
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Floriana D’Angeli | + 2378 word(s) | 2378 | 2021-05-11 11:06:45 | | | |
| 2 | Vivi Li | + 216 word(s) | 2594 | 2021-05-12 03:14:19 | | |
Video Upload Options
The difficulty to treat resistant strains-related hospital-acquired infections (HAIs) promoted the study of phytoextracts, known sources of bioactive molecules. Accordingly, in the present study, the pharmacological activities of Juglans regia (L.) pellicle extract (WPE) were investigated. The antiviral effect was tested against Herpes simplex virus type 1 and 2, Poliovirus 1, Adenovirus 2, Echovirus 9, Coxsackievirus B1 through the plaque reduction assay. The antibacterial and antifungal activities were evaluated against medically important strains, by the microdilution method.
1. Introduction
The emerging phenomenon of antimicrobial resistance (AMR) strongly limits the available therapeutic choices for the treatment of a large variety of infectious diseases, leading to an increased risk for severe infections, complications, and mortality [1]. Besides, AMR-related drug ineffectiveness can also significantly affect the success of major surgery and cancer chemotherapy [2][3]. Indeed, the precarious immune system of these patients promotes the development of opportunistic infections, which, in the absence of appropriate therapies, could result fatally. Thus, the concomitant presence of vulnerable individuals and the constant inclination to harboring pathogens make the hospital a high-risk environment for infectious diseases [4]. In this regard, the etiological agents responsible for hospital-acquired infections (HAIs) include bacteria, fungi, and viruses [5]. A recent surveillance study showed that most HAIs are provoked by Escherichia coli (18%), Staphylococcus aureus (12%), and Klebsiella spp. (9%), followed by Pseudomonas aeruginosa (8%), Enterococcus faecalis (8%), coagulase-negative staphylococci (7%), Enterobacter spp. (5%), Enterococcus faecium (4%), Proteus spp. (3.2%), and other Enterococcus spp. (3%). Moreover, this study revealed that a certain percentage of HAIs (6.3%) is due to fungal colonization and invasion, mainly to Candida albicans which account for 3.2% of the cases [6]. However, the concrete possibility to isolate antimicrobial-resistant strains among these pathogens could seriously compromise the efficacy of the treatment, with a consequent worsening of clinical conditions and, in extreme cases, even leading to a fatal outcome.
Regarding viruses, viral opportunistic infections occur when the host defenses are reduced, a condition frequently observed in hospitalized patients [7]. In this respect, the most common viruses involved in HAIs include Herpes simplex viruses 1 and 2 (HSV-1 and HSV-2), enteroviruses such as coxsackieviruses A and B, echoviruses, polioviruses, and adenoviruses [7][8][9].
In this context, much attention has been paid to the pharmacological role of natural extracts, as a source of bioactive compounds, able to hinder both pathogen and cancer cell physiology [8]. Concerning that, it has been shown that the crude extracts of the different tissues of walnut (kernel, shell, husk, bark, root, leaves, and septum) are endowed with outstanding pharmacological activities, including the anti-inflammatory, blood clotting, neuroprotective, antioxidant, antiproliferative, and antimicrobial properties [9][10][11][12][13][14][15].
In recent work, our group demonstrated a double effect of walnut septum extract, which was able to counteract both human glioblastoma cell survival and bacterial growth [16]. On the other hand, the biological effects of the edible portion covering the walnut kernel (pellicle) remain poorly investigated. In our previous paper, we characterized the chemical constituents of walnut pellicle extract (WPE), through ultra-performance liquid chromatography–tandem mass spectrometry (UPLC-Ms/Ms) method. It is worth noting that the phytochemical profile of an extract can vary depending on the geographical area in which the relative plant has grown. Accordingly, J. regia chemical composition is strictly related to cultivar and climate area [17]. Indeed, our chemical analysis showed a high content of active compounds, mostly phenols, and hydrolyzable tannins. Interestingly, we also demonstrated the ability of WPE to inhibit the growth and biofilm formation of coagulase-negative staphylococci (CoNS). Furthermore, this extract proved to be effective in eradicating the biofilm previously formed by CoNS [18]. The promising results obtained led us to deepen the antimicrobial properties of this extract, by studying its effect on the most common etiological agents involved in HIAs.
Besides, since opportunistic infections can significantly affect the clinical outcome of oncologic patients, it could be useful to find novel antimicrobial agents also able to prevent cancer cell proliferation. Accordingly, the cytotoxic effect of WPE was evaluated on two tumoral (human colorectal adenocarcinoma cell line Caco-2 and human breast cancer cell line MCF7) and one primary (human foreskin fibroblast cell line HFF1) cell lines. Finally, by DPPH and the Folin–Ciocalteau assays, we analyzed the antioxidant properties and the total phenol content of WPE, respectively.
2. Cytotoxicity on HEp-2 and Vero Cell Lines
The 50% cytotoxic doses (CD50) of WPE on HEp-2 and VERO cells were 25.00 and 28.00 µg/mL, respectively (Table 1). The non-cytotoxic doses were used to perform the antiviral evaluation.
Table 1. Antiviral activity of walnut pellicle extract.
| CD50 a | ID50 b | |||||||
|---|---|---|---|---|---|---|---|---|
| HEp-2 | VERO | HSV-1 | HSV-2 | ECHO 9 | Polio 1 | Coxsackie B1 | Adeno 2 | |
| WPE (µg/mL) c | 25.00 | 28.00 | 10.00 | 8.00 | ORC d | ORC | ORC | ORC |
| Acyclovir (µg/mL) | >600.00 | >600.00 | 0.12 | 0.36 | ORC | ORC | ORC | ORC |
a CD50, concentration which inhibited 50% cell growth compared to control cultures; b ID50, the concentration which inhibited 50% virus plaque formation and virus-induced cytopathogenicity. Values are mean ± S.D. (maximal S.D. estimated) for three separate assays. c WPE: walnut pellicle extract. d ORC: out of range of concentration.
