
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Odayme Quesada | + 2594 word(s) | 2594 | 2021-05-10 05:41:29 | | | |
| 2 | Vivi Li | Meta information modification | 2594 | 2021-05-11 03:29:29 | | |
Video Upload Options
Ischemia with non-obstructive coronary arteries (INOCA) is an increasingly recognized disease, with a prevalence of 3 to 4 million individuals, and is associated with a higher risk of morbidity, mortality, and a worse quality of life. Persistent angina in many patients with INOCA is due to coronary microvascular dysfunction (CMD), which can be difficult to diagnose and treat. A coronary flow reserve <2.5 is used to diagnose endothelial-independent CMD. Antianginal treatments are often ineffective in endothelial-independent CMD and thus novel treatment modalities are currently being studied for safety and efficacy. CD34+ cell therapy is a promising treatment option for these patients, as it has been shown to promote vascular repair and enhance angiogenesis in the microvasculature.
1. Introduction
Ischemia with non-obstructive coronary arteries (INOCA) is an increasingly recognized disease with an estimated prevalence of 3 to 4 million individuals [1]. INOCA is characterized by signs and symptoms of ischemia in the absence of obstructive disease [2][3][4][5][6][7]. Although the pathophysiology is not completely understood, coronary microvascular dysfunction (CMD) has been shown to play a critical role and is reported in 47–64% of INOCA patients [1][5][8][9][10][11]. CMD encompasses endothelial-dependent and endothelial-independent microvascular dysfunction. Endothelium-dependent CMD stems from the inability of endothelial cells to produce vasodilatory substrates, thus blunting adequate myocardial perfusion during stress. Conversely, endothelial-independent CMD is detected as abnormal microvascular dilation resulting in a reduced coronary flow reserve (CFR) in response to adenosine. Endothelium-independent CMD results from the inability of smooth muscles to dilate despite the presence of vasodilatory substrates. CMD is associated with increased rates of major adverse cardiac events (MACE), including heart failure (HF), myocardial infarction (MI), and non-fatal stroke during long-term follow-up [2][3][4][11]. However, the current treatment is limited to risk factor management and antianginal medications, with no specific therapy for ischemia, which can significantly limit quality of life.
Cell therapy using autologous stem cells expressing CD34 (CD34+) is a novel therapeutic option for INOCA patients with CMD and refractory angina given the ability of CD34+ cells to repair the microcirculation [12][13][14]. Data from animal models indicate that CD34+ stem cells differentiate into endothelial cells, which incorporate into new vasculature and aid in the release of angiogenic cytokines, thus promoting vascular repair in the microcirculation, leading to improved myocardial perfusion in tissues damaged by acute and chronic ischemia [12][13][14][15][16][17][18]. Three consecutive, randomized, double-blinded, placebo-controlled trials in the United States, in patients with obstructive coronary artery disease (CAD) and Canadian Cardiovascular Society (CCS) class 3–4 refractory angina, established the feasibility of the intra-myocardial delivery of auto-CD34+ cells, showing significant improvements in short- and long-term anginal frequency (AF) and total exercise time (TET) [15][16][18]. Meta-analysis of these three trials demonstrated significant improvement in AF and TET, and a reduction in mortality [19]. PreSERVE-AMI, a large clinical trial using CD34+ cell therapy for left ventricular dysfunction post-ST-segment elevation MI (STEMI), demonstrated the safety and potential efficacy of the intracoronary infusion of CD34+ cell therapy in post-MI patients [20]. A recent two-center, phase 1 feasibility and safety trial (ESCaPE-CMD, NCT03508609) using autologous CD34+ stem cells in INOCA patients with endothelial-independent CMD demonstrated a significant improvement in CFR, AF, CCS class and quality of life [21]. These results led to an ongoing, double-blinded, placebo-controlled trial (FREEDOM, NCT04614467) to demonstrate the safety and efficacy of the intracoronary delivery of CD34+ stem cells. We will review the preclinical and clinical data that led to the development and application of cell-based therapy for ischemic repair, with a focus on microvascular repair in INOCA patients with CMD.
