
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | QIANG PENG | + 5649 word(s) | 5649 | 2021-04-30 13:40:13 | | | |
| 2 | Conner Chen | Meta information modification | 5649 | 2021-05-11 07:16:17 | | | | |
| 3 | Conner Chen | Meta information modification | 5649 | 2021-05-13 05:53:02 | | |
Video Upload Options
Spermine, a member of polyamines, exists in all organisms and is essential for normal cell growth and function. It is highly expressed in the prostate compared with other organs and is detectable in urine, tissue, expressed prostatic secretions, and erythrocyte. A significant reduction of spermine level was observed in prostate cancer (PCa) tissue compared with benign prostate tissue, and the level of urinary spermine was also significantly lower in men with PCa. Decreased spermine level may be used as an indicator of malignant phenotype transformation from normal to malignant tissue in prostate.
1. The Sources of Spermine
1.1. Extracellular Sources
The body pool of spermine is maintained by three sources: food consumption, intestinal microbiota, and endogenous (de novo) biosynthesis arising from increased synthesis enzymes activity [1]. This external dietary source provides a larger quantity of spermine than the endogenous biosynthesis, and a wide range of foods derived from plants and animal tissues in our diet contain high spermine. Spermine contents are typically high in the internal organs and meat of warm-blooded animals and soybeans, whereas high levels of putrescine and spermidine are found in fish flesh, fruits, and vegetables [2]. Spermine-rich foods can be absorbed from the intestinal lumen and distributed in the body through systemic circulation, increasing its content in multiple systems [3]. Different dietary habits in different regions varied in polyamine intakes amount and composition. A study comparing dietary polyamines intake in six West European countries, the USA, and Japan showed that the Western-style diet had high polyamine intake, whereas the Japanese diet represented a significantly lower polyamine intake, especially in spermine [2].
1.2. Endogenous Biosynthesis
De novo biosynthesis of spermine in vivo mainly triggered by amino acids such as ornithine, methionine, and arginine (Figure 1). The pathway starts with the production of ornithine from arginine by the action of arginase. Ornithine is then decarboxylated to produce putrescine with the involvement of ornithine decarboxylase (ODC), a key rate-limiting enzyme in the first step of polyamine synthesis. Putrescine can generate spermidine and methylthioadenosine (MTA) with sequential reactions of aminopropyl group from decarboxylated S-adenosylmethionine (dcSAM) under the action of spermidine synthase (SRS). The dcSAM is converted from S-adenosylmethionine (SAM) by enzymatic role of adenosylmethionine decarboxylase (AMD) as a second rate-limiting enzyme in polyamine synthesis. Then, the biosynthesized spermidine can be sequentially converted into spermine and an additional MTA by spermine synthase (SMS) with sequential reactions of the secondary dcSAM molecule [4]. The reactions to form spermine and spermidine are irreversible, but the mutual conversion of polyamines can occur through the action of spermine oxidase (SMO) or N1-acetylpolyamine oxidase (PAO) after acetylation [5]. Spermine can also be oxidized directly to form spermidine, H2O2, and 3-aminopropanal.
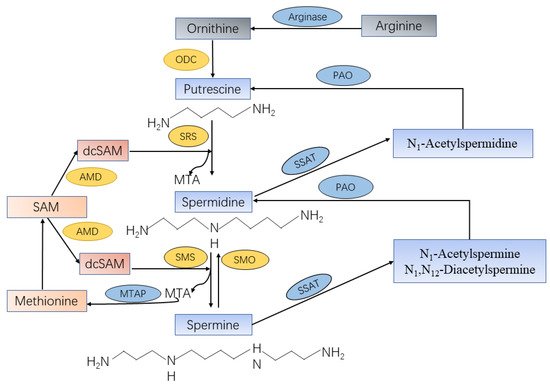
Figure 1. Spermine synthesis and catabolism in mammalian cells. Arginine produces ornithine through the action of arginase. Ornithine decarboxylase (ODC) is the first rate-limiting enzyme in spermine synthesis, in which ornithine is decarboxylated to produce putrescine. Spermidine synthase (SRS) and spermine synthase (SMS) are constitutively expressed aminopropyltransferases that catalyze the transfer of the aminopropyl group from decarboxylated S-adenosylmethionine (dcSAM) to putrescine and spermidine to form spermidine and spermine, respectively, as well as methylthioadenosine (MTA). The dcSAM is converted from S-adenosylmethionine (SAM) by enzymatic role of adenosylmethionine decarboxylase (AMD) as a second rate-limiting enzyme in polyamine synthesis. The central enzyme in the polyamine catabolic pathway is spermidine-spermine-N1-acetyltransferase (SSAT), which monoacetylates spermidine and mono-/diacetylates spermine. These acetylated polyamines are the substrates of N1-acetylpolyamine oxidase (PAO), which are catalyzed to form putrescine or spermidine, respectively. Spermine can be oxidized directly and specifically to produce spermidine by spermine oxidase (SMO).
The catabolic mechanism of spermine contains two steps that are catalyzed by rate-limiting catabolic enzymes spermidine/spermine N1-acetyltransferase (SSAT) and PAO. SSAT plays an important role in polyamine homeostasis as a propylamine acetyltransferase that convert spermine to monoacetylated metabolites (N1-acetylspermine) or diacetylated spermine (N1, N12-diacetylspermine) [6]. These acetylated spermine have at least two potential fates, either export from the cell by transporter or as PAO substrates. PAO, a constitutively expressed peroxisomal polyamine oxidase, generates putrescine or spermidine from acetylated spermine, with the production of H2O2 and 3-acetyaminopropanal [5]. Both SMO and PAO have the potential to generate substantial amounts of reactive oxygen species (ROS), resulting in oxidative damage [7]. In addition, online database cBioPortal [8] and GEPIA [9] were used to further investigate whether those enzymes that were directly related to spermine synthesis were dysregulated in PCa. Results revealed that the transcription level of SMS, SMO, and SAT1 were upregulated, whereas AMD1 expression level had no change in PCa tissues compared with normal. Furthermore, less than 1% mutation level of SMS and AMD1 in PCa were observed, but SMO and SAT1 had no alterations in PCa.
