Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Natalia Manousi | + 2912 word(s) | 2912 | 2021-05-10 08:04:56 | | | |
| 2 | Catherine Yang | Meta information modification | 2912 | 2021-05-11 03:20:46 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Manousi, N.; Plastiras, O. Metal-Organic Frameworks in Bioanalysis. Encyclopedia. Available online: https://encyclopedia.pub/entry/9453 (accessed on 08 February 2026).
Manousi N, Plastiras O. Metal-Organic Frameworks in Bioanalysis. Encyclopedia. Available at: https://encyclopedia.pub/entry/9453. Accessed February 08, 2026.
Manousi, Natalia, Orfeas-Evangelos Plastiras. "Metal-Organic Frameworks in Bioanalysis" Encyclopedia, https://encyclopedia.pub/entry/9453 (accessed February 08, 2026).
Manousi, N., & Plastiras, O. (2021, May 10). Metal-Organic Frameworks in Bioanalysis. In Encyclopedia. https://encyclopedia.pub/entry/9453
Manousi, Natalia and Orfeas-Evangelos Plastiras. "Metal-Organic Frameworks in Bioanalysis." Encyclopedia. Web. 10 May, 2021.
Copy Citation
Metal-organic frameworks (MOFs) are novel materials composed of metal ions or clusters in coordination with organic linkers. Unequivocally, MOFs are gaining more and more attention in analytical chemistry due to their superior properties, including high surface area and tunability of pore size and functionality.
metal-organic frameworks
extraction
MOFs
bioanalysis
1. Introduction
Bioanalysis is a term generally used to describe the quantitative determination of xenobiotics (i.e., exogenous chemical compounds, such as drugs and their metabolites) and biotics (i.e., endogenous compounds such as biomarkers) in biological matrices. As a result, bioanalysis is of high importance for drug discovery, since it provides crucial information regarding drug absorption, distribution, metabolism, and elimination [1]. In bioanalytical studies, conventional biofluids, such as urine, human plasma, serum, and whole blood, are usually analyzed [2]. However, other alternative matrices like oral fluid [3], keratinized matrices namely hair and nail clippings, dry blood spots and cerebrospinal fluid can be also examined. Hair and nails can provide information on chronic exposure and they can be obtained through non-invasive methods under supervision in order to prevent adulteration or substitution. Moreover, compared to the conventional samples, the collection of dried blood spots is simple and requires only a small amount of blood [2][4][5].
MOFs are a class of novel crystalline materials that exhibit structures formed from the coordination between multidentate organic groups and metal ions [6]. Compared to other solid-phase extraction sorbents, MOFs exhibit superior surface area, satisfactory thermal and mechanical stability, as well as tunability of pore size and functionality [7][8]. Moreover, MOFs can be modified with chemical groups that uniquely affect the overall selectivity and sensitivity of the extraction process [6][8]. Due to their interesting physical and chemical properties, MOFs have been employed in different scientific fields, including gas separation, gas storage, and catalysis [9]. Currently, MOFs are used in biomedical applications for various purposes, e.g., as nanocarriers for drug delivery, and various functionalization approaches can be adopted to prepare MOFs with therapeutic agents [10]. Apart from their applications in drug carrying substrate for drug delivery, MOFs are also utilized as contrast agents in magnetic resonance imaging [11]. MOFs have been also employed for the biosensing of biological markers. The applications of MOFs for the detection of biomarkers were recently discussed by Mendes et al. [12]. In the field of analytical chemistry, these materials have been employed as adsorbents in sample preparation [8], as stationary phases for GC, HPLC, and capillary electrochromatography [13], as well as column coatings for chiral separations [14]. Until now, MOFs have been utilized in multiple microextraction and miniaturized extraction techniques. Numerous publications regarding MOFs applications in sample preparation [15][8][16][17][18][19][20][21][22] have been reported in the last decade.
