
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Fabrizio Bruschi | + 1146 word(s) | 1146 | 2021-04-21 08:34:28 | | | |
| 2 | Lindsay Dong | -20 word(s) | 1126 | 2021-05-11 05:45:19 | | |
Video Upload Options
Ocular toxoplasmosis (OT) is an ocular disease caused by infection of the eye with the parasite Toxoplasma gondii and it is the most common cause of eye inflammation in the world.
1. Introduction
Ocular toxoplasmosis (OT) is caused by the parasite Toxoplasma gondii and affects many individuals throughout the world. Infection may occur through congenital or acquired routes. The parasites enter the blood circulation and reach both the retina and the retinal pigment epithelium, where they may cause cell damage and cell death. Its diagnosis is based on the detection in the patients of antibodies directed to parasite antigens or of parasite DNA [1]. A common feature reported by patients is blurred vision, and although the disease tends to resolve spontaneously, more serious, vision-threatening complications such as retinal detachment, choroidal neovascularization and glaucoma, may occur. Therefore, OT requires treatment to eliminate the parasite and the related inflammation [2]. Toxoplasma infection may take place through congenital or acquired routes. In the first case, the parasite present in an infected mother may cross the placenta and pass to her foetus.
2. General Features of Toxoplasma gondii Infection
Toxoplasma gondii, an obligate intracellular protozoan belonging to the phylum Apicomplexa, is characterised by a complex life cycle in which felines are the definitive hosts and other mammals, including humans, are intermediate hosts. In the intermediate hosts, the acute stage of the infection is characterised by tachyzoite proliferation, then the parasites form tissue cysts in various organs, including the brain, and establish a chronic infection [3].
Infection may occur by ingestion of contaminated food or water (through oocysts), raw or undercooked meat (containing tissue cysts), vertically from mother to foetus, transfusion (both through tachyzoites), or transplantation of solid organ or bone marrow/stem cells (bradyzoites) [3]. The infection usually occurs asymptomatically, and when it is clinically manifest, the symptoms are negligible (in immunocompetent individuals), mimicking a flu-like syndrome with a possible cervical lymphadenopathy, unless chorioretinitis occurs [3]. Both host factors (genetic background, gender, immunological status) and parasite factors (inoculum size, timing, genotype, virulence) may affect the course of infection in humans [3].
3. Infection of the Human Retina
Once T. gondii has established infection in the small intestine, tachyzoites enter the draining blood and lymph travelling to remote sites [4] as free bodies in the circulation, or inside leukocytes [5]. Different histopathological and clinical observations show that T. gondii tachyzoites enter the eye via the blood supply that reaches the retina through the choroidal vessels, which irrigate the photoreceptors located in the outer retina, or through the retinal vessels entering at the optic nerve head, which irrigate the inner retina [6][7] (Figure 1).
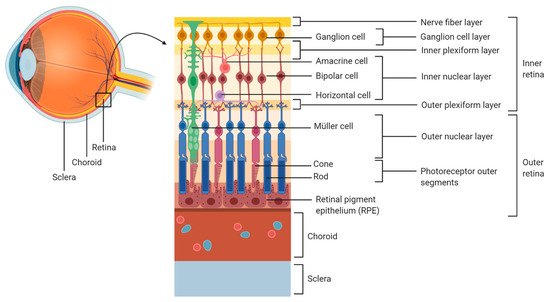
Figure 1. Organization of the human retina.
During the early course of OT, necrosis has been reported in the nerve fibre layer and in the ganglion cell layer [8][9]. In addition, other studies have reported the presence of tachyzoites in the inner retina, especially in the proximity of retinal blood vessels [10][11]. This is in line with the development of retinal vasculitis in relation to OT. Indeed, during Toxoplasma infection in utero, the posterior pole of the eye is most typically involved. Therefore, since the development of the retinal vasculature begins from the posterior pole, retinal infection mediated by the blood vessels can expand only as far as the retinal vasculature has developed [12].