2. Molecular Mechanisms of Spermine for PCa Carcinogenesis and Progression
2.1. Polyamine Metabolism, Oncogenes and Tumor Suppressors
A variety of oncogenes, including Myc, Ras, and PI3K-mTOR, etc., have been reported to target polyamine metabolites, despite their molecular regulatory mechanism is not fully understood. ODC was reported to be a transcription target of the Myc oncogene, and elevated Myc expression leads to increased ODC mRNA and protein that are necessary to drive prostate cell proliferation and malignant transformation [10]. Except for Myc, other oncogenes were also identified to be associated with enzymes of spermine metabolism. Upon Ras activation, ODC is also induced, and studies further confirmed that ODC expression is controlled by the Ras effector pathways [11][12]. As for the polyamine catabolism pathway, Ras can interfere with the transcriptional activation of SSAT by peroxisome proliferator-activated receptor-γ (PPARγ), leading to the downregulation of SSAT, thereby maintaining the elevated spermine levels in transformed cells [13]. Amaia et al. found that increased AMD enzyme occurs frequently in PTEN-deficient PCa cells, and treatment with the mTORC1 inhibitor resulted in a loss of AMD protein. Further research revealed that activated mTOR can indirectly block the proteasomal degradation of pro-AMD and stabilize pro-AMD through phosphorylation at residue S298, leading to increased AMD and spermine biosynthesis in PCa [14]. Another encouraging finding includes the potential of targeting the methionine salvage pathway such that a high level of MTAP in PCa is maintained. A combinational strategy targeting MTAP and accelerating polyamine catabolism has a synergistic inhibitory effect on androgen-sensitive and castration refractory PCa models in vitro and in vivo [15].
Compared with the numerous reports on the interaction between polyamine metabolism and oncogenes, there are few reports on the relationship between polyamine metabolism and tumor suppressor genes. p53 is a potent tumor suppressor, and its mutation is a central element in the initiation and progression in at least half of all human cancers, including PCa [16]. As a transcription target of p53, SSAT was activated by p53 to induce lipid peroxidation and ferroptosis in response to ROS-induced cell stress, which lead to tumor growth suppression. Therefore, the tumor-suppressive function of p53 appears to be partly caused by the direct transcriptional activation of SSAT and SSAT-dependent lipid peroxidation and ferroptosis [17].
2.2. Polyamine Metabolism in PCa-Associated Oxidative Stress and Inflammation
ROS, such as hydroxyl radical, superoxide, and hydrogen peroxide, may contribute to the initiation and development of PCa, as well as the conversion of PCa into castration refractory PCa, via regulation of androgen receptor (AR) signaling [18]. In human, NKX3.1 has been proposed to be an essential factor in PCa carcinogenesis, which interacts with AR to promote PCa cell viability [19][20]. NKX3.1 can either by direct modulation of gene targets or by indirect regulation of AR expression to prevent cell apoptosis. Meanwhile, activated AR-JunD complex can induce SSAT expression, which in turn initiates enhanced polyamine oxidation that produces high ROS levels in certain PCa cells [21]. ROS generated by the activation of spermidine/spermine oxidation pathway can activate more than one mechanism to help androgen-dependent PCa cell survival and promote castrate-resistant PCa growth, as well as its metastasis. As prostatic epithelia produce a large excess of spermine, androgen-induced SSAT gene expression causes spermine oxidation and H2O2 production, which could be a major reason for the high ROS levels in the prostate epithelia, and further imply that spermine catabolism may be a potential source of cancer initiation [22]. Moreover, Pegg’s study revealed that the oxidation products derived from spermine oxidation may be exacerbated by a fall in spermine, since spermine has an antioxidant effect as a free radical scavenger [7].
Although the ROS production in the polyamine metabolic pathway mainly comes from SMO and SSAT/PAO, Pledgie et al. found that the majority of damaging ROS was mainly from SMO. This suggested a pathway linking inflammation to carcinogenesis through polyamine catabolism in general, which may be mediated by SMO specifically [23]. Goodwin et al. verified that SMO staining was higher in the cancer regions of prostate tissues, especially in the prostatic intraepithelial neoplasia (PIN) compared with normal tissues, indicating that enhanced SMO expression was an early event of PCa. Thus, increased H2O2 resulting from elevated SMO confirmed a molecular link between inflammation and carcinogenesis in PCa [24]. There was also evidence indicating that the use of aspirin was inversely linked to the risk of developing PCa [25]. The responses of PCa to aspirin were varied, and the shift from resistance to sensitivity was associated with decreased SSAT activity, which provides in vitro evidence that the sensitivity of human PCa cells to aspirin was correlated with cellular SSAT activity status [26]. Aspirin is expected to decrease SSAT activity in early PCa, thereby increasing tumor cell sensitivity to aspirin and ultimately suppressing tumor growth by altering cellular polyamine content.
2.3. Anticancer Immunosurveillance
Polyamines are involved in the establishment of an immunosuppressive tumor microenvironment and as a reason for the failure of immunotherapy [27]. A polyamine-blocking therapy (PBT) combining the DFMO with a novel polyamine transport inhibitor inhibited tumor growth and promoted durable protection against tumor recurrence in immunocompetent mice, but not in T-cell-deficient athymic nude mice [28]. A study by Alexander confirmed that the antitumor effect of PBT was T-cell dependent, accompanied by increased T-cells and decreased immunosuppressive tumor infiltrating cells. This provided a novel strategy to suppress tumor growth and reverse tumor immunosuppression by targeting polyamines [29]. Increased polyamine uptake by immune cells resulted in decreased cytokine and antitumor immune molecules required for antitumor activities. Thus, in an environment with increased polyamine, immune cells may lose their antitumor immune functions that facilitate cancer cell invade and metastasize [30].
Spermine as an inhibitor of immune responses has been widely reported. For example, spermine inhibits the generation of nitric oxide in bacterial endotoxin-activated macrophages [31], proinflammatory cytokine synthesis [32] and macrophage activation [33] and show immunosuppressive to T cells in vitro and in vivo [34]. A recent study by Singh et al. showed that ODC could regulate the activation of M1 macrophages, and its deletion could lead to enhanced M1 expression that promote tumor-killing responses [35]. These findings indicated that polyamine, especially spermine, played a regulatory role as an immunosuppressive effector by targeting T cells and suppressive myeloid cells, providing a survival mechanism and restored a more favorable tumor microenvironment to escape tumor immune response.
2.4. Apoptosis
Apoptosis suppression is thought to play a central role in the development and progression of cancer. A study by Peggger et al. indicated that decreased ODC activity and polyamine levels after castration induced apoptosis in prostate epithelial cells [36], which imply a protective effect of spermine on apoptosis. Moreover, inhibiting polyamine catabolism by suppressing key polyamine catabolism enzymes could prevent cyclin-dependent kinase inhibitor-induced apoptosis in PCa [37]. Some research groups also revealed that the death of PCa cells induced by some polyamine analogues were actually based on their ability to induce apoptotic cell death, which was in line with the merging evidence that polyamines are actively involved in apoptotic cell death [38][39]. BIS, a novel spermine analogue, induced apoptosis and improved radiosensitivity in human PCa cell lines and xenografts nude mice [40]. However, a study by Mi et al. tested that analogue BE-3-3-3 treatment prevented the growth of PCa cell lines without activation of the apoptosis pathway [41]. As a structural analog of spermine, it is possible that BE-3-3-3 might still mimic protective behavior of spermine. The effect of spermine biosynthetic enzyme AMD inhibitor on the process of apoptotic cell death further support the notion that increased spermine level plays a role in cellular resistance to apoptotic cell death [42]. The nature of the protective effect of spermine is not well known, but several mechanisms, such as endonuclease inhibition [43], DNA stabilization [44], and DNA defense against oxidative stress [45] have been proposed. To conclude, spermine is involved in PCa cell apoptosis, which makes it ideal target for PCa therapeutic action.