2. Bioanalytical Applications
Until now, many MOFs have been employed for the sample preparation of biological samples. Typical examples of the most common MOFs that have been evaluated in bioanalysis include MOF-5 synthesized from a zinc salt and benzene-1,4-dicarboxylic acid, MIL-100(Cr) synthesized from a chromium salt and benzene-1,3,5-tricarboxylic acid, MIL-101(Cr) synthesized from a chromium salt and benzene-1,4-dicarboxylic acid, MIL-101(Fe) synthesized from an iron salt, and benzene-1,4-dicarboxylic acid, MIL-53(Al) synthesized from aluminum salt and benzene-1,4-dicarboxylic acid and UiO-66 synthesized from a zirconium salt and benzene-1,4-dicarboxylic acid [23][24][25]. MOFs can exhibit good affinity towards small organic molecules. Thus, high adsorption efficiency can be observed through various mechanisms, including π–π interactions between the delocalized π-electron system of the target analytes and the aromatic rings, the sorbents, and hydrophobic interactions. A further increase of the affinity towards the target analytes can be achieved through functionalization of the MOF sorbent [16].
ZIFs are a subfamily of MOFs synthesized from Zn2+ or Co2+ and imidazole linkers which are also used as extraction sorbents. ZIFs combine the benefits of zeolites and MOFs. The most common zeolitic imidazolate framework that has been evaluated in sample preparation is ZIF-8. This material can be synthesized from zinc nitrate hexahydrate 2-methylimidazole and it exhibits interesting characteristics including high surface area and permanent porosity [26][27]. It has been reported that unlikely with other zeolitic imidazole frameworks, ZIF-8 has exceptional chemical and thermal stability in aqueous alkaline solutions and water [28]. ZIF-7 is another common zeolitic imidazole framework that has been evaluated in bioanalytical applications [25].
2.1. Dispersive Solid-Phase Extraction
Dispersive solid-phase extraction is one of the most common extraction techniques used for the sample preparation of biological samples. In d-SPE, the sorbent is dispersed into the sample to adsorb the target analytes. After extraction, the sorbent is retained by a mechanical process, such as centrifugation. This technique is favored by the contact between the sorbent and the target analytes. As a result, one of the major advantages of this technique is its high extraction efficiency. Moreover, d-SPE is a simple and rapid sample preparation approach that avoids potential limitations of the conventional SPE process, such as channeling or blocking of cartridges or [29][30].
Rocío-Bautista et al. [31] evaluated three different MOFs (HKUST-1, MOF-5 and MIL-53(Al)) for the vortex-assisted d-SPE of parabens from environmental waters, cosmetic creams, and human urine samples. Vortex irradiation was chosen to avoid problems due to the non-uniform energy dispersion and due to temperature increase. Among the examined materials, HKUST-1 provided the best performance and was finally chosen. Due to the application of vortex irradiation, rapid extraction (around 5 min) was achieved.
MIL-53(Al) has been used as adsorbent for the extraction of estrogens and glucocorticoids from water and urine samples by dispersive micro-SPE prior to their determination by UPLC-MS/MS. Compared to MIL-101(Cr), MIL-100(Fe), and UiO-66(Zr) that were also studied, MIL-53(Al) provided better adsorption efficiency, as well as more significant binding ability towards the target analytes. Extraction of the target analytes took place due to hydrophobic effects among the phenyl rings of MOFs and the steroid ring system of the target analytes. Intermolecular hydrogen bonds between the carboxylic groups of MOFs and hydroxylic groups of hormones and π–π interactions between phenyl rings of MOFs and estrogens could also occur [32].
Other examples of MOFs that have been employed as d-SPE sorbents include the zirconium-based MOF UiO-66-NH2 [33] with 2-aminoterephthalic acid as linkers and ZIF-67 [34]. In the former case, the adsorbent was used for the extraction of sialic acids from serum samples. In the latter case, the sorbent was synthesized from cobalt nitrate hexahydrate and 2-methylimidazole and employed for the extraction of buprenorphine from plasma and urine [33]. A ZIF-8 derived carbon porous has been also applied for d-SPE of methamphetamine from urine samples [35]. The sorbent was prepared by the carbonization of the precursor MOF at 800 °C under nitrogen steam for 8 h. The reported method enabled the rapid and cost-effective determination of methamphetamine.
2.2. Magnetic Solid-Phase Extraction
Magnetic solid-phase extraction is a form of d-SPE, that utilizes a magnetic sorbent which is added to an aqueous sample to adsorb the target analytes. Following the adsorption step, an external magnet is employed to remove the adsorbent and the sample solution is discarded. Afterwards, elution from the sorbent takes place by the addition of an appropriate solvent, the sorbent is isolated with the assistance of the magnet and the eluent is analyzed by an instrumental technique. This extraction techniques takes advantage of the benefits of the d-SPE process, with the ease in separation due to the magnetic properties of the sorbent [36][37][38].
