells took up more nanoprisms compared to spherical NPs [12]. On the contrary, Hacat cells ingested an equal quantity of both types of silver nanomaterials. This was due to the large interaction area of platelet-like nanosprisms with the cell surface and the bending stiffness of the cell membrane. The more flexible nature of human mesenchymal stem cells membrane appeared to favor uptake of nanoprisms. In contrast, Hacat cells had stiffer cell membrane (high Young’s modulus), and therefore, the energy gain due to the large interaction area of nanoprisms is compensated by the energy spent in deforming the cell membrane to enter inside the cells [12].
It is evident from the above discussion that the physicochemical properties of silver nanomaterials should be carefully considered and assessed in order to be able to accurately modulate their interaction with cellular organelles. These properties determine whether silver nanomaterials can induce inflammation or dampen the immune response. The inflammatory properties of silver nanomaterials are discussed in detail in the following sections.
2. Pro-Inflammatory Properties of Silver Nanomaterials
Immune cells recognize silver nanomaterials as foreign particles, thus, any exposure of nanomaterials could trigger a cascade of inflammatory reactions involving the activation of neutrophils, macrophages, and helper T cells, and consequently leading to expression of a large number of cytokines, such as Tumor Necrosis factor-α (TNF-α), Interleukins (IL-1β, IL-6, IL-12, IL-18), etc. [13]. These molecules are part of the normal body natural defense to fight diseases and are utilized in immunotherapies and vaccines. However, the unwanted elevation of a cytokines level in response to nanomaterials may lead to serious side effects, such as systemic inflammation [14]. The possible signaling pathways that may be activated by silver nanomaterials to trigger pro-inflammatory cytokine release are shown in Figure 1 and include Nuclear Factor-kβ (NF-kβ), c-Jun N-terminal kinase (JKK), or Mitogen-activated protein kinase kinase (MKK) pathways. In a normal physiological situation, in order to avert diseases, it is necessary to tightly regulate the production of cytokines and prevent overstimulation of the immune system. Therefore, it is important to conduct a systematic evaluation of the propensity of silver nanomaterials in inducing cytokine release from immune cells as a major parameter for confirming their safety profile. Various in vitro and in vivo assays [15] to explore the immune responses caused by engineered silver materials are discussed in Table 1 and Table 2.
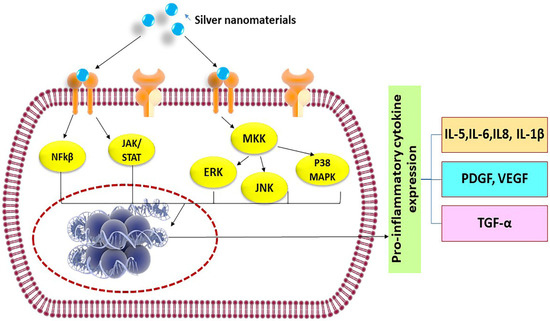
Figure 1. Possible signaling pathways activated by silver nanomaterials to release pro-inflammatory cytokines.
Table 1. In vitro evaluation of the immune response of silver nanomaterials.
| Type of Ag | Size | Reagents Used | Type of Immune Cells | Cytokines Expression | In vitro Inflammatory Assays | Ref |
|---|---|---|---|---|---|---|
| NPs | <20 nm | AgNO3, Quercetin, Polyoxyethylene Glycerol trioleate, and Tween 20 | Caco-2 cells | Decreased IL-8 expression | qRT-PCR, ELISA, total protein content, Nitrate/Nitrite Colorimetric Assay | [16] |
| Nano wires | 10 µm | AgNO3, ethylene glycol, poly (vinylpyrrolidone) | Human monocyte-derived macrophages | Up taken by macrophages and transformed to silver chloride | High angle annular dark field scanning electron microscopy, Confocal analysis | [17] |
| Nanoclusters | 1.5 nm | NaBH4, AgNO3 | RAW264.7 cells | Release TNF-α, IL-6 | ELISA | [18] |
| NPs | 14 nm | NaBH4, AgNO3, Sodium citrate |
RAW264.7 and J774.1 | Reduced TNF-α expression | ELISA | [19] |
| NPs | 10–50 nm | AgNO3, Extracts of Viburnum opulus | Hacat cells | Increased IL-1α and decreased IL-1α, IL-6 | ELISA | [20] |
| NPs | 20–80 nm | AgNO3, Extracts of Sambucus nigra | Hacat cells | Reduced IL-1α production | ELISA | [21] |
| NPs | 10 nm | Dendrimer, NaBH4, AgNO3, Sodium citrate | RAW264.7 and J774.1 | Decreased TNF-α, IL-6 | ELISA | [22] |
| NPs | 23.52–60.83 nm | AgNO3, Ethanolic petal extract of Rosa indica | Rat peritoneal macrophages | Attenuate production of NO and superoxide | Nitrate/Nitrite Colorimetric Assay, Estimate superoxide anion generation | [23] |
| NPs | 10.29–45.57 nm | AgNO3, Aqueous extracts of Phyllanthus acidus L. | Rat peritoneal macrophages | Attenuate production of IL-1α, NO and superoxide | ELISA, Immunoblotting, Nitrate/Nitrite Colorimetric Assay, Estimate superoxide anion generation | [24] |
| NPs | 4 nm | Chloroform, NaBH4, AgNO3, POPS | Bone marrow-derived macrophage cells | Decrease in IL-6 and IL-1β, no effect in TNF-α | ELISA | [25] |
Table 2. In vivo assessment of the immune response of silver nanomaterials.
