
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Adriana Nowak | + 6261 word(s) | 6261 | 2021-05-10 04:50:59 | | | |
| 2 | Peter Tang | Meta information modification | 6261 | 2021-05-11 06:00:56 | | |
Video Upload Options
Numerous honeybee (Apis mellifera) products, such as honey, propolis, and bee venom, are used in traditional medicine to prevent illness and promote healing. Therefore, this insect has a huge impact on humans’ way of life and the environment. While the population of A. mellifera is large, there is concern that widespread commercialization of beekeeping, combined with environmental pollution and the action of bee pathogens, has caused significant problems for the health of honeybee populations. One of the strategies to preserve the welfare of honeybees is to better understand and protect their natural microbiota.
1. Introduction
The honeybee Apis mellifera is a social insect species that has successfully colonized numerous ecosystems around the world and plays a crucial role in pollinating wild and cultivated plants, with substantial implications for the global economy and natural ecosystems [1]. Honeybees provide a key link in the production of food, and their economic value to the United States alone is estimated to be as much as USD 15 billion [2]. Besides their pollination value, honeybees are important because of their great agronomic and economic potential owing to the production of valuable commercial products such as wax, pollen, propolis, royal jelly, and most importantly, honey [1].
Bees are vital for the preservation of the ecosystem as they help maintain an ecological balance. They are known to have complex interactions with their environment and a diverse range of microorganisms. Understanding the relationship between honeybees and their external environment is important to maintain a hospitable environment for both humans and bees. The honeybee microbiome is central to maintaining the individual’s health, and a disrupted microbiome makes the insect susceptible to a variety of problems. Thus, research has focused on the intestinal microbiome of honeybees; its role and function in bee health, fitness, and metabolism; and its response to many physical, biological, chemical, and environmental factors [3][4][5][6][7]. Such a broad perspective is needed, considering the importance of honeybee health and the knock-on impact on environmental protection.
2. Apis mellifera Characterization
Apis mellifera is one of the most common floral visitors in natural environments worldwide. On average, honeybees account for 13% of floral visits across all networks. Five percent of plant species are visited by A. mellifera exclusively [8]. The lifespan of honeybees varies significantly depending on the moment of their emergence. Therefore, they can be classified as either short-lived summer bees or long-lived winter bees. Bees emerging in spring and midsummer live for an average of 25–40 days, while winter bees have a much longer lifespan of more than 100 days [9]. This bimodal longevity distribution presumably results from complex dynamics associated with biotic and abiotic factors, interactions between individuals in the colony, and regulatory mechanisms of individuals influenced by intracolonial conditions [10]. It has been shown to be predominantly associated with bees’ flight activity and the change in the nature of their tasks, from those performed inside the nest to the more hazardous task of foraging. This significant transition in the life cycle of an adult bee is related to both dietary and physiological changes, including a shift from a carbohydrate–protein diet to a pure carbohydrate diet [11].
The worldwide distribution of honeybees is due to the activities of beekeepers, but their native range is also large, spanning Europe, Africa, and the Middle East [12]. There are 10 species of honeybee belonging to the genus Apis. Phylogenetic analyses involving nuclear DNA and mitochondrial (mtDNA) markers clearly approved clustering these species into three distinct groups: Cavity-nesting bees (represented by A. mellifera, A. cerana, A. koschevnikovi, and A. nulensis), giant bees (A. dorsata, A. laboriosa, A. dorsata binghami, and A. nigrocincta), and dwarf bees (A. florae and A. andreniformis) [13]. Except for A. mellifera, all species are now limited to Asia, and the lineage that brought about the A. mellifera embodies an early split from different cavity-nesting bees, so it is thought that A. mellifera may have originated from Asia [12].
Honeybees live in large communities with a complex organization that depends on cooperative and altruistically motivated individuals and communication. The colony is formed by hundreds of males (drones), sterile female workers numbering between 12,000 and 90,000 depending on the season, and a single queen [14][15]. The workers are responsible for all activities that assist with reproduction: They clean combs and feed larvae; are involved in comb building, the evaporation of nectar, and guarding of the hive; and above all, they are responsible for foraging to provide the colony with food and water [14]. The duties of the queen, after nuptial flights, are limited exclusively to laying eggs. During the period of most intense development, which usually takes place at the end of spring and beginning of summer, the queen lays about 2000 eggs. Drones appear in May, and they are crucial for the reproduction process. They copulate with the queen in the air and then die. Drones that did not participate in the reproduction process are expelled from the hive at the end of July and starve to death [16][17]. The group remains consistent due to its ability to distinguish nestmates from non-nestmates, which is denoted by the presence of the guard bees at the entrance of the hive. Their function is to prevent non-nestmates from entering the nest and allow nestmates to freely move inside [15].
In the simplest terms, bee nutrition is based on nectar and pollen, the former supplying bees with carbohydrates and the latter a source of proteins, lipids, and other micronutrients. In order to obtain optimal nutrition, bees balance the intake of nutrients from these complementary food sources [18]. Adequate nutrition is crucial for the proper growth and development of a honeybee colony, while any deficits contribute to aggravation of the negative impacts of viral and fungal diseases [19]. Nutrition can be considered at three different scales, that is, in terms of colony nutrition, adult nutrition, and larval nutrition. In a colony, nutritional levels are connected by a variety of interactions between the adult bees and the brood called trophallaxis (transferring of food from one individual to another) [19]. Both larvae and adult bees are dependent on the food stores of the colony, and adult bees can adjust foraging and strategies of brood-care in accordance with the supply of the hive’s provisions [20].
Pollen is the predominant source of lipids, proteins, vitamins, and minerals. It is essential for the growth, development, and reproductive processes of honeybees [21]. It is especially important for the development of the hypopharyngeal glands and body fat in newly emerged workers, which is necessary for brood-reading and overwintering [22]. Bees collect pollen and place it on the corbiculae—structures located on the hind legs [23]. The color of the corbiculae reveals information about the flowers that were visited by bees. They most commonly appear yellow, orange, or brownish, although they can also be white, navy blue, or black. Pollen is also stored in nest cells, to which all the workers in a colony have access [24]. During pollen collection, bees display temporary specialization toward the pollen of one species. European honeybees are especially consistent in terms of the flowers on which they specialize, and their individual pollen loads usually originate from a single source. Nevertheless, at the colony level, pollen is concomitantly collected from different sources [25]. There are some plants that produce pollen that is harmful for bees. There have also been cases of poisoning of humans after ingestion of honey from poisonous plants [26]. However, poisoning occurs relatively rarely, and only when the poisonous plant is dominant in a certain area where other pollen plants are absent, and bees suffer from a lack of water. Poisoning leads to noninfectious disease of adult insects [26].
Nectar is an aqueous solution containing sugars, amino acids, organic acids, proteins fats, vitamins, and minerals. It is produced by a specialized group of cells called nectaries [27][28]. The composition of nectar is dominated by sucrose, fructose, and glucose. Honeybees are sensitive to differences in nectar composition and prefer pure sucrose over pure glucose or fructose solutions; however, in the field, nectars containing mixtures of these sugars are most commonly found [28].
Honeybees produce many different substances, namely honey, bee pollen, propolis, bee bread, royal jelly, beeswax, and bee venom, which play various functions in the life cycle of honeybees [29][30]. What makes honeybees different compared to other insects is that they hoard food. During the hoarding process, food undergoes refinement, so it differs from its original state. There are two major forms of hoarded food: honey from nectar and bee bread from pollen. They are both stored in a comb formed of wax, produced using the wax glands of adult worker bees [25].
The process of honey formation is initiated by the collection of nectar from plants. It is stored at the bottom of the esophagus in the honey stomach [31]. During transport to the hive, the nectar undergoes an enzymatic treatment. The chemical transformation is based on the hydrolysis of sucrose performed by the addition of invertase [32]. Afterward, the nectar loads are transferred from honeybee nectar collectors to food-storer bees. The food-storer bees regurgitate the nectar and deposit it into the honeycomb. The nectar then undergoes a ripening process, which consists of the further conversion of sucrose to glucose and fructose, and water evaporation [31]. The water concentration is decreased to about 17% [32]. This conversion process takes from one to three days and is finalized by the capping of the cells filled with nectar using bee wax [31].
Pollen-collecting foragers transport their pollen loads straight to cells distributed within the comb. These cells are often already packed with previous loads, which may be from different floral sources. Pollen is then processed by young hive bees that pack it tightly and add regurgitated honey, which preserves the stored pollen through its antimicrobial properties. Pollen that is packed into cells for storage is referred to as bee bread [25]. The flow of water and food in the colony of honeybees has been described in detail by Wright et al. [25].