In order to prepare appropriate MPSE sorbents, magnetization of MOFs is required to enable the possibility of magnetic separation during adsorption and elution steps. To date, there are various methods for the magnetization of MOFs for their utilization as MSPE sorbents. Examples of these approaches include the direct magnetization of MOFs, the in situ growth of magnetic nanoparticles, the single-step MOF coating and the layer-by layer MOF growth. An interesting approach for the fabrication of magnetic sorbents derived from MOF materials is their carbonization under inert atmosphere that results in the formation of magnetic porous carbons [39]. Until now, various MOFs have been employed for the fabrication of MSPE adsorbents for the extraction of small organic molecules from biological fluids.
Magnetic MIL-101(Fe) (Fe3O4/MIL-101) has been utilized for the MSPE of six organophosphorus pesticides (OPPs) from urine and hair samples prior to their determination by GC coupled with a flame photometric detector (FPD) [23]. This MOF exhibits resistance to common solvents and to water. Thus, it is a good adsorbent for the sample preparation of aqueous solutions. Prior to the extraction process, urine samples were treated with acetonitrile and acetone for protein precipitation, while hair samples were treated with acetone under ultrasonic radiation. The novel hybrid sorbent exhibited high porosity and high extraction efficiency for the OPPs due to the presence of a large amount of oxygen groups and π-electrons.
Magnetic MIL-101(Cr) (Fe3O4@MIL-101) has been used for the extraction of phthalate esters from plasma samples followed by separation and quantification by GC–MS [40]. In this case, the adsorbent was synthesized with the hydrothermal approach and it was decorated with magnetite nanoparticles. The sorbent exhibited good extraction efficiency due to the hydrophobic and π–π interactions among the phthalate esters and the terephthalic acid units of the MOF. Additionally, the coordination interaction among the oxygen atoms of the analytes and chromium metal center of the MOF, as well as the iron metal centers of the magnetite, may be also driving forces for the extraction procedure. The novel MSPE method exhibited rapid extraction dynamics, high extraction capacity and reduced consumption of organic solvents. Moreover, Wang et al. [41] prepared a Fe3O4-NH2@MIL-101(Cr) sorbent by fabricating amine-functionalized magnetite particles with MIL-101(Cr) and used it for the MSPE of monohydroxy polycyclic aromatic hydrocarbons from urine samples from coke-oven workers.
2.3. Solid-Phase Microextraction
Solid-phase microextraction is a sample preparation technique in which extraction and preconcentration of the target analytes takes place at the outer coating of a fused-silica fiber. There are two basic modes of SPME, i.e., the direct immersion SPME (DI-SPME) and the headspace SPME (HS-SPME). In the former approach, the fiber is directly immersed into the sample solution containing the target analytes, while in the latter approach the fiber is exposed to the gas phase above the sample. After the extraction step, the analytes can be desorbed either thermally or by the addition of an appropriate solvent [37]. Although there are multiple commercially available SPME fibers, most of them suffer from various limitations (e.g., short lifetime, ease of breakage, swelling in organic solvents etc.) [42]. As a result, the development of novel SPME fiber coatings is at the forefront of research. Until now, MOFs have been successfully utilized to prepare coated SPME fibers [18].
Non-steroidal anti-inflammatory drugs have been extracted from biological fluids and tablet formulation samples by a novel SPME fiber based on a capillary glass tube coated with magnetic copper benzene-1,3,5-tricarboxylate MOF. For the fabrication of the fibers, glass tube capillary fibers were used as substrate and the coating was performed by a sol-gel processing approach. The synthesized fibers showed a stable and reproducible response without interferences from the biological samples [43].
2.4. Stir Bar Sorptive Extraction
Stir bar sorptive extraction is a sample preparation technique that was introduced in 1999 by Baltussen et al. [44]. SBSE utilizes a coated stir bar that is inserted into a vial containing the sample and the adsorption of the target analytes is performed under stirring to reach equilibrium. Subsequently, elution of the adsorbed analytes is performed either by adding an appropriate solvent or thermally [45]. Among the advantages of SBSE are the simplicity of the extraction step, its overall good extraction efficiency, the reduced solvent consumption and the possibility to perform solvent-free sample preparation [45]. Currently, PDMS coated stir bars are widely used since they are commercially available, however a lot of research is focused on the development of coated stir bars with high selectivity and sensitivity [46]. MOFs have been successfully utilized to develop coatings for stir bars for the SBSE of small organic molecules from biological matrices.