2. Clinical Studies on CD34 Therapy for Ischemic Disease
Although the majority of the clinical trials have been in patients with no-option refractory angina [15][16][17][18][19], CD34+ stem cells have also been investigated in clinical studies for peripheral ischemia [22][23], nonischemic cardiomyopathy [24], myocardial infarction [20], and ischemic stroke [25][26].
2.1. CD34 Therapy for Peripheral Ischemia
In peripheral ischemia, phase 1 and phase 2 trials have shown statistically significant improvements in walking distance, limb pain, blood flow, tissue oxygenation, reduction in ulcer size, and amputation-free survival in no-option critical limb ischemia [12][13][22]. A small phase 2 trial enrolled six patients with Rutherford category 4 or 5 critical limb ischemia. All but one of the patients showed an improvement in Rutherford category from baseline that was statistically significant at 24 weeks and 52 weeks. Currently, a phase 3 trial using CD34+ stem cells for critical limb ischemia due to atherosclerosis and Buerger’s disease is near completion in Japan [23].
2.2. CD34 Therapy for Nonischemic Cardiomyopathy
In nonischemic dilated cardiomyopathy, an open-label randomized study enrolled 110 patients, including 55 treated with intracoronary delivery of CD34+ stem cells [24]. The five-year follow-up showed that CD34+ patients underwent significant improvement in cardiac function and exercise capacity, reduction in N-terminal-pro B-type natriuretic peptide, and an excellent safety profile. The five-year survival by Kaplan–Meier analysis was 2.3 times higher in the treatment group than the control group (p = 0.015). Additionally, 20% of the cell solution was labeled with a radioisotope tracer to determine intramyocardial homing. Interestingly, patients with poor homing did not show improvements in LVEF, likely due to a downregulation of homing factors in dilated cardiomyopathy, leading to poor stem cell retention in the myocardium.
2.3. CD34 Therapy for Myocardial Infarction
PreSERVE-AMI was a randomized, double-blinded, placebo-controlled, phase 2 study using intracoronary administration of bone marrow-derived autologous CD34+ cells in patients with left ventricular dysfunction post-STEMI [20]. The study enrolled 161 patients randomized to CD34+ cells versus placebo, with a primary efficacy endpoint of improvement in the mean resting total severity score by single-photon emission computerized tomography (SPECT) scan. Although the mean resting total severity score improved in both groups, the mortality rate was significantly lower in the CD34+-treated patients. There was no significant difference in overall improvement in LVEF or infarct size between the treatment and control groups. However, there was a significant relationship between CD34+ cell dose and the change in infarct size, LVEF, and days alive, with a benefit in those with higher dosages when adjusted for ischemic time. This study suggested that CD34+ stem cell therapy may improve outcomes in selected sub-populations of STEMI patients, even though it did not meet the primary efficacy endpoint.
2.4. CD34 Therapy for Ischemic Stroke
A phase 1 trial demonstrated the safety and feasibility of bone marrow-derived CD34+ cells delivered intra-arterially to stroke patients [25]. This nonrandomized, open-label, prospective trial enrolled five patients presenting within 7 days of onset of severe anterior circulation ischemic stroke. All patients had clinical improvement as assessed by modified Rankin score (median score: 4 to 2) and National Institutes of Health Stroke Scale (median score: 9 to 2) at 6 months with no significant adverse events, thus establishing preliminary safety and feasibility. Furthermore, the ongoing STROKE34 trial is a randomized, placebo-controlled, phase 2a trial using CD34+ stem cells in patients with acute ischemic stroke due to occlusion of the middle cerebral artery [26].