Another bee product is royal jelly, a substance secreted in the hypopharyngeal glands of young worker bees that is used to feed the larvae of drones and worker bees during the first three days of their lives, and to feed the queen. Worker and drone larvae are fed royal jelly along with honey and pollen. Royal jelly is the only food that the adult and larvae queen bee consumes [33][34]. The most important role of royal jelly is to provide nutrition and protection for honeybee larvae during development, and it is the crucial driving force in the process of caste determination. A fertile egg becomes either a sexually perfect future queen bee that has mature ovaries for reproduction, or a sexually immature worker, which depends strictly on the dose and timing of royal jelly consumption during larval development [34]. Fed with royal jelly exclusively, queen bees are capable of developing superior features, not only in terms of physical appearance, but also strength, stamina, and longevity (queen bees can live for up to 5–7 years) [35][36]. Proteins are the major constituent of royal jelly, most of which are water-soluble, and it is because of these that the secretion exhibits antiaging, antitumoral, and insulin-like activities [37].
3. Honeybee Microbiota
Animals that form social communities usually employ a characteristic microbiota that is essential for various processes that occur in the body [38]. The microbiota can be defined as a complex ecosystem of microorganisms that plays a critical role in a variety of metabolic functions, including modulation of glucose and lipid homeostasis, satiety regulation, management of energy, and the production of vitamins [39][40][41]. In addition, the microbiota participates in the regulation of various biochemical and physiological mechanisms by means of the production of metabolites and other substances [42]. Furthermore, the microbiota exerts anticarcinogenetic and anti-inflammatory activities, [38] and plays a significant role in the operations of the host immune system and induction of immune responses [43]. In return, the host immune system maintains a mutualistic relationship with the microbiota. This relationship enables the induction of protective responses toward pathogens and the introduction of regulatory pathways involved in the tolerance to harmless antigens [44].
While the importance of the gut microbiota is discussed more often now, the processes responsible for the beneficial features of microbial communities remain unclear [45][46][47]. The composition of the microbial communities that inhabit the gut vary significantly between different species and within them. The diversity in composition of the gut microbiota is influenced by topographical and short-term shifts in the microbial communities, with specific microorganisms inhabiting particular niches in the host during specific growth and developmental phases of the host [48].
3.1. Characteristics
Insects represent the most diverse animal clade in terms of the number of species, the ecological habitats they inhabit, and their overall biomass [3]. A. mellifera is a useful model organism with a microbial community that displays high host adaptation. While its microbiota has some similarities with those of mammals, it has a much simpler composition. The main similarities and differences in the honeybee and human gut microbiota were reviewed previously [49].
Honeybees form huge colonies that contain thousands of nonreproductive female workers, hundreds of male drones, and only one reproductive queen [14]. Newly emerged workers have a reduced core gut microbiota or may lack it entirely [50]. Their bodies are colonized by microbial communities orally by means of social interactions with nurse bees within a few days of emergence [51][52]. During metamorphosis into pupae, the gut bacteria are excreted via defecation along with the gut epithelium, and the next colonization starts due to trophallaxis, contact with other bees, as well as from the hive [53]. The abundance of bacteria in the whole gut reaches its peak 3–5 days post-adult emergence [54]. However, taxonomic shifts take place after 3–8 days, which suggests pioneer or niche construction strains. The rectum community seems to finish the development of an emergent structure after three days. The ileum is more variable, with its final structure emerging after eight days. The most important factor influencing this process is the prevalence of core species, the host immune response related to it, and the successional alternation of the environment of ileum [4]. The workers are involved in age-associated tasks, and newly emerged bees are usually associated with hive maintenance and cleaning tasks. Therefore, the interactions with adult bees, contact with the comb, and consumption of bee bread are all potential routes of inoculation [54][55]. Dong et al. [50] analyzed the succession of A. mellifera workers gut microbiota from birth to senescence, i.e., from 0–40 days postemergence (dpe). The genera Gilliamella, Frischella, and Snodgrassella colonized the honeybee gut at 1 dpe; Lactobacillus, Bifidobacterium, and Commensalibacter colonized at 3 dpe, while a simultaneous reduction in Gilliamella was observed. At 12 dpe, significant colonization by L. kunkeei and Bartonella sp. appeared, while Bacteroides sp., Escherichia sp., Shigella sp., and Porphyromonadaceae decreased between 19 and 25 dpe. Commensalibacter sp. and Bifidobacterium sp. abundance was reduced at 25 dpe [50].
The microbiota of honeybees are located in different parts of the gut, including the crop (located between the esophagus and ventriculus, and used for storage and transport of nectar to the hive; also called stomach or sack); midgut; the hindgut, consisting of the ileum (a narrow tube containing six longitudinal folds) and lumen; and the distal rectum [56][57]. Only Parasaccharibacter sp. was found in relative abundance in worker hypopharyngeal glands [58].
It was estimated that adult workers’ guts are inhabited by characteristic, specialized microorganisms belonging to nine clusters of bacterial species [59]. Each of the clusters represents a set of bacterial strains that are related. Similar to human hosts, the microbial communities in honeybees are dominated by host-adapted species, which are highly intolerant of atmospheric oxygen; therefore, the transmission of bacterial species takes place by social interactions between hosts [60]. However, unlike mammalian gut microbiota, all of the bacterial species can be cultured in a laboratory [61].
Using 16S rDNA community surveys and metagenomics of the total DNA, it was determined that guts of worker honeybees are inhabited by nine bacterial species clusters that account for 95–99.9% of the bacteria in almost all individuals [59][62][63]. Two ubiquitous Gram-negative species—Snodgrasella alvi (nonfermenting sugar bacteria that form a film directly on the gut wall; family Neisseriaceae) and Gilliamella apicola (bacteria with the ability to ferment sugar that inhabits areas directed toward the center of the lumen; family Orbaceae)—that are members of the Proteobacteria phylum can be distinguished [2][59][63]. There are two Gram-positive species belonging to phylum Firmicutes that are ubiquitous and abundant; namely, Lactobacillus Firm-4 and Lactobacillus Firm-5, which inhabit the distal rectum [2][59]. In the majority of adult workers, Bifidobacterium asteroides is also found (albeit with much lower abundance) [53][61]. The mentioned bacterial species clusters are the most essential microorganisms in the honeybee gut, the so-called “core bacteria” [64]. There are also less-abundant/stable species from Proteobacteria: The Gammaproteobacteria Frischella perrara (Orbaceae family); the Alphaproteobacteria Parasaccharibacter apium, Bombella favorum, Bombella mellum, Bombella apis (Acetobacteraceae family, Alpha 2.2); and Commensalibacter sp. (Alpha 2.1) and Bartonella apis (Alpha 1) from the Rhizobiaceae family [50][53][59][63][65][66]. Representatives of phylum Bacteroidetes have also been identified in the honeybee gut—Apibacter adventoris and Apibacter mensalis [67][68].
A previous study [69] detected 10 taxa dominant in bee samples—four representatives of Lactobacillus sp., two Gilliamella sp., one Bifidobacterium sp., and one Snodgrassella sp.—that are considered to be part of the core gut microbiome of honeybees. Two of the taxa, from Frischella sp. and Bartonella sp., may vary depending on the environment. They are noncore members of honeybee gut [64]. Wang et al. [70][71] showed that the dominant phyla in honeybee GIT are Proteobacteria (63.2%), Firmicutes—(17.6%, with 15.9% of Lactobacillus sp.), Actinobacteria (4.1%, with 3.34% of Bifidobacterium sp.), and Bacteroidetes (1.7%, with 0.23% of Bacteroides sp.). The core member Lactobacillus Firm-4 was detectable in 98.4% of all analyzed bees in the study by Kešnerová et al. [64]. Tola et al. [63] analyzed A. mellifera gut microbiota from sub-Saharan African regions of Kenya, where indigenous and traditional management methods involving very little human intervention are practiced in beekeeping, unlike those practiced in Europe. They confirmed the core honeybee gut microbiota members were from the genera Gilliamella, Snodgrassella, Lactobacillus (Firm-4 and Firm-5), Bifidobacterium, Frischella, Commensalibacter, Bombella, Apibacter, and Bartonella, and that Frischella sp. was the third most dominant genus (16.9%), while Lactobacillus (Firm-4 and Firm-5) exhibited a lower abundance than has been demonstrated in other studies [63]. A summary of the GIT microbiota in honeybees is presented in Figure 1.