3. Spermine as a Biomarker for PCa
A literature search was performed on several English databases (Pubmed, Embase, Cochrane library, Scopus, Web of Science) using the Medical Subject Heading (MeSH) terms and free text words as a combination of strategy, including “prostatic neoplasms”, “prostate neoplasm”, “prostate carcinoma”, “prostate cancer”, “prostate cancers”, “cancer of the prostate”, “prostatic cancer”, and “spermine”. (as of February 16, 2021). A total of 167 published original and review articles were identified through literature search and manual search of citations from identified articles and selected journals. Among these articles, twenty-four articles focused on the detection of spermine level in PCa from urine, tissues, expressed prostatic secretions (EPS), and erythrocyte were identified, and their characteristic are summarized in Table 1.
Table 1. Spermine studies performed in urine/tissues/EPS/erythrocyte from PCa patients.
| Publication | Sample Type | Analytical Platform | PCa Group | Control Group | Main Results | Statistical Significance | Ref. |
|---|---|---|---|---|---|---|---|
| Sanford et al., 1975 | 24-h urine | Beckman spectrophot-ometer | n = 15 PCa | n = 42 healthy controls | ↑Polyamines in 11/15 PCa | NR | [46] |
| Fair et al., 1975 | 12-h/ 24-h urine | Spectronic 20 colorimeter | n = 44 PCa | n = 13 healthy controls | Similarly low levels of spermine detected in cancer and healthy controls | NR | [47] |
| Sugimoto et al., 1995 | Morning Urine |
HPLC | n = 24 urogenital cancer, including 13 PCa | n = 43 benign urogenital disorders; n = 52 healthy controls | ↑DiAcSpm in urogenital cancer | NR | [48] |
| Hiramatsu et al., 1997 | Morning Urine |
HPLC | n = 31 urogenital cancer, including 15 PCa | n = 42 benign urogenital disorders; n = 52 healthy controls | ↑DiAcSpm in urogenital cancer ↑DiAcSpm in cancer patients with poor prognosis and recurrence |
NR | [49] |
| Tsoi et al., 2016 | Pre-biopsy urine with serum PSA level >4.0 ng/mL | UPLC–MS/MS | n = 66 PCa | n = 88 BPH, n = 11 healthy controls | ↓Spermine in PCa; AUC of spermine for PCa: 0.83 |
p < 0.0001 | [50] |
| Chiu et al., 2021 | Pre-biopsy urine with serum PSA level 4–20 ng/mL | UPLC–MS/MS | n = 185 PCa; n = 103 HGPCa | n = 415 healthy controls | ↓Spermine in PCa and HGPCa; AUC of spermine risk score: PCa 0.78, HGPCa 0.82 |
p < 0.001 | [51] |
| Graaf et al., 2000 | Tissue | HPLC | n = 7 PCa | n = 4 healthy controls, n = 3 BPH | ↓Spermine in PCa | p < 0.05 | [52] |
| Swanson et al., 2003 | Tissue | HRMAS | n = 7 PCa (gland percentage < 20) n = 13 PCa (gland percentage ≥ 20, 8 with GS ≤ 6, 5 with GS ≥ 7) | n = 33 healthy controls | ↓Spermine in PCa compared with controls ↓Spermine in PCa with higher GS | p = 0.01 p = 0.05 |
[53] |
| Swanson et al., 2006 | Tissue | HRMAS | n = 60 PCa | n = 6 healthy controls | ↓Spermine in PCa | p < 0.01 | [54] |
| Maxeiner et al., 2010 | Tissue | HRMAS | n = 16 PCa with BCR | n = 32 PCa without BCR (16 clinical-stage-matched and 16 pathological-stage-matched) | Spermine alteration predicts PCa recurrence | NR | [55] |
| Nagarajan et al., 2010 | Tissue | (2D) J-resolved spectroscopy (JPRESS) | n = 7 PCa with GS = 4 + 3 | n = 7 PCa with GS = 3 + 4 | ↑(Cho + Cr)/Spm ratio in PCa with GS = 4 + 3 | p = 0.07 | [56] |
| García-Martín et al., 2011 | Tissue | 1H-MRS | n = 30 | n = 249 | ↑Cho/(Cit + Spm) ratio in PCa | p < 0.001 | [57] |
| Giskeodegar-d et al., 2013 | Tissue | HRMAS | n = 30 PCa with GS = 6; n = 81 HGPCa with GS ≥ 7 |
n = 47 normal adjacent samples | ↓Spermine in PCa and HGPCa compared with normal ↓Spermine in HGPCa compared with PCa ↑(Cho+Spm+Cr/Cit) ratio in HGPCa |
p = 0.022 p = 0.0044 p = 2.17 × 10-4 |
[58] |
| Selnaes et al., 2013 | Tissue | In vivo MRSI and ex vivo HRMAS | n = 15 PCa with GS ≥ 4 + 3 for ex vivo HRMAS n = 19 PCa with GS ≥ 4 + 3 for in vivo MRSI |
n = 16 PCa with GS ≤ 3 + 4 for ex vivo HRMASn = 12 PCa with (GS ≤ 3 + 4) for in vivo MRSI | ↑(Cho+Spm+Cr/Cit) ratio with increasing GS | p = 0.035 (ex vivo) p = 0.001 (in vivo) |
[59] |
| Basharat et al., 2015 | Tissue | HRMAS | n = 8 PCa with T3 stage n = 19 PCa with GS = 7 |
n = 7 PCa with T1 stage, n = 11 with T2 stagen = 6 PCa with GS = 6 |
↓Spermine in PCa with advanced stage and higher GS | T3 vs. T1 p = 0.04 T3 vs. T2 p = 0.08 GS = 7 vs. GS = 6 p = 0.01 |
[60] |
| Hansen et al., 2016 | Tissue | HRMAS | n = 34 ERGhigh PCa | n = 30 ERGlow PCa | ↓Spermine in ERGhigh PCa compared with ERGlow PCa | p < 0.001 | [61] |
| Shukla-Dave et al., 2016 | Tissue | Immunofluo-rescence | n = 18 HGPIN; n = 120 PCa |
n = 103 healthy controls | ↓Spermine in HGPIN and PCa | p < 0.0001 | [62] |
| Braadland et al., 2017 | Tissue | HRMAS | n = 50 PCa with recurrence | n = 60 PCa without recurrence | ↑Spermine independently associated with better RFS ↑(Cho+Cr)/Spm independently associated with worse RFS |
RFS: HR = 0.72, p = 0.016 RFS: HR = 1.43, p = 0.014 |
[63] |
| Lynch et al., 1997 | EPS by prostatic massage | 1H-MRS | n = 4 PCa | n = 12 healthy controls; n = 10 BPH; n = 11 vasal aplasia, n = 1 prostatodynia | ↓(Cit to Spm) ratio in PCa | p < 0.02 | [64] |
| Serkova et al., 2008 | EPS by prostatic massage | 1H-MRS | n = 52 PCa | n = 26 healthy controls | ↓Spermine in PCa | p < 0.002 | [65] |
| Cipolla et al., 1990 | Erythrocyte spermine | HPLC | n = 36 PCa with metastases; n = 12 PCa with hormonal escape |
n = 17 PCa without metastases; n = 41 PCa with hormonal responsiveness |
↑Spermine in PCa with metastases ↑Spermine in hormone-refractory PCa |
p < 0.01 p < 0.001 |
[66] |
| Cipolla et al., 1993 | Erythrocyte spermine | HPLC | n = 28 endocrine-treated PCa with progression | n = 23 endocrine-treated PCa without progression | ↑Pretherapeutic spermine level in PCa with progression | p < 0.01 | [67] |
| Cipolla et al., 1994 | Erythrocyte spermine | HPLC | n = 40 newly diagnosed, stage D2 PCa | NA | ↑Spermine associated with shorter PFS and CSS in PCa | PFS: p = 0.001 CSS: p = 0.0025 |
[68] |
| Cipolla et al., 1996 | Erythrocyte spermine | HPLC | n = 88 PCa with metastases | NA | ↑Pretherapeutic spermine level predicts worse PFS and CSS in metastatic PCa | PFS: p < 0.0001 CSS: p < 0.0005 |
[69] |
Abbreviations: NR, not reported; ROC, Receiver operating characteristics; GS, Gleason score; BCR, biochemical recurrence; HGPCa, high-grade prostate cancer; HGPIN, high-grade prostatic intraepithelial neoplasia; RFS: recurrence-free survival; NA, not available; PFS: progression-free survival; CSS: cancer special survival.