2.5. CD34 Therapy for Refractory Angina
Successful phase 1 and phase 2 trials have shown the safety and efficacy of intramyocardial CD34+ stem cell therapy in treating no-option refractory angina. The phase 1, double-blinded, placebo-controlled trial enrolled 24 patients with CCS class 3 or 4 angina on optimal medical treatment, and demonstrated initial safety as well as clinical improvement in the CD34+ treated patients [15]. Under physiological conditions, the circulating CD34+ cell concentration is too low in the peripheral circulation. Thus, stimulation with granulocyte colony-stimulating factor (G-CSF) at 5 μg/kg per day for 4–5 days, followed by leukapheresis at day 5 with subsequent CD34+ enrichment, was used to harvest autologous mobilized CD34+ cells. The phase 2 ACT 34 trial enrolled 167 patients who were randomized to receive CD34+ stem cells in low doses (1 × 105 cells/kg, n = 55), high doses (5 × 105 cells/kg, n = 56), and an equal volume placebo diluent (n = 56) [16]. Treatment was distributed between 10 distinct ischemic sites with viable myocardium using a NOGA Myostar® mapping injection catheter. Both CD34+ cell therapy-treated patient groups showed significant improvements in the primary endpoint of AF at 6 months and 12 months. Compared to the placebo group, the low-dose treatment group showed a reduction in AF (6 months: 6.81 vs. 10.91 episodes per week, p = 0.02; 12 months: 6.3 vs. 11.0 episodes per week, p = 0.035) and improvement in TET at 6 and 12 months (6 months: 139 ± 151 vs. 69 ± 122 s, p = 0.014; 12 months: 140 ± 171 vs. 58 ± 146 s, p = 0.017) [16]. A two-year follow-up of 130 of those patients showed that autologous CD34+ cell therapy was associated with persistent improvements in AF in both the low-dose and high-dose groups (p = 0.03) [17]. Additionally, there was a decrease in mortality (p = 0.08) and MACE (p = 0.08).
The phase 3 RENEW, randomized, double-blinded, placebo-controlled trial was originally designed to obtain FDA approval using improvements in TET as the primary endpoint. Unfortunately, the trial was stopped early due to financial issues with the sponsor. Among the 112 enrolled patients, the improvement in TET was 61.0 s at 3 months (95% confidence interval (CI): −2.9 to 124.8, p = 0.06), 46.2 s at 6 months (95% CI: −28.0 to 120.4, p = 0.22), and 36.6 s at 12 months (95% CI: –56.1 to 129.2, p = 0.43) [18]. Furthermore, AF was significantly improved at 6 months (relative risk (RR): 0.58 by intention to treat, p = 0.02). As the study was incomplete, researchers were unable to conclusively determine the efficacy of CD34+ cell therapy for refractory angina patients. Further retrospective data analyses have shown that CD34+ treatment decreased long-term mortality (24% vs. 47%, p = 0.02), costs related to cardiac care (62% reduction translating to an average of USD 5500, p = 0.03), and interventional coronary procedures at 12 months (1.2 ± 0.91 vs. 0.32 ± 0.75 events, p < 0.0001) [27]. In all three trials, placebo patients also underwent treatment with G-CSF, leukapheresis, and intramyocardial injections. Therefore, the trials were completely blinded.
A 2018 meta-analysis of these three randomized, double-blinded trials (n = 304)—phase 1 and 2 ACT-34, ACT-34 extension and phase 3 RENEW—showed an improvement in TET, AF, and MACE in patients with obstructive coronary artery disease and refractory angina receiving intramyocardial autologous CD34+ cell therapy [19]. TET improved by 46.6 s at 3 months (p = 0.007), 49.5 s at 6 months (p = 0.016), and 44.7 s at 12 months (p = 0.065). Additionally, the relative AF decreased–0.78 at 3 months (p = 0.032), –0.66 at 6 months (p = 0.012), and –0.58 at 12 months (p = 0.011). Lastly, there was a significant decrease in mortality (12.1% vs. 2.5%, p = 0.0025) and MACE (38.9% vs. 30.0%, p = 0.14) in patients receiving intramyocardial autologous CD34+ cell therapy compared to placebo at 24 months (Figure 1).