Three possible routes have been identified for the passage of tachyzoites across the retinal endothelium (Figure 2):
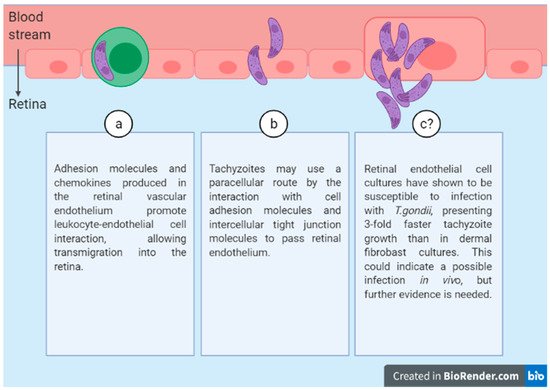
Figure 2. Three possible routes of T.gondii diffusion through the retinal endothelium.
(a) Leukocyte transport.
(b) Free parasite through a paracellular route.
(c) Endothelial cell infection.
4. Migration of T. gondii within the Human Retina
T. gondii tachyzoites can actively move through the human retina [13]. Two major retinal cell populations, i.e., Müller glial cells and neurons, were the most susceptible [13]. In particular, the results showed a preference for Müller glial cells, which harboured a bulk of parasitophorous vacuoles and the highest parasite load. Moreover, parasite growth in these cells exceeded that in human dermal fibroblasts by over 50% [14][15]. The parasite upregulates chemokine expression in infected Müller cells and, after leukocyte entry in the retina, some cell functions become activated while others are inhibited when leukocytes interact with infected Müller cells [16].
5. Infection of the RPE by T. gondii
Infection in retinal pigment epithelial cells is expected to promote choroidal neovascularization. However, this is a rare situation during OT. Indeed, a recent study showed that the RPE produces Pigment Epithelial Derived Factor (PEDF) within hours of infection [14]. PEDF is an antiangiogenic factor, and its release at an early stage in the infection may prevent choroidal neovascularization [17].
6. Immune Response during Retinal Infection
Humans have a wide range of immune response-related genes with different polymorphisms, creating a particular risk profile with different pathogenetic mechanisms for each individual. The RPE upregulates the expression of major histocompatibility complex (MHC) class I and class II to present parasite antigens in the eye [18][19]. Tachyzoite-infected human RPE produces Granulocyte-Macrophage Colony-Stimulating Factor (GM-CSF), Interleukin 6 (IL-6), and IL-18, activating neutrophils and leading to the production of reactive oxygen species and inflammatory cytokines such as Tumour Necrosis Factor (TNF)-α and IL-1β, which may trigger retinal damage associated to OT [20]. On the other hand, IL-6 has been reported to antagonize the activity of ocular Transforming Growth Factor β (TGF-β, an anti-inflammatory cytokine), which would inhibit natural killer cell activity and Nitric Oxide production, allowing parasite survival [21].
7. Toxoplasma Genotype Influences the Course of Ocular Infection
There are at least 16 haplogroups of T. gondii, grouped in six clades based on the single-nucleotid polymorphisms across the genome [22]. The most investigated types are I, II and III (haplogroups 1, 2 and 3). In the United States, Toxoplasma strain type has been identified as an important factor that determines the severity of congenital toxoplasmosis [23]. Different recombinant (atypical) strains, such as Type x (haplogroup 12), are associated with severe disease in patients with AIDS and OT in North, Central and South America [24][25]. In Europe, Type II strain accounts for 70–80% of human infections [25]. In immunocompromised individuals with OT, genotype II accounts for 85% of the cases [26]. Among Toxoplasma-infected German uveitis patients with OT, a novel nonreactive serotype in 44% of cases, and Type II in 41% of cases, were observed [27].
8. Conclusions
Even though Toxoplasma is a well-studied parasite, the mechanisms behind human OT are still far from being elucidated. So far, it has been shown that the parasite might have different strategies to successfully persist in the host, entering the retina by at least three different routes. Then, the tachyzoites migrate across the retinal layers, spreading the infection within the inner retina. However, there is still work ahead to identify the cells most affected and to gain more knowledge about the immune response. New ex vivo models to study OT will surely help answering these and other questions that might occur on the way towards a better understanding of T. gondii infection of the retina.