3. Antiviral Activity
Results obtained from our screening demonstrated that WPE had an inhibitory effect on HSV-1 and HSV-2 replication at doses below the CD50. The ID50 values were found to be 10 and 8 µg/mL for HSV-1 and HSV-2, respectively. The antiviral effect of WPE was compared to acyclovir, to which HSV-1 and HSV-2 showed an ID50 of 0.12 and 0.36 µg/mL, respectively. WPE was ineffective against Echovirus 9 (ECHO 9), Poliovirus 1 (Polio 1), Coxsackievirus B1 (Coxsackie B1), and Adenovirus 2 (Adeno 2) (Table 1). The study of the effect of the compound on neutralization of viral infectivity demonstrated that WPE had not virucidal activity against all the viruses tested.
4. Antibacterial Activity
Based on minimal inhibitory concentration (MIC) values, which ranged from 8.59 to 275.00 µg/mL, the Gram-positive strains were more sensitive to WPE than the Gram-negative ones. It is worth noting that WPE, at the concentration of 275.00 µg/mL, was able to inhibit the growth of five clinical strains resistant to ciprofloxacin (E. faecium 018/040, E. coli 024/040, E. coli 025/040, Klebsiella pneumoniae (K. pneumoniae) 035/040 and P. aeruginosa 028/040). The results of the antibacterial activity are shown in Table 2.
Table 2. Antibacterial activity of walnut pellicle extract against Gram-positive and Gram-negative strains.
|
Bacterial Strains b |
Source |
MIC a (µg/mL) |
I.C. e |
|
|---|---|---|---|---|
|
WPE c |
Cip d |
|||
|
Range [0.53–275.00] |
[0.06–32.00] |
|||
|
Gram-positive |
||||
|
E. faecalis 012/040 |
Abscess |
8.59 |
0.50 |
S |
|
E. faecium 019/040 |
Catheter cystitis |
8.59 |
1.00 |
S |
|
S. epidermidis 007/040 |
Osteomyelitis |
8.59 |
0.031 |
S |
|
S. epidermidis ATCC 14990 |
Standard |
8.59 |
0.125 |
S |
|
E. faecalis 015/040 |
Abscess |
17.18 |
0.25 |
S |
|
S. aureus 002/040 |
Endophtalmitis |
17.18 |
0.25 |
S |
|
S. aureus 004/040 |
Pneumonia |
17.18 |
0.25 |
S |
|
E. faecalis 013/040 |
Septicemia |
34.37 |
0.50 |
S |
|
S. aureus 005/040 |
Endophtalmitis |
34.37 |
0.50 |
S |
|
E. faecalis ATCC 29212 |
Standard |
68.75 |
0.50 |
S |
|
E. faecalis 014/040 |
Pneumonia |
68.75 |
1.00 |
S |
|
E. faecium 018/040 |
Catheter cystitis |
275.00 |
8.00 |
R |
|
S. aureus ATCC 29213 |
Standard |
275.00 |
0.50 |
S |
|
S. epidermidis 009/040 |
Endophtalmitis |
275.00 |
0.015 |
S |
|
E. faecium 017/040 |
Cholecystitis |
>275.00 |
>32.00 |
R |
|
E. faecium 020/040 |
Cholecystitis |
>275.00 |
16.00 |
R |
|
E. faecium ATCC 700221 |
Standard |
>275.00 |
>32.00 |
R |
|
S. aureus 003/040 |
Pneumonia |
>275.00 |
4.00 |
R |
|
S. epidermidis 008/040 |
Septicemia |
>275.00 |
8.00 |
R |
|
S. epidermidis 010/040 |
Septicemia |
>275.00 |
8.00 |
R |
|
Gram-negative |
||||
|
E. coli 024/040 |
Cystitis |
275.00 |
4.00 |
R |
|
E. coli 025/040 |
Cystitis |
275.00 |
8.00 |
R |
|
K. pneumoniae 035/040 |
Nephritis |
275.00 |
4.00 |
R |
|
P. aeruginosa 028/040 |
Septicemia |
275.00 |
4.00 |
R |
|
P. aeruginosa 029/040 |
Pneumonia |
275.00 |
0.125 |
S |
|
P. mirabilis 038/040 |
Cystitis |
275.00 |
0.015 |
S |
|
P. mirabilis ATCC 7002 |
Standard |
275.00 |
0.25 |
S |
|
E. coli 022/040 |
Septicemia |
>275.00 |
0.015 |
S |
|
E. coli 023/040 |
Septicemia |
>275.00 |
>32.00 |
R |
|
E. coli ATCC 35218 |
Standard |
>275.00 |
0.015 |
S |
|
K. pneumoniae 032/040 |
Nephritis |
>275.00 |
4.00 |
R |
|
K. pneumoniae 033/040 |
Pneumonia |
>275.00 |
32.00 |
R |
|
K. pneumoniae 034/040 |
Pneumonia |
>275.00 |
8.00 |
R |
|
K. pneumoniae ATCC 700630 |
Standard |
>275.00 |
0.25 |
S |
|
P. aeruginosa 027/040 |
Septicemia |
>275.00 |
0.06 |
S |
|
P. aeruginosa 030/040 |
Pneumonia |
>275.00 |
16.00 |
R |
|
P. aeruginosa ATCC 27853 |
Standard |
>275.00 |
0.25 |
S |
|
P. mirabilis 037/040 |
Cystitis |
>275.00 |
1.00 |
S |
|
P. mirabilis 039/040 |
Cystitis |
>275.00 |
0.015 |
S |
|
P. mirabilis 040/040 |
Cystitis |
>275.00 |
8.00 |
R |
a MIC: Minimal Inhibitory Concentration; b Strain numbers refer to an internal directory for bacteria. c WPE: walnut pellicle extract; d Cip: Ciprofloxacin; e I.C.: Interpretive criteria for Ciprofloxacin (CLSI M100-S28): ≤1 Susceptible (S); 2 Intermediate (I); ≥4 Resistant (R).