| Nature of Ag | Size | Reducing Agent Used | Animal Strain | Model | Outcome | Ref |
|---|---|---|---|---|---|---|
| NPs | 9.3 ± 3.2 nm | NaBH4, AgNO3, Sodium citrate |
Balb/c mice | Postoperative adhesion model | Decrease inflammation in peritoneal adhesion without toxic effects | [19] |
| Nano wires |
1.5 µm and 10 µm | AgNO3, ethylene glycol, polyvinyl pyrrolidone | Sprague Dawley rats | Intratracheal instillation, Lung model | Completely internalized by lung macrophages with toxic effects | [26] |
| NPs | 7–10 nm | AgNO3, Leaf extracts of Terminalia species | Wistar albino rats | Hind paw oedema model | Inhibition of oedema by 95% | [27] |
| NPs | 10–50 nm | AgNO3, Extracts of Viburnum opulus L. | Wistar rats | Carrageenan-induced inflammation models | Decreased inflammation | [20] |
| NPs | 14 ± 9.8 nm | NaBH4, AgNO3, Sodium citrate | Male Balb/c mice | Thermal injury animal models | Silver can modulate cytokine expression | [28] |
| NPs | 10 nm (5–15 nm) | Dendrimer, NaBH4, AgNO3, Sodium citrate | C57BL/6 N mice | Excisional and burn wound models | Enhanced anti-inflammatory efficacy | [22] |
| NPs | 20–80 nm | AgNO3, Extracts of Sambucus nigra | Male Wistar rats, | Carrageenan-induced inflammation models | AgNPs enhanced inflammation edema rate | [21] |
| NPs | 12–22 nm | Starch, NaOH, AgNO3, Absolute ethanol | Male and female rats | Grade II burn wound models | Reduce rat paw oedema | [29] |
| Nano crystalline silver | 10–15 nm | AgNO3, polyethene | Domestic White/Landrace swine | Porcine contact dermatitis model | Treated normal pigs have near-normal skin after 24 h | [30] |
| Silver-coated glass beads | 850–1400 µm and 5 µm | Borosilicate glass beads | Male Balb/c mice | Models mimicking Crohn’s disease and ulcerative colitis | Attenuated inflammation in colitis and Crohn’s disease models | [31] |
| NPs | 7 ± 3 nm | AgNO3, Diaminopyridiinyl Heparin, Glucose, | Male rats | Carrageenan-induced paw edema | Localization of anti-inflammatory effects[32] |
Size-dependent pro-inflammatory consequences triggered by silver nanomaterials can be noticed in several instances. When NPs enter the biological system, there is a greater neutrophil influx in case of particles of sizes below 100 nm compared to their larger counterparts. For instance, it has been demonstrated that smaller sized AgNPs (3–5 nm) could cross the cell membrane of neural cells and induce IL-1β secretion [33]. These AgNPs were internalized by endocytic uptake processes into small cellular vesicles that could release silver and induce generation of ROS and inflammation. They activated immune reaction genes, including CXCL13 and MARCO, which caused release of TNF-α in zebrafish liver cells, thus triggering pro-inflammatory events [33]. In another study, AgNPs of sizes of about 56 nm upregulated the expression of pro-inflammatory cytokines such as IL-1β and IL-6 in human lung epithelial cells (A549) [34]. Giovanni et al. reported the activation of pro-inflammatory responses in macrophages that were exposed to an ultra-low concentration of silver [35]. These studies indicate that particle size is therefore an important factor that drives inflammatory events [36].