Wang et al. [47] prepared a SBSE device by in situ immobilization of MIL-68 onto chemical resistant PEEK jacket. For this purpose, the MIL-68 material was prepared through the solvothermal approach from benzene-1,4-dicarboxylic acid and aluminum chloride hexahydrate. Moreover, the PEEK jacket was functionalized and plenty of benzoic acid groups were available to bind with the MOF material. The novel SBSE device was used for the extraction of parabens from cosmetics and rabbit plasma. Under optimum condition, the coated SBSE could extract the analytes within 2 h, while 250 μL of methanol were required for their desorption. The proposed method exhibited good performance characteristics.
Fluorouracil and phenobarbital have been extracted from urine and plasma samples by a SBSE coated with a ZIF derived nanoporous carbon [48]. For this purpose, ZIF-67 was initially prepared from cobalt nitrate hexahydrate and 2 methylimidazole, followed by carbonization at 700 °C under nitrogen flow for 6 h. Subsequently, a borosilicate glass bar containing an iron bar were activated and a silicon glue was employed as adhesive media to prepare the coated bars. Due to the strong adhesion of the sorbent onto the surface of the SBSE bar, good mechanical and chemical stability were observed and the SBSE media were found to be reusable for up to 70 times.
2.5. Pipette Tip SPE
Pipette tip SPE is a miniaturized format of SPE in which the sorbent is usually placed between two frits inside a pipette tip. Extraction of the target analytes is achieved after several repeated aspirating/dispensing cycles to complete. Pipette-tip extraction is a rapid and easy extraction technique that requires low consumption of organic solvents [49][50]. Moreover, other advantages of PT-SPE are its good overall extraction efficiency and the possibility of automation. However, only a small number of commercially available tips are currently available [51]. MOFs have been successfully employed as adsorbents for PT-SPE in bioanalysis. An important consideration for the utilization of MOFs in PT-SPE methods is the minimal back-pressure during sample and solvent aspiration [50][52].
Kahka et al. [50] evaluated an amino-functionalized UiO-66 (UiO-66-NH2) as sorbent for the PT-SPE of carbamazepine from urine and water samples prior to their determination by HPLC-UV [50]. The amino-functionalized MOF was prepared from zirconium chloride and 2-aminoterephthalic acid. Due to the amino functionality on organic linker, the novel sorbent exhibited good performance and selectivity towards carbamazepine. For this purpose, an aliquot of 5 mg of the sorbent was placed into a 20 μL pipette-tip which was further attached to 100 μL variables sampler. Extraction of the target analyte was achieved by aspirating the same sample solution in five aspirating/dispensing cycles, while elution was performed by aspirating different aliquots of the eluent for seven cycles. The developed PT-SPE combined the benefits of PT-SPE technique (e.g., rapid extraction and ease in operation) with robustness and large adsorption capacity of the novel MOF.
The same authors also proposed the utilization of a tantalum MOF as adsorbent for the PT-SPE of nicotine from saliva, urine and wastewater samples prior to their determination by HPLC-UV [52]. For the preparation of the novel sorbent, tantalum(V) chloride and benzene-1,3,5-tricarboxylic acid were employed, and the material was prepared through a microwave assisted reverse micelle procedure. The highest extraction efficiency was observed when 5 mg of the sorbent was placed into a 20 μL pipette tip. Extraction of nicotine was achieved by aspirating and dispensing the sample over the sorbent for 15 cycles, while 20 draw/eject cycles were required for the elution step. The proposed method provided high enrichment factors and low organic solvent consumption.
2.6. Other Extraction Techniques
MOFs have been also tested for the sorption of isopropanol and acetone in exhaled breath samples followed by their determination by GC coupled with flame ionization detector (GC-FID) [25]. These compounds are established biomarkers for diabetes. Three different MOFs, i.e., MOF-5, ZIF-7, and UiO-66, were examined as adsorbents in packed tubes. UiO-66 was found to be the most suitable MOF, due to its high surface area and its porous structure. Acetone and isopropanol were able to enter the main cavities of UiO-66, resulting in strong van der Waals interactions among the methyl groups in their structure and the aromatic rings in the ligands. Moreover, the O-atoms of the carbonyl group of acetone were able to donate electrons to the zirconium cation, resulting in higher adsorption efficiency compared to isopropanol. After the extraction step, thermal desorption of the analytes was performed and the developed method provided low LODs, long lifetime, and satisfactory reproducibility.