5. Discussion
Hospital-acquired infections represent a serious complication, often arising during the convalescence period in a medical facility [19]. It has been reported that HAIs amount to the first cause of death among hospitalized patients. To prevent these injurious events, the Centers for Disease Control and Prevention (CDC) constantly monitor the clinical practices correlated to an increased risk for microorganism contamination and invasion, including ventilator-associated pneumonia, catheter-associated urinary tract infections, bloodstream infections, and surgical site infections [20]. These illnesses can be caused by different pathogens, belonging to bacteria, fungi, or virus’s family. Accordingly, infectious diseases can significantly compromise the clinical outcome of the patients, including the oncologic ones. The increasing development of resistant mechanisms to the most commonly used drug promoted the research of new bioactive chemical compounds from phytoextracts. Concerning that literature data demonstrated the antimicrobial and the antiproliferative effects of walnut extracts [11][16][21][22][23]. However, despite the great attention paid to J. regia extracts, the knowledge on the biological effects of the edible pellicle is still limited.
In our previous work, we proved an antibacterial and antibiofilm activity of the present extract against CoNS. Furthermore, by UPLC-Ms/Ms, we chemically analyzed the phytoextract, revealing the presence of several biologically active molecules (Table 5) [18]. These results led us to hypothesize further antimicrobial properties. Indeed, in the present study, we found that WPE exerts an interesting activity against HSV-1 and HSV-2, with a concentration-dependent antiviral effect. It was demonstrated that natural extracts rich in polyphenols are capable to directly block virus attachment to host-cell [24][25] or interfering with the early phases of the replicative cycle [26]. Accordingly, the anti-herpes effect may be attributed to a synergistic effect of different compounds, belonging to phenols, flavonoids, and sterols. For example, among flavonoids, rutin and quercetin exerted potent anti-herpetic activity, both showing an EC50 of 5 μM against HSV-1, although only quercetin inhibited HSV-2 infection, with an EC50 of 35 μM [27][28]. A number of reports highlighted the antiviral role of quercetin against HSV. Hung et al. demonstrated the ability of quercetin to inhibit HSV-1 infection of Vero cells [29]. A further study showed that this molecule is able to modulate the expression of HSV proteins, such as the viral glycoprotein D (gD), an essential protein for the attachment of virus on host cell membrane during the infectious process, and ICP0, a viral protein expressed in the early phases of the HSV-1 replication cycle. Furthermore, the authors also demonstrated that quercetin acts at the genic level, downregulating the correlated gene-replication such as ICP0, UL13, and UL52 [30]. The WPE contains other compounds [18], including protocatechuic acid, myricetin [31], gallic acid, ellagic acid, kaempferol [32], and β-sitosterol [33], which were also associated with the anti-HSV activity. Interestingly, an in-silico analysis showed that tris-juglone, one of the previously determined constituents of WPE (Table 3) [18], showed antiviral activity against the SARS-CoV-2 virus by inhibiting cathepsin L, a lysosomal cysteine endopeptidase involved in the activation of heparinase [34]. The indirect block of this enzyme reduces the egress of the virus from the host cell. Since cathepsin L plays the same role in HSV-2, we can hypothesize that the HSV-inhibitory effect of WPE could be similarly mediated by tris-juglone [35].
Table 3. Chemical compounds identified from walnut pellicle extract through UPLC-Ms/Ms [18] and related pharmacological activities.
| Chemical Name | Chemical Class | Chemical Structure | RT * (min) | m/z (g/mol) | Pharmacological Activities |
|---|---|---|---|---|---|
| Protocatechuic acid | Benzoic acid and Phenol derivatives | 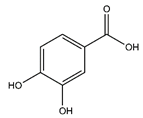 |
35.09 | 154.027 | Antiviral [31] Antibacterial [36] Antioxidant [37] |
| Gallic acid | Benzoic acid and Phenol derivatives | 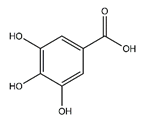 |
18.60 | 170.021 | Antiviral [32] Antibacterial [38] Antioxidant [37] |
| Ferulic acid | Cinnamic acids and derivatives | 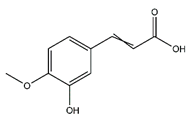 |
36.20 | 194.058 | Antibacterial [38] Antioxidant [39] |
| Sinapate | Cinnamic acids and derivatives | 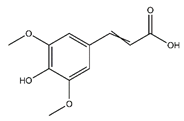 |
n.r. | 224.210 | Antibacterial, Antifungal, Antitumor [40] |
| Palmitic acid | Fatty acids | 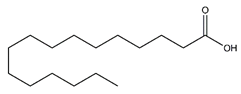 |
12.43 | 256.240 | Antioxidant [41][42] Antitumor [43] |
| Oleic acid | Fatty acids | 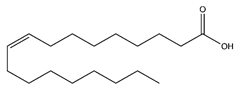 |
37.24 | 282.256 | Antioxidant [41][42] Antitumor [43] |
| Ellagic acid | Tannins | 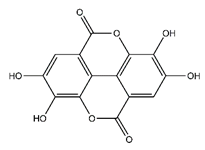 |
33.20 | 302.006 | Antiviral [32] Antioxidant [44] Antitumor [45] |
| Quercetin | Flavonoids | 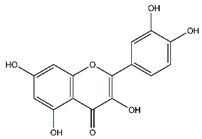 |
33.97 | 302.043 | Antiviral [27][28] Antibacterial [46] Antifungal [47] Antioxidant [39] Antitumor [45] |
| Myricetin | Flavonoids | 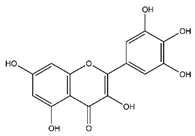 |
33.78 | 318.038 | Antiviral [31] Antifungal [47] Antitumor [45] |
| Chlorogenic acid | Cinnamate ester derivatives | 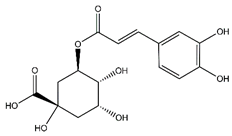 |
39.49 | 354.095 | Antioxidant [48] |
| Beta-sitosterol | Steroids and steroid derivatives | 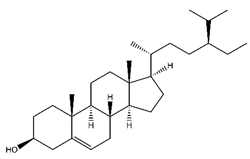 |
33.89 | 411.386 | Antiviral [33] |
| Kaempferol-arabinoside | Flavonoids | 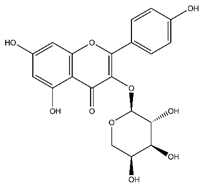 |