Surface modification of nanomaterials can also alter the response of the immune system. It is interesting to note that some of the functionalized AgNPs were found to elicit pro-inflammatory properties. Exposure to PVP-coated AgNPs induced pro-inflammatory cytokine gene expression of TNF-α, IL-1, and IL-6 in both primary blood monocytes and THP-1 cells [37]. They also resulted in the release of IL-1β by the formation of inflammasome [38][39], a multi-protein oligomer produced by myeloid cells during innate immunity. These studies showed that PVP-coated AgNPs could induce an innate immune response which could lead to the risk of inflammatory disease development. In another study, the non-methylated cytosine–phosphate–guanine (CpG)-functionalized silver nanoclusters were found to enhance immune response and were used for cell imaging [18]. CpG dinucleotide is present in bacterial and viral DNA and exhibits immune-stimulatory activities to attacking pathogens. The immune system in mammals recognizes CpG oligodeoxynucleotides via Toll-like receptor-9 and release several pro-inflammatory cytokines, including IL-6 and TNF-α, which can stimulate both the innate and adaptive immunity. CpG-functionalized silver nanoclusters exhibited minimum toxicity and were easily internalized by cells and effectively protected from nuclease degradation. These silver nanoclusters showed an immune-stimulatory effect when combined with CpG, increasing TNF-α and IL-6 production [40]. All these different scenarios explain that the choice of surface modification can determine the fate of a silver nanomaterial entering a biological system.
3. Anti-Inflammatory and Immunosuppressive Properties of Silver Nanomaterials
Anti-inflammatory cytokines are regulatory molecules that dampen the immune response. Cytokines which can have this role include IL-4, IL-10, IL-11, IL-13, and TGF-β [41]. Silver nanomaterials can be engineered to directly target immune cells and suppress their activity or avoid immune recognition. Regulation of toll-like receptor (TLR) signaling using tactfully designed silver nanomaterials is one approach to combat overpowering inflammatory response. TLRs are non-catalytic receptor proteins found mostly in macrophages and dendritic cells that help in recognizing microbes or foreign particles [42]. AgNPs capped with a monolayer of tiopronin reduced the secretion of IL-6 mediated by TLR ligands without inducing any toxicity [43]. Bergenin-loaded AgNPs and stabilized by gum xantham exhibited inhibitory effects on TLR-2 and TLR-4 and suppressed synovial inflammation in arthritic rats [44].
Anti-cytokine approaches using silver nanomaterials are yet another interesting method to reduce inflammation in which interaction between cytokines and their receptors is prevented and cytokine gene expression is reduced. Bioengineered mannan sulphate-capped AgNPs were found to downregulate both TNF-α and IL-6 expression in rats [45]. These were spherical particles with a size of 20 nm and a zeta potential of −32.4 mV. The particles were internalized by murine macrophage cell lines using receptor-mediated endocytosis via mannose receptors. These particles not only reduced inflammation but also accelerated wound healing in rats. A comparative study of nanocrystalline silver and silver nitrate in a porcine model of contact dermatitis showed that nanocrystalline silver-treated pigs had a reduced level of edema and erythema after 72 h and also a decreased expression of pro-inflammatory cytokines compared to animal ammonals treated with AgNO3 [30]. This result suggested that nanocrystalline silver could benefit wound healing. In another study, the cytokine level expression in wounds treated with genipin-crosslinked chitosan hydrogels containing silver sulfadiazine nanocrystals showed reduced IL-6 levels [46].
Appropriately designed silver nanomaterials were shown to inhibit inflammatory cell recruitment to the affected tissues, thereby preventing inflammation. This is particularly useful for implant transplantation and drug delivery. AgNPs coated onto the surface of absorbable braided suture using a layer by layer deposition showed remarkable anti-inflammatory property in mice. Immunohistochemistry results and quantitative evaluation showed reduced macrophage infiltration and decreased the production of IL-10, IL-6, and TNF-α [47].
Inhibition of T lymphocytes by silver nanomaterials is another strategy to suppress the immune system. In this context, Côté-Maurais et al. studied the effect of AgNPs on interleukin-2 (IL-2) dependent proliferation of T cells [48]. Upon activation, CD4+ T cells produce IL-2, which is a key mediator of proliferation, differentiation, and growth of effector CD4+ T cells. The authors demonstrated that AgNPs alter IL-2 release by reducing T cell proliferation due to the rise of low-affinity receptors for this cytokine [48].
Reactive oxygen species act as immune mediators affecting various immune cells [49]. When the level of ROS is elevated, immune cells become dysfunctional which often leads to immunosuppression [50]. Silver nanomaterials can be used to reduce the ROS level, thereby controlling the immune system. Manikandan et al. synthesized AgNPs using an ethanolic extract of rose petals and tested their anti-inflammatory activity on rat peritoneal macrophages in vitro [23]. They observed that exposure of the prepared NPs reduced H2O2-mediated cytotoxicity in macrophages. They also found a noticeable reduction in the liberation of potent inflammatory mediators such as superoxide anion and nitric oxide upon exposure to NPs. Another group focused on studying the impact of AgNPs on microglial inflammation as microglia are greatly affected in neurodegenerative disorders (Figure 2) [51]. Microglia are macrophage cells that act as the key active immune defense in the central nervous system. They found that after internalization of AgNPs by microglia, non-reactive silver sulphide was formed on the surface of AgNPs. Furthermore, these NPs increased the expression of hydrogen sulphide synthesizing an enzyme called cystathionine-γ-lyase and showed remarkable anti-inflammatory effects, plummeting LPS-stimulated nitric oxide, ROS, and TNF-α levels. These studies reveal the anti-inflammatory activity of various forms of silver nanomaterials.