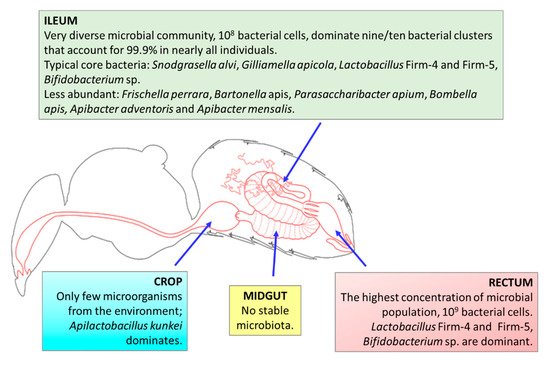
Figure 1. The gastrointestinal microbiota of an adult worker honeybee (Apis mellifera) (references in the text). Figure taken from http://honeybee.drawwing.org/book/crop (accessed on 22 March 2021) with the permission of the author.
3.2. Functions
Considering an ecological perspective, gut microorganisms play a critical role in the process of codevelopment of insect-symbiotic interactions by means of secondary metabolites. Gut microbes take part in insects’ growth, development, and reproduction, and above all they contribute significantly to their metabolism [70]. Gut microorganisms synthesize essential nutritional compounds, increase the efficiency of digestion, and support insects in absorption of nutrients [72]. Most insects are inhabited by relatively few species (in comparison to mammalian gut), of which the majority is cultivable in the laboratory, but some harbor numerous communities of specialized bacteria. The factor defining limitation in gut microbiota in most insects is the lack of transmission routes between individuals. Exceptions are social insects such as termites, ants, and most importantly, bees. Social interactions give opportunities for transfer of gut microorganisms, therefore some of the most consistent and specialized gut communities, with significant functions in nutrition and protection, have been identified in social insects, such as honeybees [73].
Studies that concentrated on the beneficial health activities that microbes confer to their host have shown that the gut microbiota of honeybees plays as important a role as it does in mammals [2][3][4][45][48][49][50][72][74][75]. Two well-established functions of gut microbiota are nutrient biosynthesis and biomass deconstruction. The nutritional function was extensively studied in experiments comprising insects feeding with unbalanced and poor diets that lacked essential nutrients like amino acids and vitamins. These studies proved that insect endosymbionts help to produce nutrients that are not present in food [76]. The second function of some insect microbiota is biomass deconstruction and digestion. Both symbiotic microorganisms and host insects can release cellulolytic enzymes responsible for the deconstruction and hydrolysis of biomass, although studies have shown that microorganism activity increases the efficiency of these processes [76]. Gut microorganisms significantly contribute to the digestion of lipids and proteins, as well as the detoxification of secondary plant compounds. They also affect survival, overall size, and egg production. Moreover, they have been shown to play an important role in insect resistance against insecticides [77].
Gut microorganisms inhabiting insects can indirectly exert beneficial health effects on humans, in the case of parasitic diseases transmitted by insect vectors [78]. It was observed that in the gut of insect vectors, parasites ingested with bloodmeal reduced in number before coming into contact with host tissues. Microbial communities are thought to be an important factor influencing this effect. It was concluded that gut microorganisms contribute to the modulation of the competence of insect vectors. One of the possible mechanisms through which microbes support insects against parasites is through modification of the gut environment to constrain parasite development or induce an immune response of the host. They are also capable of producing antimicrobial peptides, which play a key role in the control of parasites and bacterial pathogens. In the study referred to above, after bloodmeal was ingested, the population of bacteria in the vector gut expanded rapidly. However, the microbiota were able to kill all parasites present [78][79]. The application of microbial symbionts to reduce vector competence is a promising approach to control the spread of insect vector transmitted pathogens [79].
Compared with the gut microorganisms of other animals, the honeybee microbiota is heavily involved in functions associated with carbohydrates, which reflects specific adaptations to a host’s diet that is rich in sugars. It provides the honeybee with sugar uptake systems belonging to various phosphotransferase systems. Many of these transporters are classified in the mannose family [73]. This feature of bacteria is important because only trace amounts of mannose are present in nectar, but it is highly poisonous when ingested at higher concentrations [73].
Another function associated with carbohydrates is enrichment of the host with arabinose efflux permeases. This family of transporters is involved in the transfer of different compounds such as antimicrobial proteins, amino acids, and sugars. A diverse set of transporters confers protection for the bacteria against a variety of pesticides applied in agriculture and naturally occurring antimicrobial proteins ingested by bees as part of their plant-based diet [3].
Furthermore, gut microorganisms influence the transformation of both nectar into honey and plant buds and exudates into propolis, owing to their fermentation properties [80]. They are also responsible for the freshness of honey [81].
One of the ways by which the gut microbiota can affect the health of honeybees is through modulation of the immune responses of the host [82]. Microorganisms impact the development and morphogenesis of the immune system and other organs and body structures [83][84]. One of the examples of how microbes affect a host is the symbiotic interaction between the fruit fly Drosophila melanogaster and the bacteria inhabiting its gut, Acetobacter pomorum [85]. This relationship influences the host’s body size, developmental rate, metabolism, activity of stem cells, and surface area of wings [85].
The primary role of gut microbiota in the functioning of mucosal immunity is not surprising, considering that the intestinal mucosa comprises the largest surface area in contact with antigens coming from the external environment, and that the dense layer of microbiota covering the mucosa constitutes the greatest proportion of antigens presented to the resident immune cells [75]. The mucosal immune system is responsible for the realization of two seemingly contradictory functions. It must tolerate microbiota inhabiting the gut to prevent the induction of harmful systemic immune responses, while controlling the number of microorganisms to avoid overgrowth and translocation [86]. Gut microorganisms are involved in the fulfillment of these objectives [75]. They control intestinal homeostasis through a variety of mechanisms involving substances like lipopolysaccharides, flagellins, and peptidoglycans. They interact with cell receptors such as Toll-like receptors, and they activate intracellular signaling pathways associated with cell survival, replication, apoptosis, and inflammatory responses [87][88][89]. In return, the host immune system controls the composition of microbes by releasing molecules like defensins, lectins, reactive oxygen species, and bacteriocins, which effectively constrain the expansion of pathogenic microorganisms [87][88][89].
Antimicrobial peptides are crucial components of innate immunity aimed at defense against the invasion of pathogens. They are determinants of the microbiota composition, as their role is to damage pathogenic microorganisms’ cells by means of membrane perforation [90]. Four families of antimicrobial peptides (abaecin, apidaecin, defensin, and hymenoptaecin) are evoked within the honeybee hemolymph during immune challenge. In one study, bees lacking gut microbiota were compared with bees inoculated with the normal gut microbiota by feeding with hive bee guts or with the bacteria S. alvi. It was observed that apidaecin and hymenoptaecin expression was upregulated in bees inoculated with gut microbiota, which indicates that the gut microbiota induces immune responses in bees [82].
The honeybee microbiota was observed to promote body-weight gains. To examine the effect of the microbiota on the growth of hosts, body-weight measurements were made in the presence and absence of gut microorganisms. Germ-free and conventional bees were received from pupae that were collected from hives and allowed to emerge in sterile laboratory conditions [2]. Bees deprived of microbiota achieved significantly lower weight gain (by 82%) than conventional bees. The weight gain was associated with the insulin/insulin-like signaling pathway, which plays a critical role in growth, reproduction, and aging, and regulates homeostasis and behavior in bees [2].
Gut microorganisms inhabiting insects do not just affect the digestive system. Various studies proved the existence of a gut microbiota–brain axis, meaning that gut microorganisms induce alteration of neurophysiology and changes in behavior of insect hosts [91][92]. For example, microorganisms can alter volatile profiles and the olfactory behavior of their insect hosts. Consequently, they regulate the ways in which individuals interact through chemical communication, aggregate in groups, and make decisions concerning foraging and mating. For instance, lower termite Reticulitermes speratus conspecific intruders are more quickly recognized and attacked when they are colonized by foreign gut bacteria releasing unfamiliar scents. Another example is found with the leaf-cutting ant Acromyrmex echinatior, in which suppression of the gut microbiota induces aggression among non-nestmates through alterations in cuticular hydrocarbon profiles [93]. Gut microorganisms can also increase the longevity of insects. An example of such activity of microbes is in D. melanogaster, the lifespan of which was significantly elongated after application of probiotic and symbiotic formulations. These formulations rescued metabolic stress markers through management of insulin resistance and energy-regulatory pathways [91]. Gut microorganisms also affect the neurophysiological development of the host, as they support cognition by enhancing its capacity to memorize and learn. A recent study linked gut microorganisms with markers of Alzheimer’s disease [93].
The gut microbiota of honeybees was observed to impact the neurophysiology and behavior of hosts. Microbes can also affect host behavior by alteration of the levels of biogenic amines such as serotonin, octopamine, and dopamine. Levels of these compounds vary seasonally in the worker’s brains, increasing in summer when foraging activity is the highest, and at different life stages, being lower in brains of newly emerged, germ-free bees [94]. Furthermore, the gut microbiome plays a key role in the regulation of social behavioral features in honeybees [95].