n.r. | 418.080 | Antiviral [32] |
| Tocopherol | Prenol lipids | 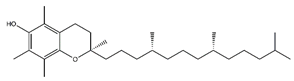 |
36.07 | 430.381 | Antioxidant [41] |
| Avicularin | Flavonoids | 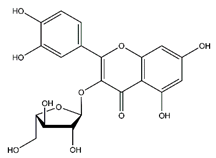 |
20.08 | 434.085 | Antifungal [49] Antitumor [50] |
| Tris-juglone | Phenanthrenes and derivatives | 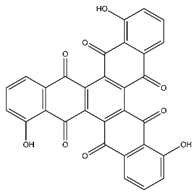 |
36.94 | 516.048 | Anti-SARS-CoV-2 (in-silico analysis) [34] |
| (+)-Procyanidin B2 | Flavonoids | 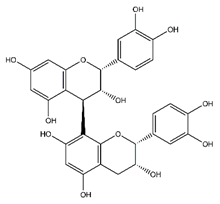 |
38.25 | 578.520 | Antibacterial, Antioxidant, Antitumor [51] |
| Rutin | Flavonoids | 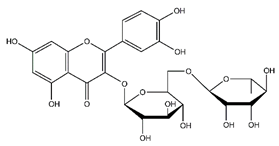 |
45.11 | 610.153 | Antibacterial [52] Antiviral [27][28][29] Antifungal [47] Antioxidant [39] Antitumor [45] |
Notes: * RT: retention time; n.r.: not reported. The retention time of the compounds was not reported, due to their low concentrations in the extract.
Furthermore, WPE was more efficient in reducing bacterial growth of Gram-positive strains compared to those Gram-negative, as indicated by the lower MIC values. This dissimilar effect reflects differences in the cell wall composition between the two bacterial groups [16][53]. Indeed, Gram-negative bacteria are characterized by a highly selective external membrane to the passage of the molecules, which makes these bacteria more resistant to drugs [53][54][55]. Nevertheless, as reported by Saraiva et al., since several bacteria strains showed MIC values under the concentration of 500 µg/mL, WPE can be considered an active antimicrobial agent [56].
Candida species are also responsible for healthcare-related infections both in immunocompetent and immunocompromised hosts [57][58]. Although new synthetic drugs are generally active against resistant Candida species, their administration can cause toxicity, interactions with other drugs, and inadequate bioavailability [59][60][61]. Experimental evidence supported the anticandidal activity of different extracts from J. regia, including leaf, bark, and root [62][63][64][65]. In agreement with these data, our findings showed an antifungal activity of WPE against different Candida species, with MICs ranging from 17.18 to 275.00 µg/mL. This effect could be attributed to the presence of the flavonoids quercetin, myricetin, and rutin [47]. Besides, in our previous chromatographic analysis, we identified the flavonol avicularin. This compound was isolated from Juglans sinensis leaves [66]. Da Silva Sa et al. demonstrated that this compound is able to inhibit C. albicans growth in concentrations of 2 to 16 µg/mL [49]. Concerning non-albicans Candida species, the extract was less efficient in reducing the growth of these pathogens. This effect could be due to both a reduced permeability of these yeasts to natural extracts and to their higher capacity to produce biofilm [67][68].
Taken together, these results clearly showed good antimicrobial effects of WPE, revealing its efficacy against different pathogens. In the oncologic field, the development of opportunistic infections is considered a common side effect of chemotherapy [69]. According to this concept, we evaluated the potential antiproliferative action of the extract, by treating two different cancer cell lines (MCF-7 and Caco-2 cells) and a primary fibroblast cell line (HFF-1) with increasing concentrations of WPE, for 24 h, 48 h, and 72 h. Interestingly, after a prolonged period (48 h and 72 h) the treatment with WPE significantly reduced Caco-2 cell viability in a dose-dependent manner. Considering that the pellicle of walnut is edible, being concomitantly ingested with the fruit and that the gastrointestinal tract is the most exposed district to the action of dietary ingredients, it is possible to hypothesize a benefic effect of this portion of the walnut, rich in polyphenols, at the intestinal level [70]. In this respect, literature data showed the ability of a large variety of classes of dietary polyphenols, including rutin, quercetin, myricetin, ellagic acid [45], and avicularin [50], all present in our extract, to affect glucose transport in Caco-2 cells via both facilitated transport proteins (GLUT 1 and 2) and sodium-dependent glucose transporter 1 (SGLT1). Several natural extracts, usually rich in these molecules, were also found to inhibit glucose uptake in Caco-2 cells through these mechanisms [50][71]. Being the main cellular energetic source, glucose plays a key role also in cancer cell growth [72]. It has been demonstrated that the inhibition of glucose uptake is able to arrest cancer progression [73]. Therefore, in our case, the cytotoxic effect of WPE on Caco-2 cells could be attributed to the reduced glucose internalization, mediated by the synergistic action of polyphenolic compounds. Conversely, the inefficacy of WPE on MCF-7 cells confirmed the results obtained in our previous studies, which showed a greater drug resistance of these cells, induced by the breast cancer resistance protein (BCRP) expression [74]. Notably, WPE did not determine any cytotoxic effect on the primary HFF-1 cells.
The protective role of WPE was further explored by evaluating its potential scavenger activity against reactive oxygen species (ROS). As expected, the extract inhibited superoxide anion formation similarly to the SOD enzyme, also showing the ability to bleach the stable DPPH radical. The free radical-scavenging activity is strictly related to the high content of phenols and flavonoids, as determined by the Folin–Ciocalteu method (Table 4). These results are consistent with other studies focusing on J. regia pellicle extracts, which revealed a high amount of phenols and flavonoids, mostly in yellow pellicle than in red ones [75][76].