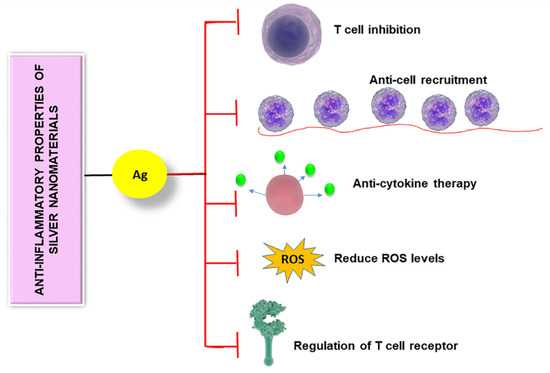
Figure 2. Anti-inflammatory properties of silver nanomaterials are controlled by different mechanisms including T cell inhibition, anti-cell recruitment, anti-cytokine therapy, regulation of T cell receptor, and reducing the level of reactive oxygen species.
4. Adjuvant Properties of Silver Nanomaterials
Adjuvants are moieties added to a vaccine to increase immune responses towards antigens. They can act in different ways to present antigens to the immune system by either acting as antigen depot or as irritants. Antigen depots present antigen over a greater period of time, thus maximizing immune response [52]. Others act as irritants which increase the body’s immune response [53]. The action of adjuvants is mainly controlled by T and B cells. For instance, Xu et al. evaluated the adjuvant effect of AgNPs both in vitro and in vivo [54]. These negatively charged NPs (−30.6 mV) with a size of ~141 nm were found to increase the serum antigen-specific IgG and IgE levels in mice, indicating that AgNPs elicited CD 4+-mediated immune response. The mechanism of an adjuvant effect is mainly attributed to the activation and recruitment of local leukocytes and macrophages by AgNPs. Asgary et al. prepared spherical AgNPs from leaf extract of Eucalyptus procera having an average size of ~60 nm and a zeta potential of −14 mV [55]. Different amounts of the prepared NPs were added to inactivated rabies virus and injected into the peritoneum of mice. The results showed that the adjuvant effect of AgNPs was enhanced by increasing their concentration and finally reached a plateau at 15–20 mg/kg. The authors proposed a mechanism for the adjuvant effect which includes accumulation and trapping of antigen by AgNPs, which leads to a better regulation of the innate immune system [55].
Silver nanomaterials can be used to develop HIV vaccines that can adequately activate critical anti-HIV immunity in a safe manner. Even though some prevailing adjuvants can assist developing immunity by HIV vaccines, they have aftereffects, as they go into host cells along with vaccine delivery; in other words, they are intracellular adjuvants that can damage host cells, ultimately causing cytotoxicity. Liu et al. demonstrated that polyvinylpyrrolidone–polyethylene glycol-modified silver nanorods can be a harmless nanocarrier adjuvant for a HIV vaccine which stays outside the cells but can still trigger immune response [56]. Compared to nanospheres, nanorods cannot be easily taken up by cells. Silver was chosen as the non-carrier adjuvant for HIV vaccine as it can inhibit HIV and can boost antibody and T cell response against ovalbumin. These nanorods improved HIV vaccine-specific IgG responses by raising the titer of IgG3. IgG3 inhibits HIV by binding to Fc receptor and mediated antibody-dependent cellular toxicity. Silver nanorods induced T cells to secrete more IFN-γ, CD107, IL-2, and IL-4. Thus, these nanorods increased critical immunities of HIV vaccine and were not cytotoxic, which is the main problem faced by other adjuvants. However, more research needs to be conducted in the future to exploit the adjuvant properties of silver nanomaterials.
References
- Geetha Bai, R.; Ninan, N.; Muthoosamy, K.; Manickam, S.; Graphene: A versatile platform for nanotheranostics and tissue engineering. Prog. Mater. Sci. 2018, 91, 24–69.
- Günter Oberdörster; Thomas Kuhlbusch; In vivo effects: Methodologies and biokinetics of inhaled nanomaterials. NanoImpact 2018, 10, 38-60, 10.1016/j.impact.2017.10.007.
- Marina A. Dobrovolskaia; Scott E. McNeil; Peter Rodgers; Immunological properties of engineered nanomaterials. Nanoscience and Technology 2009, null, 278-287, 10.1142/9789814287005_0029.
- Michelle Longmire; Peter L. Choyke; Hisataka Kobayashi; Clearance properties of nano-sized particles and molecules as imaging agents: considerations and caveats. Nanomedicine 2008, 3, 703-717, 10.2217/17435889.3.5.703.