3.1. Urine
As early as the mid-1970s, Sanford et al. discovered that the excretion of polyamines in the urine of patients harboring PCa was higher than normal individuals [46]. In the same year, Fair et al. reported a significant elevation of urinary spermidine content by Spectronic 20 colorimeter in PCa patients, but not putrescine and spermine [47]. After that, there were scattered reports about the role of spermine and polyamines in the urine of PCa patients. As shown in Figure 1, although spermine is mainly excreted into urine in the form of the monoacetyl derivative of spermine, the diacetylated derivatives of spermine (DiAcSpm) had lower secretion level in urine but with less variation in the population, thus may be a more reliable biomarker. In 1995, Sugimoto et al. found that DiAcSpm was significantly elevated in malignant tumors of the genitourinary system, including PCa, using high-performance liquid chromatography (HPLC) [48]. Soon after, a study by Hiramatsu et al. further revealed that decreased urinary DiAcSpm level occurred in PCa patients with effective treatment, and its elevation was correlated with poor prognosis and recurrence [49]. After that, there was no further report on urinary spermine in PCa patients.
A pilot study conducted in Hong Kong [50] using ultraperformance liquid chromatography–tandem mass spectrometry (UPLC–MS/MS) found a significantly decreased urine spermine content in PCa patients, showing its potential as a novel noninvasive diagnostic biomarker that can help distinguish PCa from non-cancerous cases including BPH. A large-scale validation study by Chiu et al. showed that in men with an elevated PSA level of 4.0–20.0 ng/mL, urine spermine can act as a noninvasive test to identify men at a higher risk of high grade PCa (HGPCa). The urine spermine and a multivariate Spermine Risk Score (combining urine spermine, prostate volume, PSA, and digital rectal examination) could act as a guide to predict PCa and HGPCa. Using the urine Spermine Risk Score, a negative predictive value of 95% for HGPCa was achieved and 37% unnecessary biopsies could be avoided [51].
Urinary spermine level was found to be significantly reduced in PCa and could serve as a potential biomarker for noninvasive diagnosis of PCa. Although the observation of such an elevated DiAcSpm level in PCa urine was inconsistent with spermine, it is in line with results of previous literature about PCa studies. Increased SMO and SSAT expression were well reported in the early PIN and cancer regions of PCa patients, which resulted in a depletion of spermine content [24][62]. This also supported the observation of increased urinary DiAcSpm content in PCa, which resulted from the enzymatic action of SSAT converting spermine to DiAcSpm (Figure 1).
3.2. Tissue
Polyamine measurements in PCa cell lines with different degree of differentiation revealed that poorly differentiated cell lines contained lower spermine concentrations [70]. A similar correlation was found between tissue spermine level and degree of differentiation in human PCa tissue, as well as in the urine of PCa patients [52][50][53][58] (Table 1). Metabolic studies tools such as proton magnetic resonance spectroscopy (1H-MRS) or high-resolution magic-angle spinning (HRMAS) have been used to evaluate metabolic information in the prostate, which was obtained noninvasively from multiple, distinct regions of prostatic tissue in situ or from intact biopsy material. In 2000, Graaf et al. found through HPLC that normal and BPH tissues had higher levels of spermine, whereas in tumor tissues, especially those metastatic PCa tissues, spermine levels were significantly lower, which might serve as a biomarker for malignant prostate tissues [52]. Swanson et al. also demonstrated that the content of spermine in PCa was significantly reduced or absent in 80% of higher-grade (Gleason Score ≥7) PCa samples using HRMAS, which indicated that spermine reduction is not only an early indicator of PCa development, but also an indicator of PCa aggressiveness [53]. These results were also supported by other studies, which confirmed that spermine concentration was significantly lower in PCa tissues than healthy glandular tissues [54] and can be used as a marker to assess PCa aggressiveness, in terms of cancer Gleason scores [58] and stages [60]. Maxeiner et al. had tried to assess whether spermine level could be used to predict PCa recurrence, forty-eight PCa cases were divided into 3 groups according to their clinical and pathological information, as well as their biochemical recurrence status. The principal component analysis based on HRMAS results revealed that tissue spermine level could be used to identify PCa recurrence after prostatectomy [55].
It is difficult to separate the individual metabolite signals due to overlapped choline, polyamine (mainly spermine), and citrate spectral lines between 3 and 3.2 ppm using nuclear magnetic resonance spectroscopy (NMR spectroscopy), and the ratio of choline over citrate plus spermine, namely the Cho/(Cit+Spm) ratio, was found to discriminate between PCa and healthy tissue [57], and a higher metabolite ratio of (choline+creatine)/spermine (Cho+Cr/Spm) was shown in higher Gleason scores (4+3) subgroups of PCa compared with lower Gleason scores (3+4) subgroups by (2D) J-resolved NMR [56]. In addition, several other studies revealed that the ratio of (choline plus spermine plus creatine) over citrate, (Cho+Spm+Cr)/Cit, can be used as an indicator of PCa aggressiveness [58][71][59]. Because increased choline level coincides with decreased spermine level in PCa, which may reduce overall sensitivity of the method, but higher field strengths, such as 3T or even 7T can improve its performance in PCa detection [72].