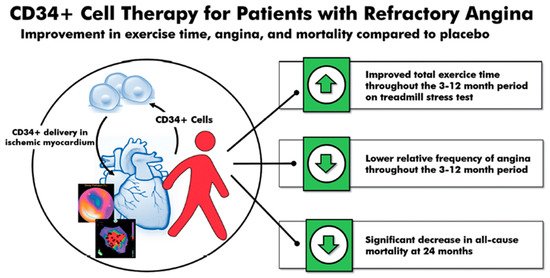
Figure 1. CD34+ cell therapy for patients with obstructive coronary artery disease and refractory angina. Results from the 2018 meta-analysis of three consecutive randomized, double-blinded, placebo-controlled trials in patients with obstructive coronary artery disease and Canadian Cardiovascular Society class 3–4 refractory angina showed that a single intracoronary infusion of autologous CD34+ cells significantly improved total exercise time, decreased angina frequency, and decreased all-cause mortality.
Although the three previous refractory angina trials used intramyocardial injection, the intracoronary delivery of CD34+ stem cells has also been shown to be safe and effective. This potentially allows for a safer and more established delivery route without losing the effectiveness of therapy. A 2010 study from China of 112 patients showed the safety and feasibility of intracoronary CD34+ stem cell therapy in refractory angina patients [28]. In this single-center study, there were no differences in the frequency of adverse events between the placebo and treatment groups, showing that the intracoronary delivery of bone-marrow-derived CD34+ cells is safely tolerated. Additionally, the study showed that patients receiving intracoronary CD34+ stem cell therapy had a greater reduction in AF at 3 months (–14.6 ± 4.8 vs. –4.5 ± 0.3 episodes, p < 0.01) and 6 months (–15.6 ± 4.0 vs. –3.0 ± 1.2 events, p < 0.01) compared to placebo. Once again, the placebo group also showed a significant decrease in AF from baseline, indicating a strong placebo effect. This study also found a statistically significant decrease in use of nitroglycerin and CCS class, as well as an increase in TET in patients receiving intracoronary CD34+ stem cells [28]. In summary, extensive clinical trial data have demonstrated the outstanding safety of CD34+ stem cell therapy, and consistently showed clinical improvements in conditions characterized by perfusion abnormalities. No studies to date have reported an increased risk of uncontrolled cell growth of CD34+ inoculated tissues, angiomas or cancer.
3. CD34 Therapy as a Novel Treatment for Coronary Microvascular Dysfunction
Unfortunately, 25% of patients with INOCA have refractory angina despite medications. Cell therapy using autologous stem cells expressing CD34+ is a novel therapeutic option for these INOCA patients with CMD and refractory angina. The recent two-center ESCaPE-CMD trial (NCT03508609), sponsored by Caladrius Biosciences, evaluated the efficacy and safety of autologous CD34+ cell therapy in 20 INOCA patients with endothelial-independent CMD, defined as CFR ≤ 2.5, and refractory angina [21]. Patients received G-CSF for 4 days followed by leukapheresis, but, in contrast to the previous refractory angina trials, the CD34+ cells were delivered via an intracoronary route. The study showed that a single intracoronary infusion of autologous CD34+ cells significantly increased the CFR from 2.08 ± 0.32 at baseline to 2.68 ± 0.79 at 6 months after treatment (p = 0.0045). This correlated clinically to decreased AF (4.42 to 2.02, p = 0.0036), improved CCS class (3.2 to 2.05, p < 0.001), and improved Seattle Angina Questionnaire (SAQ) and 36-item short-form survey (SF-36) scores (Figure 3) [21]. As with the previous CD34+ clinical trials, there were no cell-related adverse events, demonstrating the excellent safety and tolerability of CD34+ infusions. This study extends the prior findings from the pooled analysis of trials in patients with obstructive CAD and refractory angina, to INOCA patients with CMD and persistent angina. This has led to the phase 2, randomized, double-blinded, placebo-controlled FREEDOM trial (NCT04614467), sponsored by Caladrius Biosciences. The trial began enrollment in October 2020 with an anticipated 105 patients randomized 2:1 to CD34+ cell therapy, to establish a potential therapeutic role for endothelial progenitor cells in INOCA patients with endothelial-independent CMD (Figure 4). A single infusion of autologous CD34+ cells will be administered intracoronary. Patients in the treatment and placebo arms will undergo G-CSF cell mobilization, apheresis, and the intracoronary infusion of autologous CD34+ cells vs. placebo. The primary endpoint is a change in CFR at 6 months.