- Jayanth Panyam; Vinod Labhasetwar; Biodegradable nanoparticles for drug and gene delivery to cells and tissue. Advanced Drug Delivery Reviews 2003, 55, 329-347, 10.1016/s0169-409x(02)00228-4.
- Meiyu Wu; Hongbo Guo; Lin Liu; Ying Liu; Liming Xie; Size-dependent cellular uptake and localization profiles of silver nanoparticles.. International Journal of Nanomedicine 2019, 14, 4247-4259, 10.2147/IJN.S201107.
- Jeongsin Park; Dae-Hyoun Lim; Hyun-Jeong Lim; Taejung Kwon; Jin-Sil Choi; Sohee Jeong; In-Hong Choi; Jinwoo Cheon; Size dependent macrophage responses and toxicological effects of Ag nanoparticles. Chemical Communications 2011, 47, 4382, 10.1039/c1cc10357a.
- haronova, A.; Loza, K.; Surmeneva, M.; Surmenev, R.; Prymak, O.; Epple, M.; Synthesis of positively and negatively charged silver nanoparticles and their deposition on the surface of titanium. IOP Conf. Ser. Mater. Sci. Eng. 2016, 116, 012009.
- Kishor Sarkar; Sovan Lal Banerjee; Patit Paban Kundu; Giridhar Madras; Kaushik Chatterjee; Biofunctionalized surface-modified silver nanoparticles for gene delivery.. Journal of Materials Chemistry B 2015, 3, 5266-5276, 10.1039/c5tb00614g.
- Jung Soo Suk; Qingguo Xu; Namho Kim; Justin Hanes; Laura M. Ensign; PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Advanced Drug Delivery Reviews 2016, 99, 28-51, 10.1016/j.addr.2015.09.012.
- Yating Zhao; Yu Wang; Fu Ran; Yu Cui; Chang Liu; Qinfu Zhao; Yikun Gao; Da Wang; Si-Ling Wang; A comparison between sphere and rod nanoparticles regarding their in vivo biological behavior and pharmacokinetics.. Scientific Reports 2017, 7, 4131, 10.1038/s41598-017-03834-2.
- Christina Maria Graf; Daniel Nordmeyer; Christina Sengstock; Sebastian Ahlberg; Jörg Diendorf; Joerg Raabe; Matthias Epple; Manfred Köller; Jürgen Lademann; Annika Vogt; et al.Fiorenza RancanEckart Rühl Shape-Dependent Dissolution and Cellular Uptake of Silver Nanoparticles. Langmuir 2018, 34, 1506-1519, 10.1021/acs.langmuir.7b03126.
- Mahmoud Elsabahy; Karen L. Wooley; Cytokines as biomarkers of nanoparticle immunotoxicity.. Chemical Society Reviews 2013, 42, 5552-5576, 10.1039/c3cs60064e.
- Timothy M. Potter; Barry W. Neun; Jamie C. Rodriguez; Anna N. Ilinskaya; Marina A. Dobrovolskaia; Scott E. McNeil; Analysis of Pro-inflammatory Cytokine and Type II Interferon Induction by Nanoparticles. Advanced Structural Safety Studies 2017, 1682, 173-187, 10.1007/978-1-4939-7352-1_15.
- Ninan, N.; Albrecht, H.; Blencowe, A. Chapter 5—Mammalian Cell-Based Assays for Studying Bio-Nano Interactions. In Characterization of Nanomaterials; Mohan Bhagyaraj, S., Oluwafemi, O.S., Kalarikkal, N., Thomas, S., Eds.; Woodhead Publishing: Cambridge, UK, 2018; pp. 129–166.
- Alina Martirosyan; Konstantinos Grintzalis; Madeleine Polet; Laurie Laloux; Yves-Jacques Schneider; Tuning the inflammatory response to silver nanoparticles via quercetin in Caco-2 (co-)cultures as model of the human intestinal mucosa. Toxicology Letters 2016, 253, 36-45, 10.1016/j.toxlet.2016.04.018.
- Theodorou, I.G.; Müller, K.H.; Chen, S.; Goode, A.E.; Yufit, V.; Ryan, M.P.; Porter, A.E. Silver Nanowire Particle Reactivity with Human Monocyte-Derived Macrophage Cells: Intracellular Availability of Silver Governs Their Cytotoxicity. ACS Biomater. Sci. Eng. 2017, 3, 2336–2347.
- Yu Tao; Zhenhua Li; Enguo Ju; Jinsong Ren; Xiaogang Qu; One-step DNA-programmed growth of CpG conjugated silver nanoclusters: a potential platform for simultaneous enhanced immune response and cell imaging. Chemical Communications 2013, 49, 6918, 10.1039/c3cc41972j.