References
- Liu, Q.; Wang, Z.-D.; Huang, S.-Y.; Zhu, X.-Q. Diagnosis of Toxoplasmosis and Typing of Toxoplasma gondii. Parasit Vectors 2015, 8.
- Park, Y.-H.; Nam, H.-W. Clinical Features and Treatment of Ocular Toxoplasmosis. Korean J. Parasitol. 2013, 51, 393–399.
- Montoya, J.G.; Liesenfeld, O. Toxoplasmosis. Lancet 2004, 363, 1965–1976.
- Dubey, J.P. Toxoplasmosis of Animals and Humans; CRC Press: Boca Raton, FL, USA, 2016.
- Silveira, C.; Vallochi, A.; Silva, U.; Muccioli, C.; Holland, G.; Nussenblatt, R.; Belfort, R.; Rizzo, L. Toxoplasma gondii in the Peripheral Blood of Patients with Acute and Chronic Toxoplasmosis. Br. J. Ophthalmol. 2011, 95, 396–400.
- Hildebrand, G.D.; Fielder, A.R. Anatomy and Physiology of the Retina. In Pediatric Retina; Reynolds, J., Olitsky, S., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 39–65.
- Feustel, S.M.; Meissner, M.; Liesenfeld, O. Toxoplasma gondii and the Blood-Brain Barrier. Virulence 2012, 3, 182–192.
- Nicholson, D.H.; Wolchok, E.B. Ocular Toxoplasmosis in an Adult Receiving Long-Term Corticosteroid Therapy. Arch. Ophthalmol. 1976, 94, 248–254.
- Yeo, J.H.; Jakobiec, F.A.; Iwamoto, T.; Richard, G.; Kreissig, I. Opportunistic Toxoplasmic Retinochoroiditis Following Chemotherapy for Systemic Lymphoma. A Light and Electron Microscopic Study. Ophthalmology 1983, 90, 885–898.
- Holland, G.N.; Engstrom, R.E.; Glasgow, B.J.; Berger, B.B.; Daniels, S.A.; Sidikaro, Y.; Harmon, J.A.; Fischer, D.H.; Boyer, D.S.; Rao, N.A. Ocular Toxoplasmosis in Patients with the Acquired Immunodeficiency Syndrome. Am. J. Ophthalmol. 1988, 106, 653–667.
- Roberts, F.; Mets, M.B.; Ferguson, D.J.; O’Grady, R.; O’Grady, C.; Thulliez, P.; Brézin, A.P.; McLeod, R. Histopathological Features of Ocular Toxoplasmosis in the Fetus and Infant. Arch. Ophthalmol. 2001, 119, 51–58.
- Provis, J.M. Development of the Primate Retinal Vasculature. Prog. Retin. Eye Res. 2001, 20, 799–821.
- Furtado, J.M.; Ashander, L.M.; Mohs, K.; Chipps, T.J.; Appukuttan, B.; Smith, J.R. Toxoplasma gondii Migration within and Infection of Human Retina. PLoS ONE 2013, 8, e54358.
- Smith, J.R.; Ashander, L.M.; Arruda, S.L.; Cordeiro, C.A.; Lie, S.; Rochet, E.; Belfort, R.; Furtado, J.M. Pathogenesis of Ocular Toxoplasmosis. Prog. Retin. Eye Res. 2020, 100882.
- Nogueira, A.R.; Leve, F.; Morgado-Diaz, J.; Tedesco, R.C.; Pereira, M.C.S. Effect of Toxoplasma gondii Infection on the Junctional Complex of Retinal Pigment Epithelial Cells. Parasitology 2016, 143, 568–575.