Gut-microbiota involvement in xenobiotic metabolism has been known for years, and this ability sheds light on the potential ability to maintain microbiota as a target for drugs to effectively contribute to treatment for various diseases [96][97]. As honeybees are exposed to a wide range of pesticides, an important role of their gut microbiota is the detoxification of xenobiotics, especially neonicotinoid insecticides [98]. Wu et al. [98] demonstrated that honeybee gut microbiota contribute to the host’s endogenous detoxification and resistance to thiacloprid and fluvalinate, as it promotes the expression of detoxification enzymes in the midgut. The importance of honeybee gut microbiota was also illustrated by a metagenome project in which symbionts of honeybees were affected by viruses. This led to detrimental effects on the growth and development of bees, and could be a major cause of colony collapse disorder (CCD) [76]. Undigested pollen was observed in the fecal content of honeybees that died due to CCD, and it indicated a deficit in the abundance of beneficial probiotic bacteria in the GIT. This may have been caused by pesticides and antibiotic residues [99].
The microbiota synthesizes enzymes such as proteases and glycosidases, metabolizes indigestible polysaccharides, produces essential vitamins, and conducts xenobiotic metabolism. This significantly expands the host’s biochemical capacity [100]. The fermentation of indigestible carbohydrates and oligosaccharides by bacteria belonging to the genera Bacteroides, Roseburia, Bifidobacterium, and Faecalibacterium results in the formation of short-chain fatty acids (SCFAs) including butyrate, propionate, and acetate [71][101]. These substances provide rich sources of energy for the host. Butyrate helps prevent the accumulation of toxic byproducts of metabolism [101]. Honeybee gut microbiota functions are presented in Figure 2.
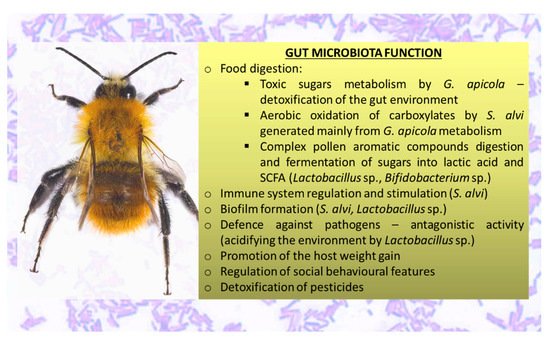
Figure 2. Summary of the main functions of Apis mellifera gut microbiota (references in the text).
3.3. Factors Affecting Honeybee Microbiota
Interactions between the honeybee gut community and the environment are complex and not well understood. There exists a huge diversity of gut microorganisms among insects, influenced by many factors such as habitat, feeding preference, life stage, and host species. Jones et al. [59] showed that the broad landscape influenced the diversity of some members of honeybee gastrointestinal microbiota, especially those belonging to Proteobacteria and Firmicutes. Muñoz-Colmenero et al. [102] demonstrated that the environment plays the main role in determining honeybee microbiota, and that agricultural treatments cause disruption to the bacterial community.
Honeybees exhibit a complex social network of microorganisms that can be characterized by variations according to geographic location [5][103]. For example, in A. mellifera jemenitica, the rural honeybee characteristic of the Kingdom of Saudi Arabia, some bacteria identified in the alimentary tract—Citrobacter sp., Providencia vermicola, Exiguobacterium acetylicum, and Planomicrobium okeanokoites—are unique to this species [104]. The core honeybee intestinal microbiota is also subjected to global seasonal variations [105]. Few studies have shown how extreme modifications impact gut microbiota dynamics during overwintering. However, seasonal changes in the honeybee microbiome in Canada were investigated by Bleau et al. [53], and they observed a decrease in the abundance of Enterobacteriaceae from September to November, while the relative abundance of Neisseriaceae increased. Subotic et al. [69] found that the honeybee microbiome changes seasonally. Another study found differences in bacterial abundance of honeybee gut community members between summer and winter months that were linked to diet [64]. The lowest diversity and highest bacterial loads were observed in winter bees (with high levels of Bartonella sp. and Commensalibacter sp.) [86]. Furthermore, diet (type of sugar used in winter forage, nutritional stressors, poor-quality diet, and propolis-rich and propolis-poor diets) has been shown to determine the profile of the dominant honeybee gut community [71][106][107]. A high-fat diet (palm oil) significantly increased the abundance of Gilliamella sp., while a decreased abundance of Bartonella sp. was observed [108]. In another study, honeybees that were subjected to feeding with “aged” pollen displayed increased mortality, a higher load of Nosema sp., a pathogen of fungal origin, and a significant shift in the gut microbiota composition [6].
Due to the increasing risk of CCD, attempts have been made to treat colonies using chemical methods. Antibiotics can influence the host by altering the species of gut microbiota. Daisley et al. [109] documented the deleterious effects of antibiotics on the gut microbiome, immunity, and productivity of honeybees. Several residues of antibiotics and veterinary chemotherapeutics are detected in honey, showing that honeybees are still exposed to them, despite many countries banning their usage in beekeeping [110][111]. These stressors prompt a reduction of bacterial species in the honeybee gut, weakening their immunity and increasing their susceptibility to infections [112]. In one study, honeybees underwent treatment with antibiotics, which resulted in the elimination of their microbiota. It was found that bees were more susceptible to infections by Nosema ceranae (a frequent honeybee pathogen) due to its negative influence on the immune system, which was illustrated by the depletion of the expression of genes that encode antimicrobial peptides [54]. Another study suggests that disturbance of gut microbiota with tetracycline decreased honeybee survival, which was associated with an elevated susceptibility to the opportunistic pathogen Serratia sp. [6]. Furthermore, antibiotic residues may be found later in honeybee products. Ortiz-Alvarado et al. [113] studied the effect of two commercial beekeeping antibiotics—Terramycin (oxytetracycline) and Tylan (tylosin tartrate)—on bee physiology and behavior throughout development. The results of the study showed that antibiotic treatments increased the amount of lipids and the rate of behavioral development. The timing of the antibiotic treatment affected the age of onset of behaviors, starting with cleaning, then nursing and foraging. Bees treated during the larva–pupa stages demonstrated an accelerated behavioral development and loss of lipids, while bees treated from larva to adulthood had a delay in behavioral development and loss of lipids. These effects of antibiotic treatments suggest a role of microbiota in the interaction between the fat body and brain, which is important for honeybee behavioral development. Zheng et al. [49] presented an overview of the recent research in the field of antibiotic use. Long-term antibiotic use may have impacted the diversity within human gut communities and has resulted in high frequencies of resistance determinants [114]. In the United States and other countries where beekeepers used antibiotics since the late 1940s to control or prevent larval bacterial diseases (foulbrood), antibiotic exposure has affected gut communities of honeybees [115][116][117]. This practice has resulted in high frequencies of antibiotic resistance determinants in core gut bacteria isolated from bees in the United States, in contrast to gut bacteria of honeybees from countries that do not permit the use of antibiotics in beekeeping [110][118]. In both human and honeybee gut communities, resistance determinants have been exchanged among community members through horizontal transfer [119]. In the European Union (EU), legal permission for the application of antibiotics is connected with the food safety and protection of consumers. The new European environmental strategy “The European Green Deal” [120] stresses the role of the “from farm to fork” approach, which entails designing a fair, healthy, and environmentally friendly food system. The strategic plans will need to reflect an increased level of ambition to reduce the use and risk of chemical pesticides, as well as the use of fertilizers and antibiotics. The EU needs to develop innovative ways to protect harvests from pests and disease, and to consider the potential role of new innovative techniques to improve the sustainability of the food system, while ensuring that they are safe. The most significant act that regulates food safety is Regulation No. 178/2002 [121], which includes the basic rules on food safety and established the European Food Safety Agency. European food safety is regulated by over a hundred legal acts, and Regulation No. 415/2014 [122] established the EU reference laboratory for bee health, which coordinates the methods employed in the member states for diagnosing relevant bee diseases. In reference to the veterinary medicinal products as antibiotics in the bee sectors, member states have to comply with the European rules on veterinary medical products. The definition of honey is regulated in the Directive 2001/110/EC [123]. The Commission stresses the limited availability of veterinary medicines for bees. According to Regulation (EC) No 470/2009 [124], the veterinary medicinal products intended for use in food-producing animals like bees have to be scientifically evaluated according to human food-safety requirements. Regulation (EU) No 37/2010 [125] outlined the EU Maximum Residue Limits (MRLs) for residues of pharmacologically active substances in honey. For some substances (e.g., amitraz and coumaphos), an MRL has been established, while for others the evaluation demonstrated that no MRL was required to protect food safety (e.g., flumethrin, oxalic acid, and tau fluvalinate). Products that have not been assessed as safe according to these requirements can neither be authorized nor used otherwise for food-producing animals. A new Regulation (EU) No 6/2019 [126] on veterinary medical products will come into effect on 22 January 2022. The regulation sets out rules for the sale, manufacture, import, export, supply, distribution, control, and use of veterinary medicinal products (VMPs), aiming to modernize legislation, stimulate innovation in and increase the availability of VMPs, and strengthen the EU’s campaign against antimicrobial resistance. The regulation specifies clear and fully harmonized labeling requirements, adopts a simpler system for making decisions on exceptions, and uses a risk-based approach to pharmacovigilance and controls among the key measures. It defines clear rules for organically sourced VMPs and novel therapies that also aim to encourage the development of new VMPs. It is important that the regulation strengthens the EU’s fight against antimicrobial resistance by banning the preventive use of antibiotics in groups of animals, banning the preventive use of antimicrobials via medicated feed, restricting the use of antimicrobials as a control treatment to prevent a further spread of infection, introducing a reinforced ban on the use of antimicrobials for promoting growth and increasing yield (in addition to the 2006 prohibition of using antibiotics as growth promoters in feed), including the possibility to reserve certain antimicrobials for humans only, obligating EU countries to collect data on the sale and use of antimicrobials, introducing science-based maximum limits for cross-contamination of feed with antimicrobials, and introducing various other measures to promote the responsible use of antimicrobials.