The TMPRSS2-ERG gene fusion is the most common gene rearrangement in PCa which is associated with cancer cell invasion and proliferation [73]. Two independent PCa patient cohorts conducted by HRMAS revealed lower concentrations of spermine in ERGhigh patients compared to ERGlow samples, indicating an increased cancer aggressiveness of ERGhigh compared to ERGlow. Further polyamine pathway study revealed that increased SRS and SSAT in ERGhigh samples is one of the reasons for a lower spermine level in ERGhigh, which can lead to rapid spermine consumption in cancer cells [61]. As for the prognosis value of spermine in PCa, Braadland et al. showed that decreased spermine concentration can be identified as an independent prognostic marker with shorter recurrence-free survival using ex vivo HRMAS on tissue samples from 110 PCa patients treated with radical prostatectomy [63].
A study by Amita et al. using immunofluorescence further confirmed that spermine content was significantly lower in high-grade prostate intraepithelial neoplasia (HGPIN) and PCa tissues. Enzymes of spermine metabolism pathway (ODC, PAO and SMS) showed opposite expression levels, with significantly higher level in HGPIN and PCa tissues [62]. Prostatic secretory granules (PSG), present in the secretory cells of the glandular prostate, are the major secretory pathway of the prostate gland [74]. Cohen et al. found that PSG consumption during the development of normal prostate epithelial cells through dysplasia to adenocarcinoma was accompanied by reduced spermine. The decreased spermine expression in untreated PCa is linked to PSG loss, and furthermore, androgen deprivation therapy can prevent spermine production in normal prostate secretory cells and paralleled PSG depletion, but spermine will continue to be produced in androgen-resistant tumor clones [75].
3.3. Human Expressed Prostatic Secretions (EPS)
Apart from direct monitoring of prostate tissues, prostatic fluid collected after prostate massage is richer in prostatic metabolites, and is less affected by confounding factors [76]. There were two reports suggested an association between spermine concentration in EPS and PCa, both of which were detected by 1H-MRS method (Table 1). They found that spermine concentration in human EPS can act as a potential marker of PCa that is independent of age, and decreased spermine level was highly predictive of PCa and negatively correlated with the risk of PCa [64][65].
3.4. Erythrocyte
Greater than 95% of circulating spermine and spermidine are transported by erythrocyte [77]. A total of four papers reported “erythrocyte spermine and PCa”, and all of them were published by the same research team [66][67][68][69] (Table 1). In 1990, Cipolla et al. found that erythrocyte spermine levels were significantly correlated with PCa stages, with a higher level in metastatic PCa and hormone-refractory PCa patients [66]. Later, further study was conducted by comparing two cohorts of hormone-treated PCa patients with or without progression, results revealed that increased pretherapeutic erythrocyte spermine level in patients with a higher risk of PCa progression/recurrence, which may help distinguish those people who may benefit more from aggressive primary treatments [67]. In addition, they found that elevated erythrocyte spermine level was an independent prognostic variable for shorter progression-free survival and cancer-special survival in PCa patients [68]. As for metastatic PCa patients, pretherapeutic erythrocyte spermine level also has a significant prognosis and hormonal escape prediction value, which can help discriminate risk of PCa recurrence after hormone treatment [69].
4. Therapeutic Potential for PCa by Targeting Spermine Metabolism Pathway
Genetic and epigenetic changes can be heterogeneous, so targeting the metabolic phenotype of cancer that is downstream of multiple common genetic changes may provide a new and effective treatment perspective [78]. The prostate has the highest level of spermine biosynthesis than any other organ, and spermine homeostasis plays an important role in the prostate, thus tumor tissues derived from this gland may have a regulatory response to spermine metabolism pathway [41].
4.1. Inhibition of Anabolism Pathway
Increased activity of polyamine metabolism enzymes has been reported in PCa, especially ODC and AMD, which are the first and second rate-limiting enzymes in spermine biosynthesis pathway, respectively [62][79]. A variety of strategies targeting these two key biosynthetic enzymes have been validated as drug targets for PCa in cell and animal models, as well as various clinical trials (Table 2). Some competitive inhibitors of AMD, such as MGBG and CGP-48664, had revealed a potent antiproliferative activity in vitro and in vivo [41][80][81][82]. Difluoromethylornithine (DFMO), the most widely studied ODC inhibitor, has significant inhibitory effects on the growth of cultured PCa cells and animal models [80][83][84][85]. However, in some clinical trials, DFMO, either alone or in combination with other chemotherapies, was generally found to have little antitumor activity [86][87][88]. The limited antitumor activity of DFMO is partly due to a compensatory extracellular polyamine uptake mechanism upon the occurrence of depleted polyamine pools [89]. In contrast to the rather moderate effect of DFMO on models with established cancer, there is increasing interest in using DFMO as a potential strategy for cancer chemoprevention. Studies showed that normal epithelial prostate cells overexpressing ODC undergone malignant transformation in vitro and in vivo [62]. The use of DFMO could suppress the chemically induced prostate carcinogenesis in transgenic adenocarcinoma mouse prostate (TRAMP) models [90]. In addition, a clinical study by Simoneau et al. also showed that DFMO treatment was associated with decreased prostate growth in healthy men with a family history of PCa [91] (Table 2).
Table 2. Inhibitors targeting spermine metabolism pathway in cell and animal models, as well as clinical trials for PCa.