UiO-66, amino-functionalized UiO-66 (UiO-66-NH2), and magnetic UiO-66-NH2@Fe3O4-SiO2 have been evaluated as adsorbents for the micro-extraction by packed sorbent (MEPS) of trans-muconic acid from urine samples followed by determination by HPLC-UV [53]. MEPs is as a miniaturized mode of SPE, in which conditioning, loading, washing and elution steps are performed at a microliter syringe instead of a SPE cartridge. Among the benefits of MEPs technique is the lower consumption of organic solvent, its simplicity and its low cost [53][54]. Among the three examined UiO-66 derivatives, the magnetic sorbent exhibited the highest sensitivity (in terms of detection limits), while all of the three examined materials showed good extraction performance.
MOF enhanced electromembrane extraction (EME) has been also utilized for biosamples’ analysis. EME is an alternative to the LPME that is based on migration of charged species in an electric field. Due to the application of voltage, ionizable compounds are transported from an aqueous solution to an acceptor phase (Figure 1). Among the advantages of EME are the speed of the extraction, the low cost, the utilization of disposable extraction units, and the negligible organic solvents consumption [55]. Fakhari et al. [56] used a MIL-101(Cr) in a supported liquid membrane. This approach was successfully employed for the extraction of basic drugs from biological fluids.
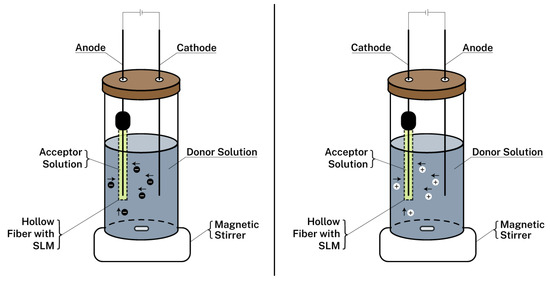
Figure 1. Main elements of the EME technique for the extraction of anions (left) and cations (right). SLM: Supported liquid membrane.
References
- Li, P.; Bartlett, M.G. A review of sample preparation methods for quantitation of small-molecule analytes in brain tissue by liquid chromatography tandem mass spectrometry (LC-MS/MS). Anal. Methods 2014, 6, 6183–6207.
- Manousi, N.; Raber, G.; Papadoyannis, I. Recent Advances in Microextraction Techniques of Antipsychotics in Biological Fluids Prior to Liquid Chromatography Analysis. Separations 2017, 4, 18.
- Drummer, O.H. Drug testing in oral fluid. Clin. Biochem. Rev. 2006, 27, 147–159.
- Kovatsi, L.; Titopoulou, A.; Tsakalof, A.; Samanidou, V. HPLC Analysis of Antipsychotic Asenapine in Alternative Biomatrices: Hair and Nail Clippings. J. Liq. Chromatogr. Relat. Technol. 2015, 38, 1666–1670.
- Livadiotou, D.; Samanidou, V. Dried blood spot sampling for therapeutic drug monitoring. New Sampl. Strateg. Toxicol. Ther. Drug Monit. 2015, 67–78.
- Giliopoulos, D.; Zamboulis, A.; Giannakoudakis, D.; Bikiaris, D.; Triantafyllidis, K. Polymer/metal organic framework (MOF) nanocomposites for biomedical applications. Molecules 2020, 25, 185.
- Zhou, H.C.; Long, J.R.; Yaghi, O.M. Introduction to metal-organic frameworks. Chem. Rev. 2012, 112, 673–674.
- Manousi, N.; Zachariadis, G.A.; Deliyanni, E.A. On the use of metal-organic frameworks for the extraction of organic compounds from environmental samples. Environ. Sci. Pollut. Res. 2020.
- Kuppler, R.J.; Timmons, D.J.; Fang, Q.R.; Li, J.R.; Makal, T.A.; Young, M.D.; Yuan, D.; Zhao, D.; Zhuang, W.; Zhou, H.C. Potential applications of metal-organic frameworks. Coord. Chem. Rev. 2009, 253, 3042–3066.