Another factor influencing honeybee gut microbiome composition is exposure to particulate-matter air pollution [127], which has been investigated for the buff-tailed bumblebee (Bombus terrestris) [128]. Likewise, there is scant evidence on the effects of heavy metals on honeybees [129][130].
In a recent study by Wang et al. [131], the authors investigated how microplastics impact honeybee fitness. They fed newly emerged bees for 14 days with microplastics under laboratory conditions. The accumulation and degradation of microplastics in the gut and interaction with gut bacteria was observed. A significant decrease in diversity and changes in the core microbial population took place. The real challenge with environmental factors affecting the honeybee microbiome, such as air pollutants, heavy metals, and microplastics, is determining the mechanism of their action and how they should be measured. Several factors influencing the honeybee gut community are presented in Figure 3.
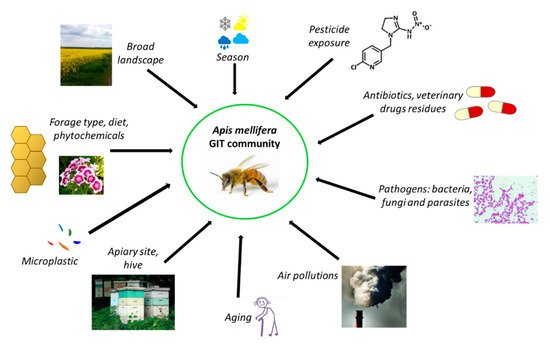
Figure 3. Possible factors affecting the microbiome of A. mellifera GIT (gastrointestinal tract) (references in the text).
References
- Villalba, A.; Maggi, M.; Ondarza, P.M.; Szawarski, N.; Miglioranza, K.S.B. Influence of land use on chlorpyrifos and persistent organic pollutant levels in honey bees. Bee bread and honey: Beehive exposure assessment. Sci. Total Environ. 2020, 713, 136554.
- Zheng, H.; Powell, J.E.; Steele, M.I.; Dietrich, C.; Moran, N.A. Honeybee gut microbiota promotes host weight gain via bacterial metabolism and hormonal signaling. Proc. Natl. Acad. Sci. USA 2017, 114, 4775–4780.
- Engel, P.; Moran, N.A. Functional and evolutionary insights into the simple yet specific gut microbiota of the honey bee from metagenomic analysis. Gut Microbes 2013, 4, 60–65.
- Anderson, K.E.; Ricigliano, V.A. Honey bee gut dysbiosis: A novel context of disease ecology. Curr. Opin. Insect Sci. 2017, 22, 125–132.
- Anjum, S.I.; Shah, A.H.; Aurongzeb, M.; Kori, J.; Azim, M.K.; Ansari, M.J.; Bin, L. Characterization of gut bacterial flora of Apis mellifera from north-west Pakistan. Saudi J. Biol. Sci. 2018, 25, 388–392.
- Bonilla-Rosso, G.; Engel, P. Functional roles and metabolic niches in the honey bee gut microbiota. Curr. Opin. Microbiol. 2018, 43, 69–76.
- Bommuraj, V.; Chen, Y.; Birenboim, M.; Barel, S.; Shimshoni, J.A. Concentration- and time-dependent toxicity of commonly encountered pesticides and pesticide mixtures to honeybees (Apis mellifera L.). Chemosphere 2021, 266, 128974.
- Hung, K.J.; Kingston, J.M.; Albrecht, M.; Holway, D.A.; Kohn, J.R. The worldwide importance of honey bees as pollinators in natural habitats. Proc. Bio. Sci. 2018, 285, 20172140.
- Matilla, H.R.; Harris, J.L.; Otis, G.W. Timing of production of winter bees in honey bee (Apis mellifera) colonies. Insectes Sociaux 2001, 48, 88–93.
- Amdam, G.V.; Omholt, S.W. The Regulatory Anatomy of Honeybee Lifespan. J. Theor. Biol. 2002, 216, 209–228.
- Cardoso-Júnior, C.A.M.; Guidugli-Lazzarini, K.R.; Hartfelder, K. DNA methylation affects the lifespan of honey bee (Apis mellifera L.) workers – evidence for a regulatory module that involves vitellogenin expression but is independent of juvenile hormone function. Insect Biochem. Mol. Biol. 2018, 92, 21–29.
- Han, F.; Wallberg, A.; Webster, M.T. From where did the Western honeybee (Apis mellifera) originate? Ecol. Evol. 2012, 2, 1949–1957.
- Gupta, R.K. Chapter 2. Taxonomy and Distribution of Different Honeybee Species. In Beekeeping for Poverty Alleviation and Livelihood Security; Gupta, R.K., Reybroeck, W., van Veen, J.W., Gupta, A., Eds.; Springer Nature: Berlin, Germany, 2014; Volume 1.
- Glenny, W.; Cavigli, I.; Daughenbaugh, K.F.; Radford, R.; Kegley, S.E.; Flenniken, M.L. Honey bee (Apis mellifera) colony health and pathogen composition in migratory beekeeping operations involved in California almond pollination. PLoS ONE 2017, 12, e0182814.
- Hewlett, S.E.; Wareham, D.M.; Barron, A.B. Honey bee (Apis mellifera) sociability and nestmate affiliation are dependent on the social environment experienced post-eclosion. J. Exp. Biol. 2017, 221, eb173054.
- Amiri, E.; Strand, M.K.; Rueppell, O.; Tarpy, D.R. Queen quality and the impact of honey bee diseases on queen health: Potential for interactions between two major threats to colony health. Insects 2017, 8, 48.
- Lee, K.V.; Goblirsch, M.; McDermott, E.; Tarpy, D.R.; Spivak, M. Is the brood pattern within a honey bee colony a reliable indicator of queen quality? Insects 2019, 10, 12.
- Negri, P.; Villalobos, E.; Szawarski, N.; Damiani, N.; Gende, L.; Garrido, M.; Maggi, M.; Quintana, S.; Lamattina, L.; Eguaras, M. Towards Precision Nutrition: A Novel Concept Linking Phytochemicals, Immune Response and Honey Bee Health. Insects 2019, 10, 401.
- Iorizzo, M.; Pannella, G.; Lombardi, S.J.; Ganassi, S.; Testa, B.; Succi, M.; Sorrentino, E.; Petrarca, S.; De Cristofaro, A.; Coppola, R.; et al. Inter- and intra-species diversity of lactic acid bacteria in Apis mellifera ligustica colonies. Microorganisms 2020, 8, 1578.
- Brodschneider, R.; Crailsheim, K. Nutrition and health in honey bees. Apidologie 2010, 41, 278–294.
- Danner, N.; Keller, A.; Härtel, S.; Steffan-Dewenter, I. Honey bee foraging ecology: Season but not landscape diversity shapes the amount and diversity of collected pollen. PLoS ONE 2017, 12, e0183716.
- Hoover, S.E.; Ovinge, L.P. Pollen Collection, Honey Production, and Pollination Services: Managing Honey Bees in an Agricultural Setting. J. Econ. Entomol. 2018, 111, 1509–1516.
- Vieira, K.I.C.; Azevedo Werneck, H.; Santos Júnior, J.E.; Silva Flores, D.S.; Serrão, J.E.; Campos, L.A.D.O.; Resende, H.C. Bees and the environmental impact of the rupture of the fundão dam. Integr. Environ. Assess. Manag. 2020, 16, 631–635.