| Publication | Inhibitor | Target | PCa Cell Lines | PCa Animal Models | Clinical Trials | Main Results | Ref. | |
|---|---|---|---|---|---|---|---|---|
| Polyamine Synthesis Inhibitor | Heston et al., 1982 | DFMO | ODC | R3327 MAT-Lua | Rat injected with R3327 MAT-Lu | NR | In vitro and in vivo: Inhibition of R3327MAT-Lu growth |
[83] |
| Dunzendorfer et al., 1983 | DFMO/MGBG/DFMO + MGBG | ODC + AMD | NR | Rat injected with R3327-Gb | NR | In vivo: Inhibition of R3327-G growth by either DFMO or MGBG; their combination was more effective |
[80] | |
| Herr et al., 1984 | DFMO/MGBG/DFMO + MGBG | ODC/AMD/ ODC + AMD |
NR | Rat injected with R3327-G | NR | In vivo: DFMO had no antitumor effect, MGBG retarded tumor growth; their combination inhibited tumor growth |
[81] | |
| Herr et al., 1986 | DFMO + MGBG | ODC + AMD | NR | NR | Phase I; 5 advanced, hormone-resistant PCa patients | Clinical trial: No antitumor effects, but drugs were well tolerated |
[86] | |
| Horn et al., 1987 | DFMO ± doxorubicin + cyclophosphamide | ODC ± conventional chemotherapy | NR | NR | Phase I-II; 9 PCa patients (DFMO + conventional chemotherapy); 5 PCa patients (conventional chemotherapy) | Clinical trial: No effect |
[87] | |
| Kadmon et al., 1992 | DFMO | ODC | NR | Rat injected with R3327 MAT-Lu | NR | In vivo: Modest inhibition of R3327 MAT-Lu growth |
[84] | |
| Delworth et al., 1995 | CGP-48664 | AMD | LNCaP, LNCaP-LN3, PC-3M, and PC-3M-MM2 | Nude mice injected with LNCaP-LN3 cells or PC-3M-MM2 cells | NR | In vitro and in vivo: Induction of cytostasis; Inhibition of tumor growth in slow-growing tumor, but not fast-growing tumor |
[82] | |
| Mi et al., 1998 | CGP-48664 | AMD | LNCaP, DU145, and PC-3 | NR | NR | In vitro: Inhibition of PCa cells growth |
[41] | |
| Carbone et al., 1998 | DFMO/PXM/ DFMO + PXM | ODC/ prostaglandin Inhibitor/ODC + prostaglandin Inhibitor |
NR | NR | Phase I; 31 cancer patients, including stage A or B PCa |
Clinical trial: Dosage toxicity assessment |
[88] | |
| Gupta et al., 2000 | DFMO | ODC | NR | TRAMP | NR | In vivo: DFMO prevent prostate tumorigenesis |
[90] | |
| Devens et al., 2000 | DFMO | ODC | PC3, LNCaP, DU145 | Nude mice injected with PC-3 cells | NR | In vitro and in vivo: Inhibition of tumor growth |
[85] | |
| Simoneau et al., 2008 | DFMO/ placebo | ODC | NR | NR | 81 men with PCa family history but without personal PCa history | Clinical trial: Induction of slow prostate growth and no grade 3 or 4 toxicities |
[91] | |
| Polyamine Analogues | Jeffers et al., 1997 | BE-4-4-4-4/BE-3-7-3/BE-3-3-3 | PA analogue | DU145, LNCaP and PC-3 | BALB/c mice injected with DU145 cells | NR | In vitro and in vivo: Inhibition of PCa cells growth by all three polyamine analogues |
[92] |
| Zagaj et al., 1998 | BE-4-4-4-4 | PA analogue | AT3.1, AT6.1 and AT6.3; DU145, DuPro-1 and TSU-Pr1 | Nude mice injected with DuPro-1 and PC-3 PCa cells | NR | In vitro and in vivo: BE-4-4-4-4 was cytotoxic against rat and human PCa cells |
[93] | |
| Mi et al., 1998 | DENSpm | PA analogue | LNCaP, DU145, and PC-3 | NR | NR | In vitro: Inhibition of PCa cells growth with varied sensitivity, DU145 > PC-3 > LNCaP |
[41] | |
| Eiseman et al., 1998 | BIS | PA analogue | PC-3 and DU-145 | Nude mice injected with PC-3 and DU-145 cells | NR | In vitro and in vivo: Inhibition of PCa growth and enhanced radiosensitivity |
[40] | |
| Schipper et al., 2000 | DENSpm | PA analogue | PC-3, TSU-pr1, DU-145, and JCA-1 | Nude mice injected with Du145 cells | NR | In vitro and in vivo: Inhibition of PCa growth |
[38] | |
| McCloskey et al., 2000 | CPENSpm, CHENSpm and BE 3-3-3 | PA analogue | LNCaP, PC3, and Du145 | NR | NR | In vitro: Inhibition of PCa cells growth, especially DU145 |
[94] | |
| Reddy et al., 2001 | BE-4-4-4-4 | PA analogue | LnCap, DU145, PC-3, and DuPro | NR | NR | In vitro: Inhibition of PCa cells growth with varied sensitivity, LnCap and DU145 > DuPro >PC-3 |
[95] | |
| Valasinas et al., 2001 | BE-4-4-4-4 | PA analogue | LnCap, DU145, DuPro, and PC-3 | NR | NR | In vitro: Inhibition of PCa cells growth, PC3 was the least sensitive |
[96] | |
| Frydman et al., 2003 | Cyclopropane-Containing Polyamine Analogues | PA analogue | DU-145, DuPro, and PC-3 | NR | NR | In vitro: Inhibition of PCa cells growth, especially DU145 |
[97] | |
| Valasinas et al., 2003 | Long-chain Polyamines (Oligoamines) | PA analogue | LnCap, DU-145, DuPro and PC-3 | NR | NR | In vitro: Inhibition of PCa cells growth with varied sensitivity, LnCaP, DU145 > DuPro and PC-3 |
[98] | |
| Frydman et al., 2004 | Macrocyclic Polyamines | PA analogue | DuPro and PC-3 | NR | NR | In vitro: The macrocycles were cytotoxic against PCa cells |
[99] |
a R3327MAT-Lu, metastatic derivative of the Dunning slow-growing, androgen-responsive prostatic adenocarcinoma; b R3327-G, G subline of the Dunning rat that is a poorly differentiated PCa cell lines. Abbreviations: NR, not reported; TRAMP: Transgenic adenocarcinoma mouse prostate.
4.2. Catabolism Pathway Activation
As an alternative to blocking biosynthesis, activation of spermine catabolism by inducing the rate-limiting enzyme SSAT may offer distinct advantages. Studies by Kee et al. found that SSAT induction in cancer cell and TRAMP mice led to PCa growth inhibition in vitro and in vivo. The effect was not attributed to polyamine pool depletion, but by a heightened metabolic flux [100][101]. A possible explanation for the growth inhibition caused by SSAT overexpression may be attributed to the accelerated metabolic flux, causing excessive consumption of important metabolites, such as acetyl-CoA and SAM, and the production of potentially harmful compounds, such as hydrogen peroxide and reactive aldehydes [100].
4.3. Development and Use of Polyamine Analogues
In addition to the development of drugs that target specific enzymes in spermine metabolism pathway, another promising option is the application of synthetic polyamine analogues, which have been evaluated to produce a significant PCa growth inhibition effect in vitro and in vivo (Table 2). Symmetrically substituted bis(ethyl) analogues of spermine, i.e., BE-3-3-3 (also known as DENSpm), BE-4-4-4-4 and BE-3-7-3, nonsymmetrically substituted alkylated analogues, CPE-3-3-3, CHE-3-3-3 and the spermine analogue 1,12-diaziridinyl-4,9-diazadodecane (BIS), all have been tested in a variety of human PCa cell lines [41][38][40][92][93][94][96][95] (Table 2). From these studies it can be concluded that these analogues have varied effects in different PCa cells. For example, the androgen-independent DU-145 cells were the most sensitive, whereas the well-differentiated androgen-dependent LNCaP cells were relatively insensitive, which indicate that these analogues might have chemotherapeutic potential for PCa that has failed hormone therapy. The effect of BE-3-3-3, BE-4-4-4-4 and BIS were also examined in the nude mouse xenografts derived from DU-145, PC-3 or DuPro-1 cells with different degrees of malignancy, and results revealed an inhibitory effect on tumor growth, as well as decreased spermine levels [38][92][93]. Moreover, polyamine analogues in other types of conformations, such as macrocyclic, long-chain, and cyclopropane-containing analogues, could also have an inhibitory effect on PCa growth [99][98][97].