Table 4. Antioxidant activity and total phenolic and flavonoid contents of walnut pellicle extract.
| Sample | SOD-like Activity | DPPH Test | Total Phenolic | Total Flavonoids |
|---|---|---|---|---|
| IC50 (μg/mL) | Gallic Acid (mg/g) | Catechin (mg/g) | ||
| WPE a | 80 ± 0.51 | 48.35 ± 1.7 | 0.377 ± 0.01 | 0.292 ± 0.08 |
| SOD b | 50 mU ± 0.85 | - | - | - |
| Trolox | - | 15 mM ± 0.62 | - | - |
a WPE: walnut pellicle extract; b SOD: superoxide dismutase.
References
- Hay, S.I.; Rao, P.C.; Dolecek, C.; Day, N.P.J.; Stergachis, A.; Lopez, A.D.; Murray, C.J.L. Measuring and mapping the global burden of antimicrobial resistance. BMC Med. 2018, 16, 1–3.
- Raymond, D.P.; Kuehnert, M.J.; Sawyer, R.G. Preventing Antimicrobial-Resistant Bacterial Infections in Surgical Patients. Surg. Infect. 2002, 3, 375–385.
- Zhou, S.; Fan, L.; Wang, Z.; Wang, Q.; Xiong, Z.; Xu, Y.; Li, D. Increasing rates of Acinetobacter baumannii infection and resistance in an oncology department. J. Cancer Res. Ther. 2018, 14, 68–71.
- Chng, K.R.; MetaSUB Consortium; Li, C.; Bertrand, D.; Ng, A.H.Q.; Kwah, J.S.; Low, H.M.; Tong, C.; Natrajan, M.; Zhang, M.H.; et al. Cartography of opportunistic pathogens and antibiotic resistance genes in a tertiary hospital environment. Nat. Med. 2020, 26, 941–951.
- Khan, H.A.; Baig, F.K.; Mehboob, R. Nosocomial infections: Epidemiology, prevention, control and surveillance. Asian Pac. J. Trop. Biomed. 2017, 7, 478–482.
- Weiner-Lastinger, L.M.; Abner, S.; Benin, A.L.; Edwards, J.R.; Kallen, A.J.; Karlsson, M.; Magill, S.S.; Pollock, D.; See, I.; Soe, M.M.; et al. Antimicrobial-resistant pathogens associated with pediatric healthcare-associated infections: Summary of data reported to the National Healthcare Safety Network, 2015–2017. Infect. Control. Hosp. Epidemiol. 2019, 41, 19–30.
- Cabrera-Cancio, M.R. Infections and the Compromised Immune Status in the Chronically Critically Ill Patient: Prevention Strategies. Respir. Care 2012, 57, 979–992.
- Genovese, C.; Acquaviva, R.; Ronsisvalle, S.; Tempera, G.; Malfa, G.A.; D’Angeli, F.; Ragusa, S.; Nicolosi, D. In vitro evaluation of biological activities of Orobanche crenata Forssk. leaves extract. Nat. Prod. Res. 2020, 34, 3234–3238.
- Papoutsi, Z.; Kassi, E.; Chinou, I.; Halabalaki, M.; Skaltsounis, L.A.; Moutsatsou, P. Walnut extract (Juglans regia L.) and its component ellagic acid exhibit anti-inflammatory activity in human aorta endothelial cells and osteoblastic activity in the cell line KS483. Br. J. Nutr. 2008, 99, 715–722.
- Jahanban-Esfahlan, A.; Ostadrahimi, A.; Tabibiazar, M.; Amarowicz, R. A Comparative Review on the Extraction, Antioxidant Content and Antioxidant Potential of Different Parts of Walnut (Juglans regia L.) Fruit and Tree. Molecules 2019, 24, 2133.
- Salimi, M.; Ardestaniyan, M.H.; Kandelous, H.M.; Saeidnia, S.; Gohari, A.R.; Amanzadeh, A.; Sanati, H.; Sepahdar, Z.; Ghorbani, S. Anti-proliferative and apoptotic activities of constituents of chloroform extract of Juglans regialeaves. Cell Prolif. 2014, 47, 172–179.
- Amirou, A.; Bnouham, M.; Legssyer, A.; Ziyyat, A.; Aziz, M.; Berrabah, M.; Mekhfi, H. Effects of Juglans regia Root Bark Extract on Platelet Aggregation, Bleeding Time, and Plasmatic Coagulation: In Vitro and Ex Vivo Experiments. Evid. Based Complement. Altern. Med. 2018, 2018, 1–7.
- Nasiry, D.; Khalatbary, A.R.; Ahmadvand, H.; Amiri, F.B.T.; Akbari, E. Protective effects of methanolic extract of Juglans regia L. leaf on streptozotocin-induced diabetic peripheral neuropathy in rats. BMC Complement. Altern. Med. 2017, 17, 476.
- Bakht, J.; Khan, S.; Shafi, M.; Iqbal, A. Fractionation of crude extracts from controlled dried and commercially available stem bark of Juglans regia and their antimicrobial effects. Pak. J. Pharm. Sci. 2017, 30, 1581–1588.
- Ahmad, S.; Wahid, M.A.; Bukhari, A.Q.S. Fungistatic Action of Juglans. Antimicrob. Agents Chemother. 1973, 3, 436–438.
- Genovese, C.; Cambria, M.T.; D’Angeli, F.; Addamo, A.P.; Malfa, G.A.; Siracusa, L.; Pulvirenti, L.; Anfuso, C.D.; Lupo, G.; Salmeri, M. The double effect of walnut septum extract (Juglans regia L.) counteracts A172 glioblastoma cell survival and bacterial growth. Int. J. Oncol. 2020, 57, 1129–1144.