- Sun, Y.; Zheng, L.; Yang, Y.; Qian, X.; Fu, T.; Li, X.; Yang, Z.; Yan, H.; Cui, C.; Tan, W. Metal–Organic Framework Nanocarriers for Drug Delivery in Biomedical Applications. Nano-Micro Lett. 2020, 12, 1–29.
- Chowdhury, M.A. Metal-Organic-Frameworks as Contrast Agents in Magnetic Resonance Imaging. ChemBioEng Rev. 2017, 4, 225–239.
- Mendes, R.F.; Figueira, F.; Leite, J.P.; Gales, L.; Almeida Paz, F.A. Metal-organic frameworks: A future toolbox for biomedicine? Chem. Soc. Rev. 2020, 49, 9121–9153.
- Zhang, J.; Chen, Z. Metal-organic frameworks as stationary phase for application in chromatographic separation. J. Chromatogr. A 2017, 1530, 1–18.
- Xie, S.M.; Zhang, Z.J.; Wang, Z.Y.; Yuan, L.M. Chiral metal-organic frameworks for high-resolution gas chromatographic separations. J. Am. Chem. Soc. 2011.
- Manousi, N.; Zachariadis, G.A.; Deliyanni, E.A.; Samanidou, V.F. Applications of metal-organic frameworks in food sample preparation. Molecules 2018, 23, 2896.
- Manousi, N.; Giannakoudakis, D.A.; Rosenberg, E.; Zachariadis, G.A. Extraction of metal ions with metal–organic frameworks. Molecules 2019, 24, 4605.
- Ma, W.; Li, X.; Bai, Y.; Liu, H. Applications of metal-organic frameworks as advanced sorbents in biomacromolecules sample preparation. TrAC Trends Anal. Chem. 2018, 109, 154–162.
- Gutiérrez-Serpa, A.; Pacheco-Fernández, I.; Pasán, J.; Pino, V. Metal–organic frameworks as key materials for solid-phase microextraction devices—A review. Separations 2019, 6, 47.
- Rocío-Bautista, P.; Pacheco-Fernández, I.; Pasán, J.; Pino, V. Are metal-organic frameworks able to provide a new generation of solid-phase microextraction coatings?—A review. Anal. Chim. Acta 2016, 939, 26–41.
- Wang, Y.; Rui, M.; Lu, G. Recent applications of metal–organic frameworks in sample pretreatment. J. Sep. Sci. 2018, 41, 180–194.
- Rocío-Bautista, P.; Termopoli, V. Metal–Organic Frameworks in Solid-Phase Extraction Procedures for Environmental and Food Analyses. Chromatographia 2019, 82, 1191–1205.
- Rocío-Bautista, P.; González-Hernández, P.; Pino, V.; Pasán, J.; Afonso, A.M. Metal-organic frameworks as novel sorbents in dispersive-based microextraction approaches. TrAC Trends Anal. Chem. 2017, 90, 114–134.
- Zhang, S.; Jiao, Z.; Yao, W. A simple solvothermal process for fabrication of a metal-organic framework with an iron oxide enclosure for the determination of organophosphorus pesticides in biological samples. J. Chromatogr. A 2014.
- Gu, Z.Y.; Chen, Y.J.; Jiang, J.Q.; Yan, X.P. Metal-organic frameworks for efficient enrichment of peptides with simultaneous exclusion of proteins from complex biological samples. Chem. Commun. 2011, 47, 4787–4789.
- Yu, L.Q.; Su, F.H.; Ma, M.Y.; Lv, Y.K. Metal-organic frameworks for the sorption of acetone and isopropanol in exhaled breath of diabetics prior to quantitation by gas chromatography. Microchim. Acta 2019, 186, 588.
- Zou, Z.; Wang, S.; Jia, J.; Xu, F.; Long, Z.; Hou, X. Ultrasensitive determination of inorganic arsenic by hydride generation-atomic fluorescence spectrometry using nanoparticles for preconcentration. Microchem. J. 2016, 124, 578–583.
- Park, K.S.; Ni, Z.; Côté, A.P.; Choi, J.Y.; Huang, R.; Uribe-Romo, F.J.; Chae, H.K.; O’Keeffe, M.; Yaghi, O.M. Exceptional chemical and thermal stability of zeolitic imidazolate frameworks. Proc. Natl. Acad. Sci. USA 2006, 103, 10186–10191.