- Carroll, M.J.; Brown, N.; Goodall, C.; Downs, A.M.; Sheenan, T.H.; Anderson, K.E. Honey bees preferentially consume freshly stored pollen. PLoS ONE 2017, 12, e0175933.
- Wright, G.A.; Nicolson, S.W.; Shafir, S. Nutritional physiology and ecology of honey bees. Annu. Rev. Entomol. 2018, 63, 327–344.
- Nuru, A.; Hepburn, H.R. Pollen grains of some bee plants of Ethiopia. In Proceedings of the 37th International Apicultural Congress, Durban, South Africa, 28 October–1 November 2001.
- Bertazzini, M.; Medrzycki, P.; Bortolotti, L.; Maistrello, L.; Forlani, G. Amino acid content and nectar choice by forager honeybees (Apis mellifera L.). Amino Acids 2010, 39, 315–318.
- Nicolson, S.W. Bee food: The chemistry and nutritional value of nectar, pollen and mixtures of the two. Afr. Zool. 2011, 46, 197–204.
- Cornara, L.; Biagi, M.; Xiao, J.; Burlando, B. Therapeutic properties of bioactive compounds from different honeybee products. Front. Pharmacol. 2017, 8.
- Kurek-Górecka, A.; Górecki, M.; Rzepecka-Stojko, A.; Balwierz, R.; Stojko, J. Bee products in dermatology and skin care. Molecules 2020, 25, 556.
- Ball, D.W. The chemical composition of honey. J. Chem. Edu. 2007, 84, 1643.
- Svečnjak, L.; Prđun, S.; Rogina, J.; Bubalo, D.; Jerković, I. Characterization of Satsuma mandarin (Citrus unshiu Marc.) nectar-to-honey transformation pathway using FTIR-ATR spectroscopy. Food Chem. 2017, 232, 286–294.
- Vezeteu, T.V.; Bobiş, O.; Moritz, R.F.A.; Buttstedt, A. Food to some. poison to others—honeybee royal jelly and its growth inhibiting effect on European Foulbrood bacteria. MicrobiologyOpen 2016, 6, e00397.
- Melliou, E.; Chinou, I. Chapter 8—Chemistry and bioactivities of royal jelly. In Studies in Natural Products Chemistry; Elsevier: Amsterdam, The Netherlands, 2014; Volume 43, pp. 261–290.
- Mannoor, M.; Shimabukuro, I.; Tsukamotoa, M.; Watanabe, H.; Yamaguchi, K.; Sato, Y. Honeybee royal jelly inhibits autoimmunity in SLE-prone NZB × NZW F1 mice. Lupus 2009, 18, 44–52.
- Anderson, K.E.; Ricigliano, V.A.; Mott, B.M.; Copeland, D.C.; Floyd, A.S.; Maes, P. The queen’s gut refines with age: Longevity phenotypes in a social insect model. Microbiome 2018, 6, 108.
- Salazar-Olivo, L.A.; Paz-González, V. Screening of biological activities present in honeybee (Apis mellifera) royal jelly. Toxicol. In Vitro 2005, 19, 645–651.
- Pascale, A.; Marchesi, N.; Marelli, C.; Coppola, A.; Luzi, L.; Govoni, S.; Gazzaruso, C. Microbiota and metabolic diseases. Endocrine 2018, 61, 357–371.
- Greiner, T.; Bäckhed, F. Effects of the gut microbiota on obesity and glucose homeostasis. Trends Endocrinol. Metab. 2011, 22, 117–123.
- LeBlanc, J.G.; Milani, C.; de Giori, G.S.; Sesma, F.; van Sinderen, D.; Ventura, M. Bacteria as vitamin suppliers to their host: A gut microbiota perspective. Curr. Opin. Biotechnol. 2013, 24, 160–168.
- Gagliardi, A.; Totino, V.; Cacciotti, F.; Iebba, V.; Neroni, B.; Bonfiglio, G.; Trancassini, M.; Passariello, C.; Pantanella, F.; Schippa, S. Rebuilding the gut microbiota ecosystem. Int. J. Environ. Res. Public Health 2018, 15, 1679.
- Agus, A.; Planchais, J.; Sokol, H. Gut microbiota regulation of tryptophan metabolism in health and disease. Cell Host Microbe 2018, 23, 716–724.
- Molloy, M.J.; Bouladoux, N.; Belkaid, Y. Intestinal microbiota: Shaping local and systemic immune responses. Semin. Immunol. 2012, 24, 58–66.
- Belkaid, Y.; Harrison, O.J. Homeostatic Immunity and the Microbiota. Immunity 2017, 46, 562–576.
- Biedermann, L.; Rogler, G. The intestinal microbiota: Its role in health and disease. Eur. J. Pediatr. 2015, 174, 151–167.
- El Aidy, S.; Dinan, T.G.; Cryan, J.F. Gut microbiota: The conductor in the orchestra of immune–neuroendocrine communication. Clin. Ther. 2015, 37, 954–967.
- Mu, C.; Yang, Y.; Zhu, W. Gut Microbiota: The Brain Peacekeeper. Front. Microbiol. 2016, 7, 345.
- Nicholson, J.K.; Holmes, E.; Kinross, J.; Burcelin, R.; Gibson, G.; Jia, W.; Pettersson, S. Host-gut microbiota metabolic interactions. Science 2012, 336, 1262–1267.
- Zheng, H.; Steele, M.I.; Leonard, S.P.; Motta, E.V.S.; Moran, N.A. Honey bees as models for gut microbiota research. Lab. Animal. 2018, 47, 317–325.
- Dong, Z.X.; Li, H.Y.; Chen, Y.F.; Wang, F.; Deng, X.Y.; Lin, L.B.; Zhang, Q.L.; Li, J.L.; Guo, J. Colonization of the gut microbiota of honey bee (Apis mellifera) workers at different developmental stages. Microbiol. Res. 2020, 231, 126370.
- Martinson, V.G.; Moy, J.; Moran, N.A. Establishment of characteristic gut bacteria during development of the honeybee worker. Appl. Environ. Microbiol. 2012, 78, 2830–2840.
- Motta, E.V.S.; Raymann, K.; Moran, N.A. Glyphosate perturbs the gut microbiota of honey bees. Proc. Natl. Acad. Sci. 2018, 115, 10305–10310.
- Bleau, N.; Bouslama, S.; Giovenazzo, P.; Derome, N. Dynamics of the honeybee (Apis mellifera) gut microbiota throughout the overwintering period in Canada. Microorganisms 2020, 29, 1146.
- Li, J.H.; Evans, J.D.; Li, W.F.; Zhao, Y.Z.; DeGrandi-Hoffman, G.; Huang, S.K.; Li, Z.G.; Hamilton, M.; Chen, Y.P. New evidence showing that the destruction of gut bacteria by antibiotic treatment could increase the honey bee’s vulnerability to Nosema infection. PLoS ONE 2017, 12, e0187505.
- Powell, J.E.; Martinson, V.G.; Urban-Mead, K.; Moran, N.A. Routes of Acquisition of the gut microbiota of the honey bee Apis mellifera. Appl. Environ. Microbiol. 2014, 80, 7378–7387.
- Engel, P.; Martinson, V.G.; Moran, N.A. Functional diversity within the simple gut microbiota of the honey bee. Proc. Natl. Acad. Sci. 2012, 109, 11002–11007.
- Vásquez, A.; Forsgren, E.; Fries, I.; Paxton, R.J.; Flaberg, E.; Szekely, L.; Olofsson, T.C. Symbionts as major modulators of insect health: Lactic acid bacteria and honeybees. PLoS ONE 2012, 7, e33188.
- Corby-Harris, V.; Snyder, L.A.; Schwan, M.R.; Maes, P.; McFrederick, Q.S.; Anderson, K.E. Origin and effect of Alpha 2.2 Acetobacteraceae in honey bee larvae and description of Parasaccharibacter apium gen. nov., sp. nov. Appl. Environ. Microbiol. 2014, 80, 7460–7472.
- Jones, J.C.; Fruciano, C.; Hildebrand, F.; Al Toufalilia, H.; Balfour, N.J.; Bork, P.; Engel, P.; Ratnieks, F.L.W.; Hughes, W.O. Gut microbiota composition is associated with environmental landscape in honey bees. Ecol. Evol. 2017, 8, 441–451.
- Alberoni, D.; Gaggìa, F.; Baffoni, L.; Di Gioia, D. Beneficial microorganisms for honey bees: Problems and progresses. App. Microbiol. Biotechnol. 2016, 100, 9469–9482.