- Wu, S.; Ni, Z.; Wang, R.; Zhao, B.; Han, Y.; Zheng, Y.; Liu, F.; Gong, Y.; Tang, F.; Liu, Y. The effects of cultivar and climate zone on phytochemical components of walnut (Juglans regia L.). Food Energy Secur. 2020, 9.
- Acquaviva, R.; D’Angeli, F.; Malfa, G.A.; Ronsisvalle, S.; Garozzo, A.; Stivala, A.; Ragusa, S.; Nicolosi, D.; Salmeri, M.; Genovese, C. Antibacterial and anti-biofilm activities of walnut pellicle extract (Juglans regia L.) against coagulase-negative staphylococci. Nat. Prod. Res. 2019, 10, 1–6.
- Lobdell, K.W.; Stamou, S.; Sanchez, J.A. Hospital-Acquired Infections. Surg. Clin. North. Am. 2012, 92, 65–77.
- Boev, C.; Kiss, E. Hospital-Acquired Infections. Crit. Care Nurs. Clin. N. Am. 2017, 29, 51–65.
- Kaur, K.; Michael, H.; Arora, S.; Härkönen, P.L.; Kumar, S. Studies on Correlation of Antimutagenic and Antiproliferative Activities of Juglans regia L. J. Environ. Pathol. Toxicol. Oncol. 2003, 22, 57–66.
- Jagtap, A.G.; Karkera, S.G. Extract ofJuglandaceae regiaInhibits Growth, In-vitro Adherence, Acid Production and Aggregation ofStreptococcus mutans. J. Pharm. Pharmacol. 2000, 52, 235–242.
- Moghaddam, P.Z.; Mohammadi, A.; Feyzi, P.; Alesheikh, P. In vitro antioxidant and antibacterial activity of various extracts from exocarps and endocarps of walnut. Pak. J. Pharm. Sci. 2017, 30, 1725–1731.
- Bisignano, C.; Mandalari, G.; Smeriglio, A.; Trombetta, D.; Pizzo, M.M.; Pennisi, R.; Sciortino, M.T. Almond Skin Extracts Abrogate HSV-1 Replication by Blocking Virus Binding to the Cell. Viruses 2017, 9, 178.
- Astani, A.; Reichling, J.; Schnitzler, P. Melissa officinalis Extract Inhibits Attachment of Herpes Simplex Virus in vitro. Chemotherapy 2012, 58, 70–77.
- Yang, C.-M.; Cheng, H.-Y.; Lin, T.-C.; Chiang, L.-C.; Lin, C.-C. The in vitro activity of geraniin and 1,3,4,6-tetra-O-galloyl-β-d-glucose isolated from Phyllanthus urinaria against herpes simplex virus type 1 and type 2 infection. J. Ethnopharmacol. 2007, 110, 555–558.
- Lyu, S.-Y.; Rhim, J.-Y.; Park, W.-B. Antiherpetic activities of flavonoids against herpes simplex virus type 1 (HSV-1) and type 2 (HSV-2) in vitro. Arch. Pharmacal Res. 2005, 28, 1293–1301.
- Jakub, T.; Gazdová, M.; Smejkal, K.; Šudomová, M.; Kubatka, P.; Hassan, S.T. Natural Products-Derived Chemicals: Breaking Barriers to Novel Anti-HSV Drug Development. Viruses 2020, 12, 154.
- Hung, P.-Y.; Ho, B.-C.; Lee, S.-Y.; Chang, S.-Y.; Kao, C.-L.; Lee, S.-S.; Lee, C.-N. Houttuynia cordata Targets the Beginning Stage of Herpes Simplex Virus Infection. PLoS ONE 2015, 10, e0115475.
- Lee, S.; Lee, H.H.; Shin, Y.-S.; Kang, H.; Cho, H. The anti-HSV-1 effect of quercetin is dependent on the suppression of TLR-3 in Raw 264.7 cells. Arch. Pharmacal Res. 2017, 40, 623–630.
- Li, W.; Xu, C.; Hao, C.; Zhang, Y.; Wang, Z.; Wang, S.; Wang, W. Inhibition of herpes simplex virus by myricetin through targeting viral gD protein and cellular EGFR/PI3K/Akt pathway. Antivir. Res. 2020, 177, 104714.
- El-Toumy, S.A.; Salib, J.Y.; El-Kashak, W.A.; Marty, C.; Bedoux, G.; Bourgougnon, N. Antiviral effect of polyphenol rich plant extracts on herpes simplex virus type 1. Food Sci. Hum. Wellness 2018, 7, 91–101.
- Parvez, M.K.; Alam, P.; Arbab, A.H.; Al-Dosari, M.S.; Alhowiriny, T.A.; Alqasoumi, S.I. Analysis of antioxidative and antiviral biomarkers β-amyrin, β-sitosterol, lupeol, ursolic acid in Guiera senegalensis leaves extract by validated HPTLC methods. Saudi Pharm. J. 2018, 26, 685–693.
- Vivek-Ananth, R.P.; Rana, A.; Rajan, N.; Biswal, H.S.; Samal, A. In Silico Identification of Potential Natural Product Inhibitors of Human Proteases Key to SARS-CoV-2 Infection. Molecules 2020, 25, 3822.
- Hopkins, J.; Yadavalli, T.; Agelidis, A.M.; Shukla, D. Host Enzymes Heparanase and Cathepsin L Promote Herpes Simplex Virus 2 Release from Cells. J. Virol. 2018, 92.
- Stojković, D.; Živković, J.; Sokovic, M.; Glamočlija, J.; Ferreira, I.C.F.R.; Jankovic, T.; Maksimović, Z. Antibacterial activity of Veronica montana L. extract and of protocatechuic acid incorporated in a food system. Food Chem. Toxicol. 2013, 55, 209–213.
- Zhang, Z.; Liao, L.; Moore, J.; Wu, T.; Wang, Z. Antioxidant phenolic compounds from walnut kernels (Juglans regia L.). Food Chem. 2009, 113, 160–165.