- Ge, D.; Lee, H.K. Water stability of zeolite imidazolate framework 8 and application to porous membrane-protected micro-solid-phase extraction of polycyclic aromatic hydrocarbons from environmental water samples. J. Chromatogr. A 2011, 1218, 8490–8495.
- Parisis, N.A.; Giokas, D.L.; Vlessidis, A.G.; Evmiridis, N.P. Concentration of organic compounds in natural waters with solid-phase dispersion based on advesicle modified silica prior to liquid chromatography. J. Chromatogr. A 2005, 1097, 17–24.
- Román, I.P.; Chisvert Alberto, A.; Canals, A. Dispersive solid-phase extraction based on oleic acid-coated magnetic nanoparticles followed by gas chromatography-mass spectrometry for UV-filter determination in water samples. J. Chromatogr. A 2011, 1218, 2467–2475.
- Rocío-Bautista, P.; Martínez-Benito, C.; Pino, V.; Pasán, J.; Ayala, J.H.; Ruiz-Pérez, C.; Afonso, A.M. The metal-organic framework HKUST-1 as efficient sorbent in a vortex-assisted dispersive micro solid-phase extraction of parabens from environmental waters, cosmetic creams, and human urine. Talanta 2015, 139, 13–20.
- Gao, G.; Li, S.; Li, S.; Wang, Y.; Zhao, P.; Zhang, X.; Hou, X. A combination of computational−experimental study on metal-organic frameworks MIL-53(Al) as sorbent for simultaneous determination of estrogens and glucocorticoids in water and urine samples by dispersive micro-solid-phase extraction coupled to UPLC-MS/MS. Talanta 2018, 180, 358–367.
- Qu, F.; Xia, L.; Wu, C.; Liu, L.; Li, G.; You, J. Sensitive and accurate determination of sialic acids in serum with the aid of dispersive solid-phase extraction using the zirconium-based MOF of UiO-66-NH2 as sorbent. RSC Adv. 2016, 6, 64895–64901.
- Mohammadi, F.; Shabani, A.M.H.; Dadfarnia, S.; Ansari, M.; Asgharinezhad, A.A. Dispersive solid-phase extraction of buprenorphine from biological fluids using metal-organic frameworks and its determination by ultra-performance liquid chromatography. J. Sep. Sci. 2020, 43, 3045–3052.
- Taghvimi, A.; Tabrizi, A.B.; Dastmalchi, S.; Javadzadeh, Y. Metal organic framework based carbon porous as an efficient dispersive solid phase extraction adsorbent for analysis of methamphetamine from urine matrix. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2019, 1109, 149–154.
- Manousi, N.; Rosenberg, E.; Deliyanni, E.; Zachariadis, G.A.; Samanidou, V. Magnetic Solid-Phase Extraction of Organic Compounds Based on Graphene Oxide Nanocomposites. Molecules 2020, 25, 1148.
- Giakisikli, G.; Anthemidis, A.N. Magnetic materials as sorbents for metal/metalloid preconcentration and/or separation. A review. Anal. Chim. Acta 2013, 789, 1–16.
- Li, N.; Du, J.; Wu, D.; Liu, J.; Li, N.; Sun, Z.; Li, G.; Wu, Y. Recent advances in facile synthesis and applications of covalent organic framework materials as superior adsorbents in sample pretreatment. TrAC Trends Anal. Chem. 2018, 108, 154–166.
- Maya, F.; Palomino Cabello, C.; Frizzarin, R.M.; Estela, J.M.; Turnes Palomino, G.; Cerdà, V. Magnetic solid-phase extraction using metal-organic frameworks (MOFs) and their derived carbons. TrAC Trends Anal. Chem. 2017, 90, 142–152.
- Dargahi, R.; Ebrahimzadeh, H.; Asgharinezhad, A.A.; Hashemzadeh, A.; Amini, M.M. Dispersive magnetic solid-phase extraction of phthalate esters from water samples and human plasma based on a nanosorbent composed of MIL-101(Cr) metal–organic framework and magnetite nanoparticles before their determination by GC–MS. J. Sep. Sci. 2018, 41, 948–957.
- Wang, Y.; Yan, M.; Ji, Q.; Wang, M.; Wang, Q.; Wang, X.; Hao, Y. Fast magnetic solid-phase extraction using an Fe3O4 material for monohydroxy polycyclic aromatic hydrocarbons in urine of coke-oven workers. Anal. Methods 2020, 12, 2872–2880.
