
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Arianna Giannetti | + 1621 word(s) | 1621 | 2021-05-10 04:16:28 | | | |
| 2 | Nora Tang | Meta information modification | 1621 | 2021-05-11 02:54:14 | | |
Video Upload Options
Cow’s milk allergy (CMA) is one of the most common food allergies in infants, and its prevalence has increased over recent years. Understanding the diagnostic features of CMA is essential in order to manage patients with this disorder, guide the use of an elimination diet, and find the best moment to start an oral food challenge (OFC) and liberalize the diet. To date, no shared tolerance markers for the diagnosis of food allergy have been identified, and OFC remains the gold standard. Recently, oral immunotherapy (OIT) has emerged as a new therapeutic strategy and has changed the natural history of CMA. Before this, patients had to strictly avoid the food allergen, resulting in a decline in quality of life and subsequent nutritional, social, and psychological impairments. Thanks to the introduction of OIT, the passive approach involving rigid exclusion has changed to a proactive one. Both the heterogeneity in the diagnostic process among the studies and the variability of OIT data limit the comprehension of the real epidemiology of CMA, and, consequentially, its natural history. Therefore, well-planned randomized controlled trials are needed to standardize CMA diagnosis, prevention, and treatment strategies.
1. Introduction
The prevalence of food allergies (FAs) and especially cow’s milk allergy (CMA, currently one of the most common FAs among children [1][2]) has increased in recent decades.
CMA is defined as a reproducible adverse reaction to one or more cow’s milk (CM) protein (usually casein or serum β-lactoglobulin) [3]. The underlying immunological mechanism, presentation times, and organs involved differentiate CMA from other adverse reactions to CM such as lactose intolerance [4].
CMA, like all FAs, can be divided into two main categories according to the type of immunological mechanism underlying it: immunoglobulin (Ig)E-mediated or non-IgE mediated [5] (Figure 1). IgE-mediated reactions are the most common. On the other hand, there are non-IgE mediated reactions that can arise from other cellular processes involving eosinophils or T-cells.
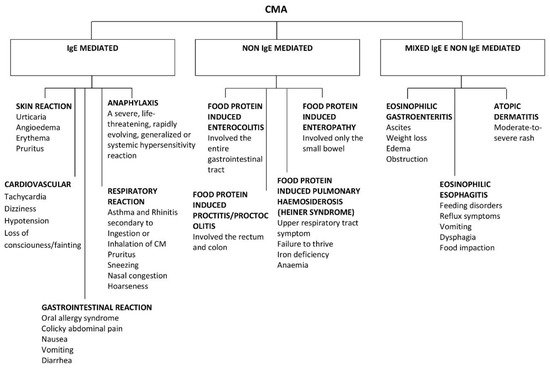
Figure 1. Clinical presentation of IgE and non IgE CMA.
CMA usually occurs in the first 2 years of life and especially within the first year, unlike other allergies, such as peanut, tree nuts, fish and shellfish allergies which may develop later in childhood or adulthood. Most allergies (including CM allergies) resolve spontaneously during childhood or adolescence, whereas peanut and tree nut allergies are more likely to persist into adulthood [6][7].
2. Cow’s Milk Composition
CM contains from 30 to 35 g of protein per liter and many proteins, which are all potential allergens [8].
Through the acidification of raw skim milk to pH 4.6 at 20 °C it is possible to obtain two different fractions: the coagulum, containing the casein proteins which account for 80% of the fraction, and the lactoserum (whey proteins), representing 20% of the total milk proteins [9]. These proteins can also be divided into soluble and insoluble proteins [10].
The major milk allergens are soluble proteins, named whey proteins, which represent approximately 20% of total proteins [11]. Allergens present in the serum fraction include α-lactalbumin (Bos d 4) and β-lactoglobulin (Bos d 5), which are the most abundant, and immunoglobulins (Bos d 7), serum albumin (BSA, Bos d 6), and traces of lactoferrin, lysozyme, proteose-peptone, and transferrin [9]. In particular lactoferrin, lactoperoxidase, and lysozyme are important antimicrobial agents, while lactoferrin, β-lactoglobulin, and α-lactalbumin are important tumor suppressors [12].