- Kenneth K Y Wong; Stephanie O. F. Cheung; Liuming Huang; Jun Niu; Chang Tao; Chi-Ming Ho; Dr. Chi‐Ming Che; Paul K. H. Tam; Further Evidence of the Anti-inflammatory Effects of Silver Nanoparticles. ChemMedChem 2009, 4, 1129-1135, 10.1002/cmdc.200900049.
- Bianca Moldovan; Luminita David; Adriana Vulcu; Liliana Olenic; Maria Perde-Schrepler; Eva Fischer-Fodor; Ioana Baldea; Simona Clichici; Gabriela Adriana Filip; In vitro and in vivo anti-inflammatory properties of green synthesized silver nanoparticles using Viburnum opulus L. fruits extract. Materials Science and Engineering: C 2017, 79, 720-727, 10.1016/j.msec.2017.05.122.
- Luminita David; Bianca Moldovan; Adriana Vulcu; Liliana Olenic; Maria Perde-Schrepler; Eva Fischer-Fodor; Adrian Florea; Maria Crisan; Ioana Chiorean; Simona Clichici; et al.Gabriela Adriana Filip Green synthesis, characterization and anti-inflammatory activity of silver nanoparticles using European black elderberry fruits extract. Colloids and Surfaces B: Biointerfaces 2014, 122, 767-777, 10.1016/j.colsurfb.2014.08.018.
- Xuelai Liu; Wei Hao; Chun-Nam Lok; Yue Chun Wang; Ruizhong Zhang; Kenneth K Y Wong; Dendrimer encapsulation enhances anti-inflammatory efficacy of silver nanoparticles. Journal of Pediatric Surgery 2014, 49, 1846-1851, 10.1016/j.jpedsurg.2014.09.033.
- Ramar Manikandan; Beulaja Manikandan; Thiagarajan Raman; Koodalingam Arunagirinathan; Narayanan Marimuthu Prabhu; Muthuramalingam Jothi Basu; Muthulakshmi Perumal; Subramanian Palanisamy; Arumugam Munusamy; Biosynthesis of silver nanoparticles using ethanolic petals extract of Rosa indica and characterization of its antibacterial, anticancer and anti-inflammatory activities. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 2015, 138, 120-129, 10.1016/j.saa.2014.10.043.
- Ramar Manikandan; M. Beulaja; R. Thiagarajan; S. Palanisamy; G. Goutham; A. Koodalingam; N.M. Prabhu; E. Kannapiran; M. Jothi Basu; C. Arulvasu; et al.Arumugam Munusamy Biosynthesis of silver nanoparticles using aqueous extract of Phyllanthus acidus L. fruits and characterization of its anti-inflammatory effect against H 2 O 2 exposed rat peritoneal macrophages. Process Biochemistry 2017, 55, 172-181, 10.1016/j.procbio.2017.01.023.
- Shima Taheri; Alex Cavallaro; Susan N. Christo; Peter Majewski; Mary Barton; J. D. Hayball; Krasimir Vasilev; Antibacterial Plasma Polymer Films Conjugated with Phospholipid Encapsulated Silver Nanoparticles. ACS Biomaterials Science & Engineering 2015, 1, 1278-1286, 10.1021/acsbiomaterials.5b00338.
- Kian Fan Chung; Joanna Seiffert; Shu Chen; Ioannis G. Theodorou; Angela Erin Goode; Bey Fen Leo; Catriona M. McGilvery; Farhana Hussain; Coen Wiegman; Christos Rossios; et al.Jie ZhuJicheng GongFarid TariqVladimir YufitAlexander George MonteithTeruo HashimotoJeremy N. SkepperMary P. RyanJunfeng ZhangTeresa D. TetleyAlexandra E. Porter Inactivation, Clearance, and Functional Effects of Lung-Instilled Short and Long Silver Nanowires in Rats. ACS Nano 2017, 11, 2652-2664, 10.1021/acsnano.6b07313.
- Hanaa Mohamed El-Rafie; Manal A. Hamed; Antioxidant and anti-inflammatory activities of silver nanoparticles biosynthesized from aqueous leaves extracts of four Terminalia species. Advances in Natural Sciences: Nanoscience and Nanotechnology 2014, 5, 35008, 10.1088/2043-6262/5/3/035008.
- Jun Tian; Kenneth Kak-Yuen Wong; Chi-Ming Ho; Chun-Nam Lok; Wing-Yiu Yu; Dr. Chi‐Ming Che; Jen-Fu Chiu; Paul K. H. Tam; Topical Delivery of Silver Nanoparticles Promotes Wound Healing. ChemMedChem 2007, 2, 129-136, 10.1002/cmdc.200600171.