- Chen, C.; Liang, X.; Wang, J.; Yang, S.; Yan, Z.; Cai, Q.; Yao, S. Development of a highly robust solid phase microextraction fiber based on crosslinked methyl methacrylate-polyhedral oligomeric silsesquioxane hybrid polymeric coating. Anal. Chim. Acta 2013, 792, 45–51.
- Mirzajani, R.; Kardani, F.; Ramezani, Z. Preparation and characterization of magnetic metal–organic framework nanocomposite as solid-phase microextraction fibers coupled with high-performance liquid chromatography for determination of non-steroidal anti-inflammatory drugs in biological fluids an. Microchem. J. 2019, 144, 270–284.
- Baltussen, E.; Sandra, P.; David, F.; Cramers, C. Stir bar sorptive extraction (SBSE), a novel extraction technique for aqueous samples: Theory and principles. J. Microcolumn Sep. 1999, 11, 737–747.
- Nazyropoulou, C.; Samanidou, V. Stir bar sorptive extraction applied to the analysis of biological fluids. Bioanalysis 2015, 7, 2241–2250.
- Manousi, N.; Zachariadis, G.A. Recent Advances in the Extraction of Polycyclic Aromatic Hydrocarbons from Environmental Samples. Molecules 2020, 25, 2182.
- Wang, C.; Zhou, W.; Liao, X.; Wang, X.; Chen, Z. Covalent immobilization of metal organic frameworks onto chemical resistant poly(ether ether ketone) jacket for stir bar extraction. Anal. Chim. Acta 2018, 1025, 124–133.
- Ghani, M.; Ghoreishi, S.M.; Shahin, M.; Azamati, M. Zeolitic imidazole framework templated synthesis of nanoporous carbon as a coating for stir bar sorptive extraction of fluorouracil and phenobarbital in human body fluids. Microchem. J. 2019, 146, 798–806.
- Shen, Q.; Dong, W.; Wang, Y.; Gong, L.; Dai, Z.; Cheung, H.Y. Pipette tip solid-phase extraction and ultra-performance liquid chromatography/mass spectrometry based rapid analysis of picrosides from Picrorhiza scrophulariiflora. J. Pharm. Biomed. Anal. 2013, 80, 136–140.
- Rezaei Kahkha, M.R.; Oveisi, A.R.; Kaykhaii, M.; Rezaei Kahkha, B. Determination of carbamazepine in urine and water samples using amino-functionalized metal–organic framework as sorbent. Chem. Cent. J. 2018, 12, 1–12.
- Bordin, D.C.M.; Alves, M.N.R.; De Campos, E.G.; De Martinis, B.S. Disposable pipette tips extraction: Fundamentals, applications and state of the art. J. Sep. Sci. 2016, 39, 1168–1172.
- Rezaei Kahkha, M.R.; Kaykhaii, M.; Sargazi, G.; Kahkha, B.R. Determination of nicotine in saliva, urine and wastewater samples using tantalum metal organic framework pipette tip micro-solid phase extraction. Anal. Methods 2019, 11, 6168–6175.
- Rahimpoor, R.; Bahrami, A.; Nematollahi, D.; Ghorbani Shahna, F.; Farhadian, M. Application of zirconium-based metal–organic frameworks for micro-extraction by packed sorbent of urinary trans, trans-muconic acid. J. Iran. Chem. Soc. 2020, 17, 2345–2358.
- Moein, M.M.; Abdel-Rehim, A.; Abdel-Rehim, M. Microextraction by packed sorbent (MEPS). TrAC Trends Anal. Chem. 2015, 67, 34–44.
- Drouin, N.; Kubáň, P.; Rudaz, S.; Pedersen-Bjergaard, S.; Schappler, J. Electromembrane extraction: Overview of the last decade. TrAC Trends Anal. Chem. 2019, 113, 357–363.
- Fakhari, A.R.; Asadi, S.; Kosalar, H.M.; Sahragard, A.; Hashemzadeh, A.; Amini, M.M. Metal-organic framework enhanced electromembrane extraction-a conceptual study using basic drugs as model substances. Anal. Methods 2017, 9, 5646–5652.
More
Information
Subjects:
Chemistry, Analytical
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
828
Revisions:
2 times
(View History)
Update Date:
11 May 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