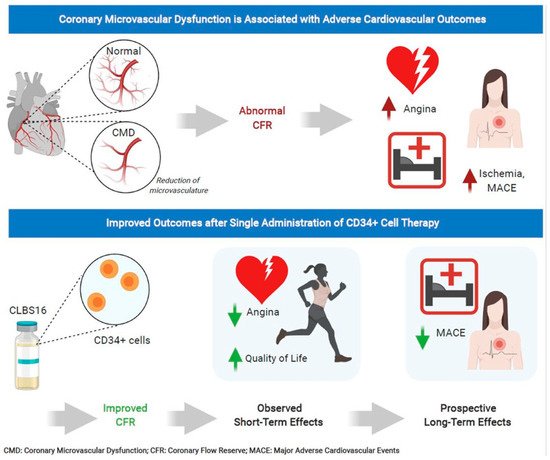
Figure 3. CD34+ cell therapy for patients with coronary microvascular dysfunction and refractory angina with no obstructive coronary artery disease. Results from the phase 1 ESCaPE-CMD trial (NCT03508609) showed that a single intracoronary infusion of autologous CD34+ cells in patients with coronary microvascular dysfunction and refractory angina with no obstructive coronary artery disease significantly improved coronary flow reserve, decreased angina frequency, and improved quality of life at 6 months.
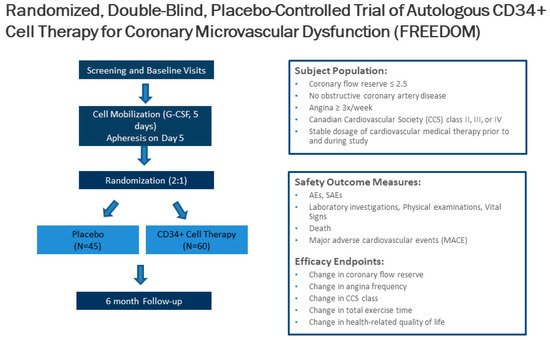
Figure 4. Trial design of the CD34+ cell therapy FREEDOM trial. The FREEDOM trial (NCT04614467) is a randomized, double-blinded, placebo-controlled trial of CD34+ cell therapy for patients with coronary microvascular dysfunction and refractory angina with no obstructive coronary artery disease.
Improvements in coronary microvascular function, as measured by CFR after CD34+ cell therapy, has led to the hypothesis that CD34+ cells repair the microvasculature in patients with CMD similarly to ischemic tissue. CD34+ cells promote vascular repair and enhance angiogenesis in the microvasculature, which restores the microcirculation and improves myocardial tissue perfusion, as evidenced by the improvement in CFR. Further, studies on the mechanisms of autologous CD34+ stem cells in CMD are needed in the future.