- Ali Hebeish; M.H. El-Rafie; M.A. El-Sheikh; Amany A. Seleem; Mehrez E. El-Naggar; Mohammed El-Rafie; Manal A. El-Sheikh; Antimicrobial wound dressing and anti-inflammatory efficacy of silver nanoparticles. International Journal of Biological Macromolecules 2014, 65, 509-515, 10.1016/j.ijbiomac.2014.01.071.
- Patricia L Nadworny; Jianfei Wang; Edward E. Tredget; Robert Burrell; Anti-inflammatory activity of nanocrystalline silver in a porcine contact dermatitis model. Nanomedicine: Nanotechnology, Biology and Medicine 2008, 4, 241-251, 10.1016/j.nano.2008.04.006.
- Krzysztof Siczek; Hubert Zatorski; Anna Chmielowiec-Korzeniowska; Radzisław Kordek; L. Tymczyna; Jakub Fichna; Evaluation of anti-inflammatory effect of silver-coated glass beads in mice with experimentally induced colitis as a new type of treatment in inflammatory bowel disease.. Pharmacological Reports 2017, 69, 386-392, 10.1016/j.pharep.2017.01.003.
- Melissa M. Kemp; Ashavani Kumar; Shaymaa Mousa; Tae-Joon Park; Pulickel Ajayan; Natsuki Kubotera; Shaker A. Mousa; Robert J. Linhardt; Synthesis of Gold and Silver Nanoparticles Stabilized with Glycosaminoglycans Having Distinctive Biological Activities. Biomacromolecules 2009, 10, 589-595, 10.1021/bm801266t.
- Chin-Lin Huang; I-Lun Hsiao; Ho-Chen Lin; Chu-Fang Wang; Yuh-Jeen Huang; Chun-Yu Chuang; Silver nanoparticles affect on gene expression of inflammatory and neurodegenerative responses in mouse brain neural cells. Environmental Research 2015, 136, 253-263, 10.1016/j.envres.2014.11.006.
- Al Omar Suliman Y; Daoud Ali; Saud Alarifi; Abdel Halim Harrath; Lamjed Mansour; Saleh Hamad Alwasel; Evaluation of cytotoxic, oxidative stress, proinflammatory and genotoxic effect of silver nanoparticles in human lung epithelial cells. Environmental Toxicology 2013, 30, 149-160, 10.1002/tox.21880.
- Marcella Giovanni; Junqi Yue; Lifeng Zhang; Jianping Xie; Choon Nam Ong; David Tai Leong; Pro-inflammatory responses of RAW264.7 macrophages when treated with ultralow concentrations of silver, titanium dioxide, and zinc oxide nanoparticles. Journal of Hazardous Materials 2015, 297, 146-152, 10.1016/j.jhazmat.2015.04.081.
- D.M. Brown; M.R. Wilson; William MacNee; V. Stone; K. Donaldson; Size-Dependent Proinflammatory Effects of Ultrafine Polystyrene Particles: A Role for Surface Area and Oxidative Stress in the Enhanced Activity of Ultrafines. Toxicology and Applied Pharmacology 2001, 175, 191-199, 10.1006/taap.2001.9240.
- A. Murphy; Alan Casey; Greg Byrne; G. Chambers; Orla Howe; Silver nanoparticles induce pro-inflammatory gene expression and inflammasome activation in human monocytes. Journal of Applied Toxicology 2016, 36, 1311-1320, 10.1002/jat.3315.
- Susan Christo; Akash Bachhuka; Kerrilyn R. Diener; Krasimir Vasilev; J. D. Hayball; The contribution of inflammasome components on macrophage response to surface nanotopography and chemistry. Scientific Reports 2016, 6, 26207, 10.1038/srep26207.
- Susan N. Christo; Kerrilyn R. Diener; Jim Manavis; Michele A. Grimbaldeston; Akash Bachhuka; Krasimir Vasilev; J. D. Hayball; Inflammasome components ASC and AIM2 modulate the acute phase of biomaterial implant-induced foreign body responses. Scientific Reports 2016, 6, 20635, 10.1038/srep20635.
- Jingjing Li; Xiaoqin Zhong; Fangfang Cheng; Jianrong Zhang; Li-Ping Jiang; Jun-Jie Zhu; One-Pot Synthesis of Aptamer-Functionalized Silver Nanoclusters for Cell-Type-Specific Imaging. Analytical Chemistry 2012, 84, 4140-4146, 10.1021/ac3003402.
- Steven M. Opal; Vera A. DePalo; Anti-Inflammatory Cytokines. Chest 2000, 117, 1162-1172, 10.1378/chest.117.4.1162.
- Taro Kawai; Shizuo Akira; Toll-like Receptors and Their Crosstalk with Other Innate Receptors in Infection and Immunity. Immunity 2011, 34, 637-650, 10.1016/j.immuni.2011.05.006.