- Borges, A.; Ferreira, C.; Saavedra, M.J.; Simões, M. Antibacterial Activity and Mode of Action of Ferulic and Gallic Acids Against Pathogenic Bacteria. Microb. Drug Resist. 2013, 19, 256–265.
- Ho, K.-V.; Roy, A.; Foote, S.; Vo, P.H.; Lall, N.; Lin, C.-H. Profiling Anticancer and Antioxidant Activities of Phenolic Compounds Present in Black Walnuts (Juglans nigra) Using a High-Throughput Screening Approach. Molecules 2020, 25, 4516.
- Chen, C. Sinapic Acid and Its Derivatives as Medicine in Oxidative Stress-Induced Diseases and Aging. Oxidative Med. Cell. Longev. 2016, 2016, 1–10.
- Li, L.; Tsao, R.; Yang, R.; Kramer, A.J.K.G.; Hernandez, M. Fatty Acid Profiles, Tocopherol Contents, and Antioxidant Activities of Heartnut (Juglans ailanthifolia Var. cordiformis) and Persian Walnut (Juglans regia L.). J. Agric. Food Chem. 2007, 55, 1164–1169.
- Cotticelli, M.G.; Forestieri, R.; Xia, S.; Joyasawal, S.; Lee, T.; Xu, K.; Smith, I.A.B.; Huryn, D.M.; Wilson, R.B. Identification of a Novel Oleic Acid Analog with Protective Effects in Multiple Cellular Models of Friedreich Ataxia. ACS Chem. Neurosci. 2020, 11, 2535–2542.
- Batirel, S.; Yilmaz, A.M.; Sahin, A.; Perakakis, N.; Ozer, N.K.; Mantzoros, C.S. Antitumor and antimetastatic effects of walnut oil in esophageal adenocarcinoma cells. Clin. Nutr. 2018, 37, 2166–2171.
- Negi, A.S.; Luqman, S.; Srivastava, S.; Krishna, V.; Gupta, N.; Darokar, M.P. Antiproliferative and antioxidant activities of Juglans regiafruit extracts. Pharm. Biol. 2011, 49, 669–673.
- Johnston, K.; Sharp, P.; Clifford, M.; Morgan, L. Dietary polyphenols decrease glucose uptake by human intestinal Caco-2 cells. FEBS Lett. 2005, 579, 1653–1657.
- Pereira, J.A.; Oliveira, I.; Sousa, A.; Valentão, P.; Andrade, P.B.; Ferreira, I.C.F.R.; Ferreres, F.; Bento, A.; Seabra, R.M.; Estevinho, L. Walnut (Juglans regia L.) leaves: Phenolic compounds, antibacterial activity and antioxidant potential of different cultivars. Food Chem. Toxicol. 2007, 45, 2287–2295.
- Al-Aboody, M.S.; Mickymaray, S. Anti-Fungal Efficacy and Mechanisms of Flavonoids. Antibiotics 2020, 9, 45.
- Nakatani, N.; Kayano, S.-I.; Kikuzaki, H.; Sumino, K.; Katagiri, A.K.; Mitani, T. Identification, Quantitative Determination, and Antioxidative Activities of Chlorogenic Acid Isomers in Prune (Prunusdomestica L.). J. Agric. Food Chem. 2000, 48, 5512–5516.
- Sa, F.A.D.S.; de Paula, J.A.M.; dos Santos, P.A.; Oliveira, L.D.A.R.; Oliveira, G.D.A.R.; Lião, L.M.; Paula, J.R.; Silva, M. Phytochemical Analysis and Antimicrobial Activity of Myrcia tomentosa (Aubl.) DC. Leaves. Molecules 2017, 22, 1100.
- Müller, U.; Stübl, F.; Schwarzinger, B.; Sandner, G.; Iken, M.; Himmelsbach, M.; Schwarzinger, C.; Ollinger, N.; Stadlbauer, V.; Höglinger, O.; et al. In Vitro and In Vivo Inhibition of Intestinal Glucose Transport by Guava (Psidium Guajava) Extracts. Mol. Nutr. Food Res. 2018, 62, e1701012.
- Luca, S.V.; Bujor, A.; Miron, A.; Aprotosoaie, A.C.; Skalicka-Woźniak, K.; Trifan, A. Preparative separation and bioactivity of oligomeric proanthocyanidins. Phytochem. Rev. 2020, 19, 1093–1140.
- Han, K.-I.; Jung, E.-G.; Patnaik, B.B.; Hong, C.-I.; Kim, Y.-J.; Jung, S.; Han, M.-D. Antibacterial and Antioxidant Activities of Leaf Extracts from Juglans sinensis, and its Phenolic Compositions. Nat. Prod. Commun. 2017, 12, 1797–1800.
- Auer, G.K.; Weibel, D.B. Bacterial Cell Mechanics. Biochemistry 2017, 56, 3710–3724.
- Exner, M.; Bhattacharya, S.; Christiansen, B.; Gebel, J.; Goroncy-Bermes, P.; Hartemann, P.; Heeg, P.; Ilschner, C.; Kramer, A.; Larson, E.; et al. Antibiotic resistance: What is so special about multidrug-resistant Gram-negative bacteria? GMS Hyg. Infect. Control. 2017, 12.
- Caporarello, N.; Olivieri, M.; Cristaldi, M.; Scalia, M.; Toscano, M.A.; Genovese, C.; Addamo, A.; Salmeri, M.; Lupo, G.; Anfuso, C.D. Blood–Brain Barrier in a Haemophilus influenzae Type a In Vitro Infection: Role of Adenosine Receptors A2A and A2B. Mol. Neurobiol. 2017, 55, 5321–5336.
- Saraiva, M.; Castro, R.H.A.; Cordeiro, R.P.; Sobrinho, T.J.S.P.; Amorim, E.L.C.; Xavier, H.S.; Pisciottano, M.N.C. In vitro evaluation of antioxidant, antimicrobial and toxicity properties of extracts of Schinopsis brasiliensis Engl. (Anacardiaceae). Afr. J. Pharm. Pharmacol. 2011, 5, 1724–1731.