4. Conclusions
Endothelial-independent CMD, associated with a reduced CFR in response to intracoronary adenosine, increases the risk of MACE, MI, heart failure hospitalizations, and mortality in INOCA patients. However, no effective specific therapy to date exists for CMD. Cell therapy using autologous CD34+ stem cells is a promising new therapeutic option for INOCA patients with CMD. Through the induction of capillary growth, direct incorporation into damaged vasculature, and the upregulation of proliferative cytokines, CD34+ stem cell therapy can enhance angiogenesis and restore the microcirculation in acute and chronic ischemia. Three consecutive randomized and double-blinded trials have demonstrated the safety and efficacy of the reduction in CCS class 3–4 refractory angina from obstructive CAD via the delivery of intramyocardial CD34+ stem cells, and a pooled analysis of these studies showed significant improvements in AF and TET and a reduction of mortality. Feasibility and safety studies on the use of autologous CD34+ cell therapy in INOCA patients with endothelial-independent CMD and persistent angina showed significant improvement in CFR in 6 months, with no cell-related adverse events. The use of CD34+ cell therapy in CMD and persistent angina will be determined by the results of the ongoing phase 2 FREEDOM trial (NCT04614467). Given the high prevalence and the lack of specific treatment options in patient with INOCA and underlying CMD, additional research is needed to identify novel therapies.
References
- Bairey Merz, C.N.; Pepine, C.J.; Walsh, M.N.; Fleg, J.L. Ischemia and No Obstructive Coronary Artery Disease (INOCA): Developing Evidence-Based Therapies and Research Agenda for the Next Decade. Circulation 2017, 135, 1075–1092.
- Widmer, R.J.; Samuels, B.; Samady, H.; Price, M.J.; Jeremias, A.; Anderson, R.D.; Jaffer, F.A.; Escaned, J.; Davies, J.; Prasad, M.; et al. The functional assessment of patients with non-obstructive coronary artery disease: Expert review from an international microcirculation working group. EuroIntervention 2019, 14, 1694–1702.
- Kunadian, V.; Chieffo, A.; Camici, P.G.; Berry, C.; Escaned, J.; Maas, A.; Prescott, E.; Karam, N.; Appelman, Y.; Fraccaro, C.; et al. An EAPCI Expert Consensus Document on Ischaemia with Non-Obstructive Coronary Arteries in Collaboration with European Society of Cardiology Working Group on Coronary Pathophysiology & Microcirculation Endorsed by Coronary Vasomotor Disorders International Study Group. EuroIntervention 2021, 16, 1049–1069.
- Jespersen, L.; Hvelplund, A.; Abildstrøm, S.Z.; Pedersen, F.; Galatius, S.; Madsen, J.K.; Jørgensen, E.; Kelbæk, H.; Prescott, E. Stable angina pectoris with no obstructive coronary artery disease is associated with increased risks of major adverse cardiovascular events. Eur. Heart J. 2012, 33, 734–744.
- Sara, J.D.; Widmer, R.J.; Matsuzawa, Y.; Lennon, R.J.; Lerman, L.O.; Lerman, A. Prevalence of Coronary Microvascular Dysfunction Among Patients With Chest Pain and Nonobstructive Coronary Artery Disease. Jacc. Cardiovasc. Interv. 2015, 8, 1445–1453.
- Recio-Mayoral, A.; Mason, J.C.; Kaski, J.C.; Rubens, M.B.; Harari, O.A.; Camici, P.G. Chronic inflammation and coronary microvascular dysfunction in patients without risk factors for coronary artery disease. Eur. Heart J. 2009, 30, 1837–1843.
- Ong, P.; Camici, P.G.; Beltrame, J.F.; Crea, F.; Shimokawa, H.; Sechtem, U.; Kaski, J.C.; Bairey Merz, C.N.; Coronary Vasomotion Disorders International Study Group. International standardization of diagnostic criteria for microvascular angina. Int. J. Cardiol. 2018, 250, 16–20.
- Shaw, L.J.; Shaw, R.E.; Merz, C.N.; Brindis, R.G.; Klein, L.W.; Nallamothu, B.; Douglas, P.S.; Krone, R.J.; McKay, C.R.; Block, P.C.; et al. Impact of ethnicity and gender differences on angiographic coronary artery disease prevalence and in-hospital mortality in the American College of Cardiology-National Cardiovascular Data Registry. Circulation 2008, 117, 1787–1801.