References
- Cipolla, B.; Guilli, F.; Moulinoux, J.P. Polyamine-reduced diet in metastatic hormone-refractory prostate cancer (HRPC) patients. Biochem. Soc. Trans. 2003, 31, 384–387.
- Kalac, P. Health effects and occurrence of dietary polyamines: A review for the period 2005-mid 2013. Food Chem. 2014, 161, 27–39.
- Milovic, V. Polyamines in the gut lumen: Bioavailability and biodistribution. Eur. J. Gastroenterol. Hepatol. 2001, 13, 1021–1025.
- Ikeguchi, Y.; Bewley, M.C.; Pegg, A.E. Aminopropyltransferases: Function, structure and genetics. J. Biochem. 2006, 139, 1–9.
- Casero, R.A.; Pegg, A.E. Polyamine catabolism and disease. Biochem. J. 2009, 421, 323–338.
- Holst, C.M.; Nevsten, P.; Johansson, F.; Carlemalm, E.; Oredsson, S.M. Subcellular distribution of spermidine/spermine N1-acetyltransferase. Cell Biol. Int. 2008, 32, 39–47.
- Pegg, A.E. Toxicity of polyamines and their metabolic products. Chem. Res. Toxicol. 2013, 26, 1782–1800.
- Cerami, E.; Gao, J.; Dogrusoz, U.; Gross, B.E.; Sumer, S.O.; Aksoy, B.A.; Jacobsen, A.; Byrne, C.J.; Heuer, M.L.; Larsson, E.; et al. The cBio cancer genomics portal: An open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012, 2, 401–404.
- Tang, Z.; Li, C.; Kang, B.; Gao, G.; Li, C.; Zhang, Z. GEPIA: A web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 2017, 45, W98–W102.
- Bello-Fernandez, C.; Packham, G.; Cleveland, J.L. The ornithine decarboxylase gene is a transcriptional target of c-Myc. Proc. Natl. Acad. Sci. USA 1993, 90, 7804–7808.
- Origanti, S.; Nowotarski, S.L.; Carr, T.D.; Sass-Kuhn, S.; Xiao, L.; Wang, J.Y.; Shantz, L.M. Ornithine decarboxylase mRNA is stabilized in an mTORC1-dependent manner in Ras-transformed cells. Biochem. J. 2012, 442, 199–207.
- Shantz, L.M. Transcriptional and translational control of ornithine decarboxylase during Ras transformation. Biochem. J. 2004, 377, 257–264.
- Ignatenko, N.A.; Babbar, N.; Mehta, D.; Casero, R.A., Jr.; Gerner, E.W. Suppression of polyamine catabolism by activated Ki-ras in human colon cancer cells. Mol. Carcinog. 2004, 39, 91–102.
- Zabala-Letona, A.; Arruabarrena-Aristorena, A.; Martin-Martin, N.; Fernandez-Ruiz, S.; Sutherland, J.D.; Clasquin, M.; Tomas-Cortazar, J.; Jimenez, J.; Torres, I.; Quang, P.; et al. mTORC1-dependent AMD1 regulation sustains polyamine metabolism in prostate cancer. Nature 2017, 547, 109–113.
- Affronti, H.C.; Rowsam, A.M.; Pellerite, A.J.; Rosario, S.R.; Long, M.D.; Jacobi, J.J.; Bianchi-Smiraglia, A.; Boerlin, C.S.; Gillard, B.M.; Karasik, E.; et al. Pharmacological polyamine catabolism upregulation with methionine salvage pathway inhibition as an effective prostate cancer therapy. Nat. Commun. 2020, 11, 52.
- Kandoth, C.; McLellan, M.D.; Vandin, F.; Ye, K.; Niu, B.; Lu, C.; Xie, M.; Zhang, Q.; McMichael, J.F.; Wyczalkowski, M.A.; et al. Mutational landscape and significance across 12 major cancer types. Nature 2013, 502, 333–339.
- Ou, Y.; Wang, S.J.; Li, D.; Chu, B.; Gu, W. Activation of SAT1 engages polyamine metabolism with p53-mediated ferroptotic responses. Proc. Natl. Acad. Sci. USA 2016, 113, E6806–E6812.
- Shiota, M.; Yokomizo, A.; Naito, S. Oxidative stress and androgen receptor signaling in the development and progression of castration-resistant prostate cancer. Free Radic. Biol. Med. 2011, 51, 1320–1328.
- Bhatia-Gaur, R.; Donjacour, A.A.; Sciavolino, P.J.; Kim, M.; Desai, N.; Young, P.; Norton, C.R.; Gridley, T.; Cardiff, R.D.; Cunha, G.R.; et al. Roles for Nkx3.1 in prostate development and cancer. Genes Dev. 1999, 13, 966–977.
- Tan, P.Y.; Chang, C.W.; Chng, K.R.; Wansa, K.D.; Sung, W.K.; Cheung, E. Integration of regulatory networks by NKX3-1 promotes androgen-dependent prostate cancer survival. Mol. Cell Biol. 2012, 32, 399–414.
- Mehraein-Ghomi, F.; Basu, H.S.; Church, D.R.; Hoffmann, F.M.; Wilding, G. Androgen receptor requires JunD as a coactivator to switch on an oxidative stress generation pathway in prostate cancer cells. Cancer Res. 2010, 70, 4560–4568.
- Basu, H.S.; Thompson, T.A.; Church, D.R.; Clower, C.C.; Mehraein-Ghomi, F.; Amlong, C.A.; Martin, C.T.; Woster, P.M.; Lindstrom, M.J.; Wilding, G. A small molecule polyamine oxidase inhibitor blocks androgen-induced oxidative stress and delays prostate cancer progression in the transgenic adenocarcinoma of the mouse prostate model. Cancer Res. 2009, 69, 7689–7695.
- Pledgie, A.; Huang, Y.; Hacker, A.; Zhang, Z.; Woster, P.M.; Davidson, N.E.; Casero, R.A., Jr. Spermine oxidase SMO(PAOh1), Not N1-acetylpolyamine oxidase PAO, is the primary source of cytotoxic H2O2 in polyamine analogue-treated human breast cancer cell lines. J. Biol. Chem. 2005, 280, 39843–39851.
- Goodwin, A.C.; Jadallah, S.; Toubaji, A.; Lecksell, K.; Hicks, J.L.; Kowalski, J.; Bova, G.S.; De Marzo, A.M.; Netto, G.J.; Casero, R.A., Jr. Increased spermine oxidase expression in human prostate cancer and prostatic intraepithelial neoplasia tissues. Prostate 2008, 68, 766–772.