The remaining 80% is represented by insoluble proteins known as caseins (known as Bos d 8). Total caseins can be divided into four proteins, representing different percentages of the whole fraction: αS1-casein, which is the most important (Bos d 9, 32%), as well as αS2-casein (Bos d 10, 10%), β-casein (Bos d 11, 28%), and κ-casein (Bos d 12, 10%). The main role of caseins relates to their mineral binding and carrier capacity, specifically for calcium and phosphorus [12].
CMAs are most frequently caused by whey proteins, but they can also be promoted by caseins [13]. As a matter of fact, most patients are sensitized to casein (Bos d 8), β-lactoglobulin (Bos d 5), and α-lactalbumin (Bos d 4), which are the major milk allergens. There are only a few studies that describe allergies to minor serum proteins such as immunoglobulin, bovine serum albumin, or lactoferrin [14].
CM contains at least 20 potentially allergenic proteins [15]. Most children with milk-allergy are sensitized to more than one allergen, with a greater variability of symptoms.
Table 1 provides a short synthesis of cow’s milk allergens and their characteristics.
Table 1. Main characteristics of CM allergens, adapted from Hochwallner [8].
| Allergen Name | Protein | Concentration (g/L) | Size (kDa) | Prevalence (% of Patients) | Allergenic Activity (% of Patients) | |
|---|---|---|---|---|---|---|
| Whey (20%) (5 g/L) |
Bos d 4 | α–Lactalbumin | 1–1.5 | 14.2 | 0–67 | 12 |
| Bos d 5 | β–Lactoglobulin | 3–4 | 18.3 | 13–62 | 19 | |
| Bos d 6 | Bovine serum albumin | 0.1–0.4 | 66.3 | 0–76 | 1 | |
| Bos d 7 | Immunoglobulins | 0.6–1 | 160 | 12–36 | ||
| Lactoferrin | 0.09 | 80 | 0–35 | 3 | ||
| Whole casein (80%) (30 g/L) | Bos d 9 | αS1–casein | 12–15 | 23.6 | 65–100 | 26 |
| Bos d 10 | αS2–casein | 3–4 | 25.2 | |||
| Bos d 11 | β–casein | 9–11 | 24 | 35–44 | 35 | |
| Bos d 12 | k–casein | 3–4 | 19 | 35–41 | 26 |
3. Subtypes of Immune-Mediated Reactions to CM
CMAs have a range of clinical manifestations, with variable intensity. Moreover, clinical features can differ from “immediate” to “delayed” reactions, and this reflects the different pathogenesis (Figure 1).
Approximately 60% of CMA are IgE-mediated, although estimates change according to the study population and age [1].
The remaining 40% is divided into non IgE-mediated and mixed forms. The latter have different underlying mechanisms, presentations, and implications, which complicate the attempts to estimate the prevalence of CMA.
Non IgE-mediated CMAs are caused by less clear immune mechanisms. In this case, clinical findings are deferred and may occur 48 h or days after CM ingestion. Moreover, there are no specific symptoms or biomarkers that can guide the diagnosis, making it difficult to reach a conclusion. Typical non-IgE-mediated forms of CMA include CM enteropathy, food protein-induced proctitis/proctocolitis (FPIAP), food protein-induced enterocolitis syndrome (FPIES), and Heiner syndrome (pulmonary hemosiderosis) [1].
There are also mixed forms of CMA (both IgE- and non-IgE-mediated) that may have either humoral and/or cell-mediated mechanisms and may present with acute and/or chronic symptoms. These include atopic dermatitis, allergic eosinophilic esophagitis, and eosinophilic gastritis [1].
4. Prevalence of CMA
Before 1950, CMA was rarely diagnosed. Since 1970, significantly varying estimates of the incidence have been reported (ranging from 1.8% to 7.5%), reflecting the differences in diagnostic criteria and study design [16].
As a matter of fact, the prevalence estimates of CMA are affected by many factors. There is a marked heterogeneity in the prevalence of FA in the majority of papers. This could be the result of misleading differences in study design or methodology (including use of different definitions of CMA), or differences between populations and geographic areas [17].
Alongside the IgE, non-IgE, and mixed forms, there are also non-immune mediated reactions (i.e., intolerance), which are sometimes misclassified.