- Reis, S.E.; Holubkov, R.; Lee, J.S.; Sharaf, B.; Reichek, N.; Rogers, W.J.; Walsh, E.G.; Fuisz, A.R.; Kerensky, R.; Detre, K.M.; et al. Coronary flow velocity response to adenosine characterizes coronary microvascular function in women with chest pain and no obstructive coronary disease. Results from the pilot phase of the Women’s Ischemia Syndrome Evaluation (WISE) study. J. Am. Coll. Cardiol. 1999, 33, 1469–1475.
- Reis, S.E.; Holubkov, R.; Conrad Smith, A.J.; Kelsey, S.F.; Sharaf, B.L.; Reichek, N.; Rogers, W.J.; Merz, C.N.; Sopko, G.; Pepine, C.J.; et al. Coronary microvascular dysfunction is highly prevalent in women with chest pain in the absence of coronary artery disease: Results from the NHLBI WISE study. Am. Heart J. 2001, 141, 735–741.
- Pepine, C.J.; Anderson, R.D.; Sharaf, B.L.; Reis, S.E.; Smith, K.M.; Handberg, E.M.; Johnson, B.D.; Sopko, G.; Bairey Merz, C.N. Coronary microvascular reactivity to adenosine predicts adverse outcome in women evaluated for suspected ischemia results from the National Heart, Lung and Blood Institute WISE (Women’s Ischemia Syndrome Evaluation) study. J. Am. Coll. Cardiol. 2010, 55, 2825–2832.
- Sietsema, W.K.; Kawamoto, A.; Takagi, H.; Losordo, D.W. Autologous CD34+ Cell Therapy for Ischemic Tissue Repair. Circ. J. 2019, 83, 1422–1430.
- Prasad, M.; Corban, M.T.; Henry, T.D.; Dietz, A.B.; Lerman, L.O.; Lerman, A. Promise of autologous CD34+ stem/progenitor cell therapy for treatment of cardiovascular disease. Cardiovasc. Res. 2020, 116, 1424–1433.
- Asahara, T.; Murohara, T.; Sullivan, A.; Silver, M.; van der Zee, R.; Li, T.; Witzenbichler, B.; Schatteman, G.; Isner, J.M. Isolation of putative progenitor endothelial cells for angiogenesis. Science 1997, 275, 964–967.
- Losordo, D.W.; Schatz, R.A.; White, C.J.; Udelson, J.E.; Veereshwarayya, V.; Durgin, M.; Poh, K.K.; Weinstein, R.; Kearney, M.; Chaudhry, M.; et al. Intramyocardial transplantation of autologous CD34+ stem cells for intractable angina: A phase I/IIa double-blind, randomized controlled trial. Circulation 2007, 115, 3165–3172.
- Losordo, D.W.; Henry, T.D.; Davidson, C.; Sup Lee, J.; Costa, M.A.; Bass, T.; Mendelsohn, F.; Fortuin, F.D.; Pepine, C.J.; Traverse, J.H.; et al. Intramyocardial, autologous CD34+ cell therapy for refractory angina. Circ. Res. 2011, 109, 428–436.
- Henry, T.D.; Schaer, G.L.; Traverse, J.H.; Povsic, T.J.; Davidson, C.; Lee, J.S.; Costa, M.A.; Bass, T.; Mendelsohn, F.; Fortuin, F.D.; et al. Autologous CD34(+) Cell Therapy for Refractory Angina: 2-Year Outcomes From the ACT34-CMI Study. Cell Transpl. 2016, 25, 1701–1711.
- Povsic, T.J.; Henry, T.D.; Traverse, J.H.; Fortuin, F.D.; Schaer, G.L.; Kereiakes, D.J.; Schatz, R.A.; Zeiher, A.M.; White, C.J.; Stewart, D.J.; et al. The RENEW Trial: Efficacy and Safety of Intramyocardial Autologous CD34(+) Cell Administration in Patients With Refractory Angina. Jacc. Cardiovasc. Interv. 2016, 9, 1576–1585.