- Kwong, W.K.; Moran, N.A. Gut microbial communities of social bees. Nat. Rev. Microbiol. 2016, 14, 374–384.
- Moran, N.A.; Hansen, A.K.; Powell, J.E.; Sabree, Z.L. Distinctive gut microbiota of honey bees assessed using deep sampling from individual worker bees. PLoS ONE 2020, 7, e36393.
- Tola, Y.H.; Waweru, J.W.; Hurst, G.D.D.; Slippers, B.; Paredes, J.C. Characterization of the Kenyan honey bee (Apis mellifera) gut microbiota: A first look at tropical and Sub-Saharan African bee associated microbiomes. Microorganisms 2020, 8, 1721.
- Kešnerová, L.; Emery, O.; Troilo, M.; Liberti, J.; Erkosar, B.; Engel, P. Gut microbiota structure differs between honeybees in winter and summer. ISME J. 2020, 14, 801–814.
- Kešnerová, L.; Moritz, R.; Engel, P. Bartonella apis sp. nov., a honey bee gut symbiont of the class Alphaproteobacteria. Int. J. Syst. Evol. Microbiol. 2016, 66, 414–421.
- Hilgarth, M.; Redwitz, J.; Ehrmann, M.A.; Vogel, R.F.; Jakob, F. Bombella favorum sp. nov. and Bombella mellum sp. nov., two novel species isolated from the honeycombs of Apis mellifera. Int. J. Syst. Evol. Microbiol. 2021.
- Kwong, W.K.; Moran, N.A. Apibacter adventoris gen. nov., sp. nov., a member of the phylum Bacteroidetes isolated from honey bees. Int. J. Syst. Evol. Microbiol. 2016, 66, 1323–1329.
- Kwong, W.K.; Steele, M.I.; Moran, N.A. Genome sequences of Apibacter spp., gut symbionts of Asian honey bees. Genome Biol. Evol. 2018, 10, 1174–1179.
- Subotic, S.; Boddicker, A.M.; Nguyen, V.M.; Rivers, J.; Briles, C.E.; Mosier, A.C. Honey bee microbiome associated with different hive and sample types over a honey production season. PLoS ONE 2019, 14, e0223834.
- Wang, S.; Wang, L.; Fan, X.; Yu, C.; Feng, L.; Yi, L. An insight into diversity and functionalities of gut microbiota in insects. Curr. Microbiol. 2020, 77, 1976–1986.
- Wang, H.; Liu, C.; Liu, Z.; Wan, Y.; Ma, L.; Xu, B. The different dietary sugars modulate the composition of the gut microbiota in honeybee during overwintering. BMC Microbiol. 2020, 20, 61.
- Pernice, M.; Simpson, S.J.; Ponton, F. Towards an integrated understanding of gut microbiota using insects as model systems. J. Insect Physiol. 2014, 69, 12–18.
- Engel, P.; Moran, N.A. The gut microbiota of insects – diversity in structure and function. FEMS Microbiol. Rev. 2013, 37, 699–735.
- Cianci, R.; Pagliari, D.; Piccirillo, C.A.; Fritz, J.H.; Gambassi, G. The microbiota and immune system crosstalk in health and disease. MediatorsInflamm 2018, 2018, 2912539.
- Sekirov, I.; Russell, S.L.; Antunes, L.C.M.; Finlay, B.B. Gut microbiota in health and disease. Physiol. Rev. 2010, 90, 859–904.
- Shi, W.; Syrenne, R.; Sun, J.-Z.; Yuan, J.S. Molecular approaches to study the insect gut symbiotic microbiota at the “omics” age. Insect Sci. 2010, 17, 199–219.
- Jing, T.-Z.; Qi, F.-H.; Wang, Z.-Y. Most dominant roles of insect gut bacteria: Digestion. detoxification or essential nutrient provision? Microbiome 2020, 8.
- Azambuja, P.; Garcia, E.S.; Ratcliffe, N.A. Gut microbiota and parasite transmission by insect vectors. Trends in Parasitology 2005, 21, 568–572.
- Cirimotich, C.M.; Ramirez, J.L.; Dimopoulos, G. Native microbiota shape insect vector competence for human pathogens. Cell Host Microbe 2011, 10, 307–310.
- Silva, M.S.; Rabadzhiev, Y.; Renon Eller, M.; Iliev, I.; Ivanova, I.; Santana, W.C. Microorganisms in honey. Honey analysis. IntechOpen 2017, S233–S258.
- Pachila, A.; Ptaszyńska, A.A.; Wicha, M.; Oleńska, E.; Małek, W. Fascinating fructophilic lactic acid bacteria associated with various fructose-rich niches. Ann. Univ. Mariae Curie Sklodowska Med. 2017, 72, S41–S50.
- Kwong, W.K.; Mancenido, A.L.; Moran, N.A. Immune system stimulation by the native gut microbiota of honey bees. R. Soc. Open Sci. 2017, 4, 170003.
- Egert, M.; Simmering, R. The microbiota of the human skin. advances in experimental medicine and biology. Adv. Exp. Med. Biol. 2016, 90, 61–81.
- Schroeder, B.O.; Bäckhed, F. Signals from the gut microbiota to distant organs in physiology and disease. Nature Med. 2016, 22, 1079–1089.
- Shin, S.C.; Kim, S.H.; You, H.; Kim, B.; Kim, A.C.; Lee, K.A.; Yoon, J.H.; Ryu, J.H.; Lee, W.J. Drosophila microbiome modulates host developmental and metabolic homeostasis via insulin signaling. Science 2011, 4, 334–670.
- Purchiaroni, F.; Tortora, A.; Gabrielli, M.; Bertucci, F.; Gigange, G.; Laniro, G.; Ojetti, V.; Scarpellini, E.; Gasbarrini, A. The role of intestinal microbiota and the immune system. Eur. Rev. Med. Pharmacol. Sci. 2013, 17, 323–333.
- Evans, J.D.; Aronstein, K.; Chen, Y.P.; Hetru, C.; Imler, J.L.; Jiang, H.; Kanost, M.; Thompson, G.J.; Zou, Z.; Hultmark, D. Immune pathways and defence mechanisms in honey bees Apis mellifera. Insect Mol. Biol. 2006, 15, 645–656.
- Valentini, M.; Piermattei, A.; Di Sante, G.; Migliara, G.; Delogu, G.; Ria, F. Immunomodulation by gut microbiota: Role of toll-like receptor expressed by T cells. J. Immunol. Res. 2014, 586939, 1–8.
- Yiu, J.H.C.; Dorweiler, B.; Woo, C.W. Interaction between gut microbiota and toll-like receptor: From immunity to metabolism. J. Mol. Med. 2016, 95, 13–20.
- Cederlund, A.; Gudmundsson, G.H.; Agerberth, B. Antimicrobial peptides important in innate immunity. FEBS J. 2011, 78, 3942–3951.
- Westfall, S.; Lomis, N.; Prakash, S. Longevity extension in Drosophila through gut-brain communication. Sci. Rep. 2018, 8, 8362.
- Leger, L.; McFrederick, Q.S. The gut–brain–microbiome axis in bumble bees. Insects 2020, 11, 517.
- Liberti, J.; Engel, P. The gut microbiota—brain axis of insects. Curr. Opin. Insect Sci. 2020, 39, 6–13.
- Harris, J.W.; Woodring, J. Effects of stress, age, season, and source colony on levels of octopamine, dopamine and serotonin in the honey bee (Apis mellifera L.) brain. J. Ins. Physiol. 1992, 38, 29–35.
- Vernier, C.L.; Chin, I.M.; Adu-Oppong, B.; Krupp, J.J.; Levine, J.; Dantas, G.; Ben-Shahar, Y. The gut microbiome defines social group membership in honey bee colonies. Sci. Adv. 2020, 6, eabd3431.
- Backhed, F.; Ding, H.; Wang, T.; Hooper, L.V.; Koh, G.Y.; Nagy, A.; Semenkovich, C.F.; Gordon, J.I. The gut microbiota as an environmental factor that regulates fat storage. Proc. Natl. Acad. Sci. USA 2004, 101, 15718–15723.
- Vergnolle, N. Protease inhibition as new therapeutic strategy for GI diseases. Gut 2016, 65, 1215–1224.
- Wu, Y.; Zheng, Y.; Chen, Y.; Wang, S.; Chen, Y.; Hu, F.; Zheng, H. Honey bee (Apis mellifera) gut microbiota promotes host endogenous detoxification capability via regulation of P450 gene expression in the digestive tract. Microb. Biotechnol. 2020, 13, 1201–1212.
- Van Engelsdorp, D.; Traynor, K.S.; Andree, M.; Lichtenberg, E.M.; Chen, Y.; Saegerman, C.; Cox-Foster, D.L. Colony Collapse Disorder (CCD) and bee age impact honey bee pathophysiology. PLoS ONE 2017, 12, e0179535.