- Rudramurthy, S.M.; Singh, S. Candida Infections in Immunocompetent Hosts: Pathogenesis and Diagnosis. Curr. Fungal Infect. Rep. 2020, 14, 233–245.
- Genovese, C.; Corsello, S.; Nicolosi, D.; Aidala, V.; Falcidia, E.; Tempera, G. Alterations of the vaginal microbiota in the third trimester of pregnancy and pPROM. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 3336–3343.
- Gintjee, T.J.; Donnelley, M.A.; Thompson, G.R. Aspiring Antifungals: Review of Current Antifungal Pipeline Developments. J. Fungi 2020, 6, 28.
- Genovese, C.; Cianci, A.; Corsello, S.; Ettore, G.; Mattana, P.; Tempera, G. Combined systemic (fluconazole) and topical (metronidazole + clotrimazole) therapy for a new approach to the treatment and prophylaxis of recurrent candidiasis. Minerva Ginecol. 2019, 71, 321–328.
- Genovese, C.; Pulvirenti, L.; Cardullo, N.; Muccilli, V.; Tempera, G.; Nicolosi, D.; Tringali, C. Bioinspired benzoxanthene lignans as a new class of antimycotic agents: Synthesis and Candida spp. growth inhibition. Nat. Prod. Res. 2018, 34, 1653–1662.
- Sytykiewicz, H.; Chrzanowski, G.; Czerniewicz, P.; Leszczyński, B.; Sprawka, I.; Krzyżanowski, R.; Matok, H. Antifungal Activity of Juglans regia (L.) Leaf Extracts Against Candida albicans Isolates. Pol. J. Environ. Stud. 2015, 24, 1339–1348.
- Noumi, E.; Snoussi, M.; Hajlaoui, H.; Valentin, E.; Bakhrouf, A. Antifungal properties of Salvadora persica and Juglans regia L. extracts against oral Candida strains. Eur. J. Clin. Microbiol. Infect. Dis. 2009, 29, 81–88.
- Raja, V.; Ahmad, S.; Irshad, M.; Wani, W.; Siddiqi, W.; Shreaz, S. Anticandidal activity of ethanolic root extract of Juglans regia (L.): Effect on growth, cell morphology, and key virulence factors. J. Med. Mycol. 2017, 27, 476–486.
- Jafer, F.N.; A Naser, L. The Biological Activity of Aqueous and Methanolic Extracts of Juglans regia on Yeasts and Pathologic Bacteria. Arch. Clin. Microbiol. 2020, 11, 2405–2410.
- An, R.-B.; Kim, H.-C.; Tian, Y.-H.; Kim, Y.-C. Free Radical Scavenging and Hepatoprotective Constituents from the Leaves of Juglans sinensis. Arch. Pharmacal. Res. 2005, 28, 529–533.
- Thevissen, K.; Ghazi, A.; de Samblanx, G.W.; Brownlee, C.; Osborn, R.W.; Broekaert, W.F. Fungal Membrane Responses Induced by Plant Defensins and Thionins. J. Biol. Chem. 1996, 271, 15018–15025.
- Tay, L.Y.; Jorge, J.H.; Herrera, D.R.; Campanha, N.H.; Gomes, B.P.F.A.; dos Santos, F.A. Evaluation of different treatment methods against denture stomatitis: A randomized clinical study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2014, 118, 72–77.
- Taplitz, R.A.; Kennedy, E.B.; Bow, E.J.; Crews, J.; Gleason, C.; Hawley, D.K.; Langston, A.A.; Nastoupil, L.J.; Rajotte, M.; Rolston, K.V.; et al. Antimicrobial Prophylaxis for Adult Patients with Cancer-Related Immunosuppression: ASCO and IDSA Clinical Practice Guideline Update. J. Clin. Oncol. 2018, 36, 3043–3054.
- Chen, C.-Y.; Milbury, P.E.; Lapsley, K.; Blumberg, J.B. Flavonoids from Almond Skins Are Bioavailable and Act Synergistically with Vitamins C and E to Enhance Hamster and Human LDL Resistance to Oxidation. J. Nutr. 2005, 135, 1366–1373.
- Schreck, K.; Melzig, M. Intestinal Saturated Long-Chain Fatty Acid, Glucose and Fructose Transporters and Their Inhibition by Natural Plant Extracts in Caco-2 Cells. Molecules 2018, 23, 2544.
- Marbaniang, C.; Kma, L. Dysregulation of Glucose Metabolism by Oncogenes and Tumor Suppressors in Cancer Cells. Asian Pac. J. Cancer Prev. 2018, 19, 2377–2390.
- Reckzeh, E.S.; Karageorgis, G.; Schwalfenberg, M.; Ceballos, J.; Nowacki, J.; Stroet, M.C.; Binici, A.; Knauer, L.; Brand, S.; Choidas, A.; et al. Inhibition of Glucose Transporters and Glutaminase Synergistically Impairs Tumor Cell Growth. Cell Chem. Biol. 2019, 26, 1214–1228.e25.
- Taviano, M.F.; Miceli, N.; Acquaviva, R.; Malfa, G.A.; Ragusa, S.; Giordano, D.; Cásedas, G.; Les, F.; López, V. Cytotoxic, Antioxidant, and Enzyme Inhibitory Properties of the Traditional Medicinal Plant Matthiola incana (L.) R. Br. Biol. 2020, 9, 163.
- Trandafir, I.; Cosmulescu, S.; Botu, M.; Nour, V. Antioxidant activity, and phenolic and mineral contents of the walnut kernel (Juglans regia L.) as a function of the pellicle color. Fruits 2016, 71, 177–184.
- Akbari, V.; Jamei, R.; Heidari, R.; Esfahlan, A.J. Antiradical activity of different parts of Walnut (Juglans regia L.) fruit as a function of genotype. Food Chem. 2012, 135, 2404–2410.