- Henry, T.D.; Losordo, D.W.; Traverse, J.H.; Schatz, R.A.; Jolicoeur, E.M.; Schaer, G.L.; Clare, R.; Chiswell, K.; White, C.J.; Fortuin, F.D.; et al. Autologous CD34+ cell therapy improves exercise capacity, angina frequency and reduces mortality in no-option refractory angina: A patient-level pooled analysis of randomized double-blinded trials. Eur. Heart J. 2018, 39, 2208–2216.
- Quyyumi, A.A.; Vasquez, A.; Kereiakes, D.J.; Klapholz, M.; Schaer, G.L.; Abdel-Latif, A.; Frohwein, S.; Henry, T.D.; Schatz, R.A.; Dib, N.; et al. PreSERVE-AMI: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial of Intracoronary Administration of Autologous CD34+ Cells in Patients With Left Ventricular Dysfunction Post STEMI. Circ. Res. 2017, 120, 324–331.
- Henry, T.D.; Noel Bairey Merz, C.; Wei, J.; Corban, M.T.; Quesada, O.; Joung, S.; Kotynski, C.L.; Wang, J.; Lewis, M.; Schumacher, A.M.; et al. CD34+ stem cell therapy increases coronary flow reserve and reduces angina in patients with coronary microvascular dysfunction. Circ. Cardiovasc. Interv. 2021. under review.
- Ohtake, T.; Mochida, Y.; Ishioka, K.; Oka, M.; Maesato, K.; Moriya, H.; Hidaka, S.; Higashide, S.; Ioji, T.; Fujita, Y.; et al. Autologous Granulocyte Colony-Stimulating Factor-Mobilized Peripheral Blood CD34 Positive Cell Transplantation for Hemodialysis Patients with Critical Limb Ischemia: A Prospective Phase II Clinical Trial. Stem Cells Transl. Med. 2018, 7, 774–782.
- Kawamoto, A.; Fujita, Y.; Sietsema, W.K.; Wang, J.; Takagi, H.; Losordo, D.W. Design of a potentially registrational study of sakigake-designated GCSF-mobilized autologous CD34 cell (CLBS12) therapy of no-option critical limb ischemia including arteriosclerosis obliterans and buerger’s disease. Cytotherapy 2020, 22, S61.
- Vrtovec, B.; Poglajen, G.; Lezaic, L.; Sever, M.; Domanovic, D.; Cernelc, P.; Socan, A.; Schrepfer, S.; Torre-Amione, G.; Haddad, F.; et al. Effects of intracoronary CD34+ stem cell transplantation in nonischemic dilated cardiomyopathy patients: 5-year follow-up. Circ. Res. 2013, 112, 165–173.
- Banerjee, S.; Bentley, P.; Hamady, M.; Marley, S.; Davis, J.; Shlebak, A.; Nicholls, J.; Williamson, D.A.; Jensen, S.L.; Gordon, M.; et al. Intra-Arterial Immunoselected CD34+ Stem Cells for Acute Ischemic Stroke. Stem Cells Transl. Med. 2014, 3, 1322–1330.
- Sargento-Freitas, J.; Pereira, A.; Gomes, A.; Amorim, P.; Matos, T.; Cardoso, C.M.P.; Silva, F.; Santo, G.C.; Nunes, C.; Galego, O.; et al. STROKE34 Study Protocol: A Randomized Controlled Phase IIa Trial of Intra-Arterial CD34+ Cells in Acute Ischemic Stroke. Front. Neurol. 2018, 9, 302.
- Johnson, G.L.; Henry, T.D.; Povsic, T.J.; Losordo, D.W.; Garberich, R.F.; Stanberry, L.I.; Strauss, C.E.; Traverse, J.H. CD34(+) cell therapy significantly reduces adverse cardiac events, health care expenditures, and mortality in patients with refractory angina. Stem Cells Transl. Med. 2020, 9, 1147–1152.
- Wang, S.; Cui, J.; Peng, W.; Lu, M. Intracoronary autologous CD34+ stem cell therapy for intractable angina. Cardiology 2010, 117, 140–147.