Another confounding factor while assessing prevalence is that many studies come from self-reports, with the consequent limitations linked to the subjective nature of the data [17]. Actually, the majority of studies on the epidemiology or natural history of FA have limitations. A precise evaluation requires a prospective ascertainment with a confirmatory oral food challenge (OFC) at predetermined intervals over time. For these reasons, studies such as these are rarely conducted due to their intrinsically reduced feasibility and ethical issues [17].
Therefore, determining an accurate diagnosis of CMA is fundamental. The process begins with an allergy-focused history that will guide all further investigations. In case that personal history is suggestive of allergy, a skin prick test (SPT) or specific IgE (sIgE) blood assay should be performed.
Evidence of sensitization (positive SPT or sIgE) with a suggestive history is usually sufficient to confirm the diagnosis, although OFC remains the gold standard [18].
It is important to emphasize that sensitization, i.e., raised sIgE directed against a specific antigen or positive SPT, in the absence of a supporting clinical history, is common in the general population but insufficient for a diagnosis of CMA. If diagnostic uncertainty remains even after a focused history and SPT/sIgE, an OFC is recommended to confirm diagnosis [18].
Other issues that may affect the estimates are the study design (prospective cohort vs cross-sectional), study population (demographic factors, geography, genetic/environmental factors), and natural history (incomplete identification of resolved cases) [1].
Despite these limitations during the assessment, a large number of studies in the United States and worldwide have attempted to estimate the prevalence of CMA [1].
An important contribution to prevalence studies was made by a meta-analysis performed by Rona et al., who analyzed publications from 1990 to 2005 and included only original studies (a total of 51 papers were considered appropriate for inclusion). The prevalence of self-reported FA was very high compared to that obtained using objective measures. Self-reported prevalence of CMA varied from 1.2% to 17%. The prevalence of CMA using SPT alone and sIgE alone, instead, was from 0.2% to 2.5% and from 2% to 9%, respectively. Studies using symptoms and sensitization (SPT ≥ 3 mm or sIgE > 0.35 kU/L) ranged from 0% to 2% prevalence, and those relying on OFC from 0% to 3% [19].
Another important meta-analysis and systematic review of CMA prevalence in Europe was performed by Nwaru et al., who analyzed publications from 2000 to 2012 (including 42 papers) [20]. The prevalence of self-reported CMA was 2.3% (95% CI 2.1–2.5), greater than that using SPT alone (0.3%, 95% CI 0.03–0.6) and sIgE alone (4.7%, 95% CI 4.2–5.1). The prevalence of CMA diagnosed by OFC was 0.6% (95% CI 0.5–0.8) and that using OFC or reported history of CMA was 1.6% (95% CI 1.2–1.9).
These meta-analyses show that prevalence estimates can be influenced by many factors such as geographic region, source population (high risk of referral bias vs general population), age and participation rates, and limitations of diagnosis [6].
Another important contribution was the EuroPrevall birth cohort study, published in 2015. In this study 12,049 children from nine different countries were enrolled, and 9336 (77.5%) were followed up until the age of 2 years. The authors calculated an overall incidence of challenge-proven CMA of 0.54% (95% CI 0.41–0.70) and showed differences in national incidences ranging from 1% (in the Netherlands and United Kingdom) to <0.3% (in Lithuania, Germany, and Greece). In this unique cohort study, they also showed that affected infants, without detectable specific antibodies to CM, were very likely to tolerate CM 1 year after diagnosis, whereas only half of those with specific antibodies in serum overcame their disease in the same period of time [21].
References
- Flom, J.D.; Sicherer, S.H. Epidemiology of cow’s milk allergy. Nutrients 2019, 11, 1051.
- Mousan, G.; Kamat, D. Cow’s milk protein allergy. Clin. Pediatr. 2016, 55, 1054–1063.
- Hill, D.J.; Firer, M.A.; Shelton, M.J.; Hosking, C.S. Manifestations of milk allergy in infancy: Clinical and immunologic findings. J. Pediatr. 1986, 109, 270–276.
- Lomer, M.C.; Parkes, G.C.; Sanderson, J.D. Review article: Lactose intolerance in clinical practice—Myths and realities. Aliment. Pharmacol. Ther. 2008, 27, 93–103.