- Knight, B.C.; Kissane, S.; Falciani, F.; Salmon, M.; Stanford, M.R.; Wallace, G.R. Expression Analysis of Immune Response Genes of Müller Cells Infected with Toxoplasma gondii. J. Neuroimmunol. 2006, 179, 126–131.
- Zhang, S.X.; Ma, J. Ocular Neovascularization: Implication of Endogenous Angiogenic Inhibitors and Potential Therapy. Prog. Retin. Eye Res. 2007, 26, 1–37.
- Lyons, R.E.; Anthony, J.P.; Ferguson, D.J.P.; Byrne, N.; Alexander, J.; Roberts, F.; Roberts, C.W. Immunological Studies of Chronic Ocular Toxoplasmosis: Up-Regulation of Major Histocompatibility Complex Class I and Transforming Growth Factor β and a Protective Role for Interleukin-6. Infect. Immun. 2001, 69, 2589–2595.
- Osusky, R.; Dorio, R.J.; Arora, Y.K.; Ryan, S.J.; Walker, S.M. MHC Class II Positive Retinal Pigment Epithelial(RPE) Cells Can Function as Antigen-Presenting Cells for Microbial Superantigen. Ocul. Immunol. Inflamm. 1997, 5, 43–50.
- Nagineni, C.N.; Detrick, B.; Hooks, J.J. Toxoplasma gondii Infection Induces Gene Expression and Secretion of Interleukin 1(IL-1), IL-6, Granulocyte-Macrophage Colony-Stimulating Factor, and Intercellular Adhesion Molecule 1 by Human Retinal Pigment Epithelial Cells. Infect. Immun. 2000, 68, 407–410.
- Ohta, K.; Yamagami, S.; Taylor, A.W.; Streilein, J.W. IL-6 Antagonizes TGF-β and Abolishes Immune Privilege in Eyes with Endotoxin-Induced Uveitis. Investig. Ophthalmol. Vis. Sci. 2000, 41, 2591–2599.
- Behnke, M.S.; Dubey, J.P.; Sibley, L.D. Genetic Mapping of Pathogenesis Determinants in Toxoplasma gondii. Annu. Rev. Microbiol. 2016, 70, 63–81.
- McLeod, R.; Boyer, K.M.; Lee, D.; Mui, E.; Wroblewski, K.; Karrison, T.; Noble, A.G.; Withers, S.; Swisher, C.N.; Heydemann, P.T.; et al. Toxoplasmosis Study Group. Prematurity and Severity Are Associated with Toxoplasma gondii Alleles (NCCCTS, 1981–2009). Clin. Infect. Dis. 2012, 54, 1595–1605.
- Khan, A.; Jordan, C.; Muccioli, C.; Vallochi, A.L.; Rizzo, L.V.; Belfort, R.; Vitor, R.W.A.; Silveira, C.; Sibley, L.D. Genetic Divergence of Toxoplasma gondii Strains Associated with Ocular Toxoplasmosis, Brazil. Emerg. Infect. Dis. 2006, 12, 942–949.
- Lehmann, T.; Marcet, P.L.; Graham, D.H.; Dahl, E.R.; Dubey, J.P. Globalization and the Population Structure of Toxoplasma gondii. Proc. Natl. Acad. Sci. USA 2006, 103, 11423–11428.
- Fekkar, A.; Ajzenberg, D.; Bodaghi, B.; Touafek, F.; Le Hoang, P.; Delmas, J.; Robert, P.Y.; Dardé, M.L.; Mazier, D.; Paris, L. Direct Genotyping of Toxoplasma gondii in Ocular Fluid Samples from 20 Patients with Ocular Toxoplasmosis: Predominance of Type II in France. J. Clin. Microbiol. 2011, 49, 1513–1517.
- Shobab, L.; Pleyer, U.; Johnsen, J.; Metzner, S.; James, E.R.; Torun, N.; Fay, M.P.; Liesenfeld, O.; Grigg, M.E. Toxoplasma Serotype Is Associated with Development of Ocular Toxoplasmosis. J. Infect. Dis. 2013, 208, 1520–1528.