- Sommer, F.; Bäckhed, F. The gut microbiota—Masters of host development and physiology. Nature Rev. Microbiol. 2013, 11, 227–238.
- Jandhyala, S.M.; Talukdar, R.; Subramanyam, C.; Vuyyuru, H.; Sasikala, M.; Nageshwar Reddy, D. Role of the normal gut microbiota. World J. Gastroenterol. 2015, 21, 8787–8803.
- Muñoz-Colmenero, M.; Baroja-Careaga, I.; Kovačić, M.; Filipi, J.; Puškadija, Z.; Kezić, N.; Estonba, A.; Büchler, R.; Zarraonaindia, I. Differences in honey bee bacterial diversity and composition in agricultural and pristine environments—A field study. Apidologie 2020, 51, 1018–1037.
- Diaz, T.; Del-Val, E.; Ayala, R.; Larsen, J. Alterations in honey bee gut microorganisms caused by Nosema spp. and pest control methods. Pest. Manag. Sci. 2019, 75, 835–843.
- Khan, K.A.; Ansari, M.J.; Al-Ghamdi, A.; Nuru, A.; Harakeh, S.; Iqbal, J. Investigation of gut microbial communities associated with indigenous honey bee (Apis mellifera jemenitica) from two different eco-regions of Saudi Arabia. Saudi J. Biol. Sci. 2017, 24, 1061–1068.
- Rouzé, R.; Moné, A.; Delbac, F.; Belzunces, L.; Blot, N. The honeybee gut microbiota is altered after chronic exposure to different families of insecticides and infection by Nosema ceranae. Microbes Environ. 2019, 34, 226–233.
- Castelli, L.; Branchiccela, B.; Garrido, M.; Invernizzi, C.; Porrini, M.; Romero, H.; Santos, E.; Zunino, P.; Antúnez, K. Impact of nutritional stress on honeybee gut microbiota, immunity, and Nosema ceranae infection. Microb. Ecol. 2020, 80, 908–919.
- Saelao, P.; Borba, R.S.; Ricigliano, V.; Spivak, M.; Simone-Finstrom, M. Honeybee microbiome is stabilized in the presence of propolis. Biol. Lett. 2020, 16, 20200003.
- Wang, X.; Zhong, Z.; Chen, X.; Hong, Z.; Lin, W.; Mu, X.; Hu, X.; Zheng, H. High-Fat Diets with Differential Fatty Acids Induce Obesity and Perturb Gut Microbiota in Honey Bee. Int. J. Mol. Sci. 2021, 22, 834.
- Daisley, B.A.; Pitek, A.P.; Chmiel, J.A.; Gibbons, S.; Chernyshova, A.M.; Al, K.F.; Faragalla, K.M.; Burton, J.P.; Thompson, G.J.; Reid, G. Lactobacillus spp. attenuate antibiotic-induced immune and microbiota dysregulation in honey bees. Commun. Biol. 2020, 3, 534.
- Ludvigsen, J.; Porcellato, D.; L’Abée-Lund, T.M.; Amdam, G.V.; Rudi, K. Geographically widespread honeybee-gut symbiont subgroups show locally distinct antibiotic-resistant patterns. Mol. Ecol. 2017, 26, 6590–6607.
- Reybroeck, W. Residues of antibiotics and chemotherapeutics in honey. J. Api. Res. 2017, 57, 97–112.
- Raymann, K.; Bobay, L.M.; Moran, N.A. Antibiotics reduce genetic diversity of core species in the honeybee gut microbiome. Mol. Ecol. 2018, 27, 2057–2066.
- Ortiz-Alvarado, Y.; Clark, D.R.; Vega-Melendez, C.J.; Flores-Cruz, Z.; Domingez-Bello, M.G.; Giray, T. Antibiotics in hives an their effect on honey bee physiology and behavioral development. Biol. Open 2020, 9, bio053884.
- Blaser, M.J. Antibiotic use and its consequences for the normal microbiome. Science 2016, 352, 544–545.
- Evans, J.; Armstrong, T.N. Inhibition of the American foulbrood bacterium, Paenibacillus larvae, by bacteria isolated from honey bees. J. Apic. Res. 2005, 44, 168–171.
- Evans, J.D.; Spivak, M. Socialized medicine: Individual and communal disease barriers in honey bees. J. Invertebr. Pathol. 2010, 103, S62–S72.
- Reybroeck, W.; Daeseleire, E.; De Brabander, H.F.; Herman, L. Antimicrobials in beekeeping. Vet. Microbiol. 2012, 158, 1–11.
- Tian, B.; Fadhil, N.H.; Powell, J.E.; Kwong, W.K.; Moran, N.A. Long-term exposure to antibiotics has caused accumulation of resistance determinants in the gut microbiota of honeybees. mBio 2012, 3, e00377-12.
- Ludvigsen, J.; Amdam, G.V.; Rudi, K.; L’Abée-Lund, T.M. Detection and characterization of streptomycin resistance (strA-strB) in a honeybee gut symbiont (Snodgrassella alvi) and the associated risk of antibiotic resistance transfer. Microb. Ecol. 2018, 76, 588–591.
- The European Green Deal. Communication from the Commission to the European Parliament, the European Council. In Proceedings of the European Economic and Social Committee and the Committee of the Regions, Brussels, Belgium, 11 December 2019.
- Regulation (EC) No 178/2002 of the European Parliament and of the Council of 28 January 2002 Laying down the General Principles and Requirements of Food Law, Establishing the European Food Safety Authority and Laying down Procedures in Matters of Food Safety, O.J.L. 31. 1 February 2002; 1–24.
- Commission Regulation (EU) No 415/2013 of 6 May 2013 Laying down Additional Responsibilities and Tasks for the EU Reference Laboratories for Rabies, Bovine Tuberculosis and Bee Health, Amending Regulation (EC) No 737/2008 and Repealing Regulation (EU) No 87/2011, O.J.L. 125. 7 May 2013; 7–12.
- Council Directive 2001/110/EC of 20 December 2001 Relating to Honey, O.J.L. 10. 12 January 2002; 47–52.
- Regulation (EC) No 470/2009 of the European Parliament and of the Council of 6 May 2009 Laying down Community Procedures for the Establishment of Residue Limits of Pharmacologically Active Substances in Foodstuffs of Animal Origin, Repealing Council Regulation (EEC) No 2377/90 and Amending Directive 2001/82/EC of the European Parliament and of the Council and Regulation (EC) No 726/2004 of the European Parliament and of the Council, O.J.L. 152. 16 June 2009; 11–22.
- Commission Regulation (EU) No 37/2010 of 22 December 2009 on Pharmacologically Active Substances and Their Classification Regarding Maximum Residue Limits in Foodstuffs of Animal Origin, O.J.L. 15. 20 January 2010; 1–72.
- Regulation (EU) 2019/6 of the European Parliament and of the Council of 11 December 2018 on Veterinary Medicinal Products and Repealing Directive 2001/82/EC, O.J.L. 4. 7 January 2019; 43–167.
- Mutlu, E.A.; Comba, I.Y.; Cho, T.; Engen, P.A.; Yazıcı, C.; Soberanes, S.; Hamanaka, R.B.; Niğdelioğlu, R.; Meliton, A.Y.; Ghio, A.J.; et al. Inhalational exposure to particulate matter air pollution alters the composition of the gut microbiome. Environ. Pollut. 2018, 240, 817–830.
- Sampson, H.; Ketley, J.; Mallon, E.; Morrissey, J. Impact of air pollution on buff-tailed bumblebees (Bombus terrestris) and their gut microbiome. Access Microbiol. 2020, 2, 7A.
- Costa, A.; Veca, M.; Barberis, M.; Tosti, A.; Notaro, G.; Nava, S.; Lazzari, M.; Agazzi, M.; Tangorra, F.M. Heavy metals on honeybees indicate their concentration in the atmosphere. a proof of concept. Ital. J. Anim. Sci. 2019, 18, 309–315.
- Rothman, J.A.; Leger, L.; Kirkwood, J.S.; McFrederick, Q.S. Cadmium and Selenate Exposure Affects the Honey Bee Microbiome and Metabolome, and Bee-Associated Bacteria Show Potential for Bioaccumulation. Appl. Environ. Microbiol. 2019, 85, e01411-19.
- Wang, K.; Li, J.; Zhao, L.; Mu, X.; Wang, C.; Wang, M.; Xue, X.; Qi, S.; Wu, L. Gut microbiota protects honey bees (Apis mellifera L.) against polystyrene microplastics exposure risks. J. Hazard. Mater. 2021, 402, 123828.