- Paula M Castillo; Juan L Herrera; Rafael Fernández-Montesinos; Carlos Caro; Ana P Zaderenko; Jose A Mejías; David Pozo; Carlos Caro; David Pozo Perez; Tiopronin monolayer-protected silver nanoparticles modulate IL-6 secretion mediated by Toll-like receptor ligands. Nanomedicine 2008, 3, 627-635, 10.2217/17435889.3.5.627.
- Komal Rao; Talat Roome; Sabahat Aziz; Anam Razzak; Ghulam Abbas; Muhammad Imran; Tooba Jabri; Jasra Gul; Munawar Hussain; Bushra Sikandar; et al.Shaheen SharafatMuhammad Shah Bergenin loaded gum xanthan stabilized silver nanoparticles suppress synovial inflammation through modulation of the immune response and oxidative stress in adjuvant induced arthritic rats.. Journal of Materials Chemistry B 2018, 6, 4486-4501, 10.1039/c8tb00672e.
- Megha Mugade; Milind Patole; Varsha Pokharkar; Bioengineered mannan sulphate capped silver nanoparticles for accelerated and targeted wound healing: Physicochemical and biological investigations. Biomedicine & Pharmacotherapy 2017, 91, 95-110, 10.1016/j.biopha.2017.04.017.
- Gina El-Feky; Samar Sharaf; Amira El-Shafei; Aisha A. Hegazy; Using chitosan nanoparticles as drug carriers for the development of a silver sulfadiazine wound dressing. Carbohydrate Polymers 2017, 158, 11-19, 10.1016/j.carbpol.2016.11.054.
- Xuelai Liu; Peng Gao; Juan Du; Xin Zhao; Kenneth K Y Wong; Long-term anti-inflammatory efficacy in intestinal anastomosis in mice using silver nanoparticle-coated suture. Journal of Pediatric Surgery 2017, 52, 2083-2087, 10.1016/j.jpedsurg.2017.08.026.
- Côté-Maurais, G.; Bernier, J. Silver and fullerene nanoparticles’ effect on interleukin-2-dependent proliferation of CD4 (+) T cells. Toxicol. In Vitro 2014, 28, 1474–1481.
- Paola Chiarugi; Giovanni Pani; Elisa Giannoni; Letizia Taddei; Renata Colavitti; G. Raugei; Mark Symons; Silvia Borrello; Tommaso Galeotti; Giampietro Ramponi; et al. Reactive oxygen species as essential mediators of cell adhesion. Journal of Cell Biology 2003, 161, 933-944, 10.1083/jcb.200211118.
- Michael Schieber; Navdeep S. Chandel; ROS function in redox signaling and oxidative stress.. Current Biology 2014, 24, R453-R462, 10.1016/j.cub.2014.03.034.
- Daniel A. Gonzalez-Carter; Bey Fen Leo; Pakatip Ruenraroengsak; Shu Chen; Angela E. Goode; Ioannis G. Theodorou; Kian Fan Chung; Raffaella Carzaniga; Milo S. P. Shaffer; David T. Dexter; et al.Mary P. RyanAlexandra E. Porter Silver nanoparticles reduce brain inflammation and related neurotoxicity through induction of H2S-synthesizing enzymes. Scientific Reports 2017, 7, 42871, 10.1038/srep42871.
- Awate, S.; Babiuk, L.A.; Mutwiri, G.; Mechanisms of action of adjuvants. Front. Immunol. 2013, 4, 114.
- Nikolai Petrovsky; Comparative Safety of Vaccine Adjuvants: A Summary of Current Evidence and Future Needs. Drug Safety 2015, 38, 1059-1074, 10.1007/s40264-015-0350-4.
- Yingying Xu; Huan Tang; Jia-Hui Liu; Haifang Wang; Yuanfang Liu; Evaluation of the adjuvant effect of silver nanoparticles both in vitro and in vivo. Toxicology Letters 2013, 219, 42-48, 10.1016/j.toxlet.2013.02.010.
- Reza Ahangari Cohan; Alireza Shoari; Fahimeh Baghbani-Arani; Seyed Ataollah Sadat Shandiz; Mohammad Sadeq Khosravy; Alireza Janani; Razieh Bigdeli; Rouzbeh Bashar; Vahid Asgary; Green synthesis and evaluation of silver nanoparticles as adjuvant in rabies veterinary vaccine. International Journal of Nanomedicine 2016, 11, 3597-3605, 10.2147/IJN.S109098.
- Ye Liu; Yekkuni L. Balachandran; Dan Li; Yiming Shao; Xingyu Jiang; Polyvinylpyrrolidone–Poly(ethylene glycol) Modified Silver Nanorods Can Be a Safe, Noncarrier Adjuvant for HIV Vaccine. ACS Nano 2016, 10, 3589-3596, 10.1021/acsnano.5b08025.