- Burks, A.; Tang, M.; Sicherer, S.; Muraro, A.; Eigenmann, P.A.; Ebisawa, M.; Fiocchi, A.; Chiang, W.; Beyer, K.; Wood, R.; et al. ICON: Food allergy. J. Allergy Clin. Immunol. 2012, 129, 129906.
- Sicherer, S.H.; Sampson, H.A. Food allergy: A review and update on epidemiology, pathogenesis, diagnosis, prevention, and management. J. Allergy Clin. Immunol. 2018, 141, 41–58.
- Savage, J.; Sicherer, S.; Wood, R. The natural history of food allergy. J. Allergy Clin. Immunol. Pract. 2016, 4, 196–203.
- Hochwallner, H.; Schulmeister, U.; Swoboda, I.; Spitzauer, S.; Valenta, R. Cow’s milk allergy: From allergens to new forms of diagnosis, therapy and prevention. Methods 2014, 66, 22–33.
- Wal, J.M. Bovine milk allergenicity. Ann. Allergy Asthma Immunol. 2004, 93, S2–S11.
- Séverin, S.; Wenshui, X. Milk biologically active components as nutraceuticals: 546 review. Crit. Rev. food Sci. Nutr. 2005, 45, 645–656.
- Haug, A.; Høstmark, A.T.; Harstad, O.M. Bovine milk in human nutrition—A review. Lipids Health Dis. 2007, 6, 1–16.
- Pereira, P.C. Milk nutritional composition and its role in human health. Nutrition 2014, 30, 619–627.
- Caffarelli, C.; Baldi, F.; Bendandi, B.; Calzone, L.; Marani, M.; Pasquinelli, P. Cow’s milk protein allergy in children: A practical guide. Ital. J. Pediatr. 2010, 36, 1–7.
- Restani, P.; Ballabio, C.; Di Lorenzo, C.; Tripodi, S.; Fiocchi, A. Molecular aspects of milk allergens and their role in clinical events. Anal. Bioanal. Chem. 2009, 395, 47–56.
- Fiocchi, A.; Schünemann, H.J.; Brozek, J.; Restani, P.; Beyer, K.; Troncone, R.; Martelli, A.; Terracciano, L.; Bahna, S.L.; Rancé, F.; et al. Diagnosis and rationale for action against cow’s milk allergy (DRACMA): A summary report. J. Allergy Clin. Immunol. 2010, 126, 1119–1128.e12.
- Host, A. Cow’s milk protein allergy and intolerance in infancy. Some clinical, epidemiological and immunological aspects. Pediatr. Allergy Immunol. 1994, 5, 1–36.
- Savage, J.; Johns, C.B. Food allergy epidemiology and natural history food allergy epidemiology natural history peanut milk egg. Immunol. Allergy Clin. NA 2015, 35, 45–59.
- Helyeh, S.; David, L.; Gary, S. Advances in the management of food allergy in children. Curr. Pediatr. Rev. 2018, 14, 150–155.
- Rona, R.J.; Keil, T.; Summers, C.; Gislason, D.; Zuidmeer, L.; Sodergren, E.; Sigurdardottir, S.T.; Lindner, T.; Goldhahn, K.; Dahlstrom, J.; et al. The prevalence of food allergy: A meta-analysis. J. Allergy Clin. Immunol. 2007, 120, 638–646.
- Nwaru, B.I.; Hickstein, L.; Panesar, S.S.; Roberts, G.; Muraro, A.; Sheikh, A. Prevalence of common food allergies in Europe: A systematic review and meta-analysis. Allergy Eur. J. Allergy Clin. Immunol. 2014, 69, 992–1007.
- Schoemaker, A.A.; Sprikkelman, A.B.; Grimshaw, K.E.; Roberts, G.; Grabenhenrich, L.; Rosenfeld, L.; Siegert, S.; Dubakiene, R.; Rudzeviciene, O.; Reche, M.; et al. Incidence and natural history of challenge-proven cow’s milk allergy in European children—EuroPrevall birth cohort. Allergy Eur. J. Allergy Clin. Immunol. 2015, 70, 963–972.