- Lin, D.W.; Nelson, P.S. The role of cyclooxygenase-2 inhibition for the prevention and treatment of prostate carcinoma. Clin. Prostate Cancer 2003, 2, 119–126.
- Li, J.; Cameron, G.A.; Wallace, H.M. Decreased sensitivity to aspirin is associated with altered polyamine metabolism in human prostate cancer cells. Amino Acids 2016, 48, 1003–1012.
- Hayes, C.S.; Defeo, K.; Dang, H.; Trempus, C.S.; Morris, R.J.; Gilmour, S.K. A prolonged and exaggerated wound response with elevated ODC activity mimics early tumor development. Carcinogenesis 2011, 32, 1340–1348.
- Hayes, C.S.; Shicora, A.C.; Keough, M.P.; Snook, A.E.; Burns, M.R.; Gilmour, S.K. Polyamine-blocking therapy reverses immunosuppression in the tumor microenvironment. Cancer Immunol. Res. 2014, 2, 274–285.
- Alexander, E.T.; Minton, A.; Peters, M.C.; Phanstiel, O.T.; Gilmour, S.K. A novel polyamine blockade therapy activates an anti-tumor immune response. Oncotarget 2017, 8, 84140–84152.
- Soda, K. The mechanisms by which polyamines accelerate tumor spread. J. Exp. Clin. Cancer Res. 2011, 30, 95.
- Southan, G.J.; Szabo, C.; Thiemermann, C. Inhibition of the induction of nitric oxide synthase by spermine is modulated by aldehyde dehydrogenase. Biochem. Biophys. Res. Commun. 1994, 203, 1638–1644.
- Zhang, M.; Caragine, T.; Wang, H.; Cohen, P.S.; Botchkina, G.; Soda, K.; Bianchi, M.; Ulrich, P.; Cerami, A.; Sherry, B.; et al. Spermine inhibits proinflammatory cytokine synthesis in human mononuclear cells: A counterregulatory mechanism that restrains the immune response. J. Exp. Med. 1997, 185, 1759–1768.
- Zhang, M.; Wang, H.; Tracey, K.J. Regulation of macrophage activation and inflammation by spermine: A new chapter in an old story. Crit. Care Med. 2000, 28, N60–N66.
- Quan, C.P.; Roux, C.; Pillot, J.; Bouvet, J.P. Delineation between T and B suppressive molecules from human seminal plasma: II. Spermine is the major suppressor of T-lymphocytes in vitro. Am. J. Reprod. Immunol. 1990, 22, 64–69.
- Singh, K.; Coburn, L.A.; Asim, M.; Barry, D.P.; Allaman, M.M.; Shi, C.; Washington, M.K.; Luis, P.B.; Schneider, C.; Delgado, A.G.; et al. Ornithine Decarboxylase in Macrophages Exacerbates Colitis and Promotes Colitis-Associated Colon Carcinogenesis by Impairing M1 Immune Responses. Cancer Res. 2018, 78, 4303–4315.
- Pegg, A.E.; Lockwood, D.H.; Williams-Ashman, H.G. Concentrations of putrescine and polyamines and their enzymic synthesis during androgen-induced prostatic growth. Biochem. J. 1970, 117, 17–31.
- Arisan, E.D.; Obakan, P.; Coker-Gurkan, A.; Calcabrini, A.; Agostinelli, E.; Unsal, N.P. CDK inhibitors induce mitochondria-mediated apoptosis through the activation of polyamine catabolic pathway in LNCaP, DU145 and PC3 prostate cancer cells. Curr. Pharm. Des. 2014, 20, 180–188.
- Schipper, R.G.; Deli, G.; Deloyer, P.; Lange, W.P.; Schalken, J.A.; Verhofstad, A.A. Antitumor activity of the polyamine analog N(1), N(11)-diethylnorspermine against human prostate carcinoma cells. Prostate 2000, 44, 313–321.
- Schipper, R.G.; Penning, L.C.; Verhofstad, A.A. Involvement of polyamines in apoptosis. Facts and controversies: Effectors or protectors? Semin. Cancer Biol. 2000, 10, 55–68.
- Eiseman, J.L.; Rogers, F.A.; Guo, Y.; Kauffman, J.; Sentz, D.L.; Klinger, M.F.; Callery, P.S.; Kyprianou, N. Tumor-targeted apoptosis by a novel spermine analogue, 1,12-diaziridinyl-4,9-diazadodecane, results in therapeutic efficacy and enhanced radiosensitivity of human prostate cancer. Cancer Res. 1998, 58, 4864–4870.
- Mi, Z.; Kramer, D.L.; Miller, J.T.; Bergeron, R.J.; Bernacki, R.; Porter, C.W. Human prostatic carcinoma cell lines display altered regulation of polyamine transport in response to polyamine analogs and inhibitors. Prostate 1998, 34, 51–60.
- Redman, C.; Xu, M.J.; Peng, Y.M.; Scott, J.A.; Payne, C.; Clark, L.C.; Nelson, M.A. Involvement of polyamines in selenomethionine induced apoptosis and mitotic alterations in human tumor cells. Carcinogenesis 1997, 18, 1195–1202.
- Ribeiro, J.M.; Carson, D.A. Ca2+/Mg(2+)-dependent endonuclease from human spleen: Purification, properties, and role in apoptosis. Biochemistry 1993, 32, 9129–9136.
- Basu, H.S.; Smirnov, I.V.; Peng, H.F.; Tiffany, K.; Jackson, V. Effects of spermine and its cytotoxic analogs on nucleosome formation on topologically stressed DNA in vitro. Eur. J. Biochem. 1997, 243, 247–258.
- Ha, H.C.; Sirisoma, N.S.; Kuppusamy, P.; Zweier, J.L.; Woster, P.M.; Casero, R.A., Jr. The natural polyamine spermine functions directly as a free radical scavenger. Proc. Natl. Acad. Sci. USA 1998, 95, 11140–11145.
- Sanford, E.J.; Drago, J.R.; Rohner, T.J.; Kessler, G.F.; Sheehan, L.; Lipton, A. Preliminary evaluation of urinary polyamines in the diagnosis of genitourinary tract malignancy. J. Urol. 1975, 113, 218–221.
- Fair, W.R.; Wehner, N.; Brorsson, U. Urinary polyamine levels in the diagnosis of carcinoma of the prostate. J. Urol. 1975, 114, 88–92.
- Sugimoto, M.; Hiramatsu, K.; Kamei, S.; Kinoshita, K.; Hoshino, M.; Iwasaki, K.; Kawakita, M. Significance of urinary N1,N8-diacetylspermidine and N1,N12-diacetylspermine as indicators of neoplastic diseases. J. Cancer Res. Clin. Oncol. 1995, 121, 317–319.
- Hiramatsu, K.; Sugimoto, M.; Kamei, S.; Hoshino, M.; Kinoshita, K.; Iwasaki, K.; Kawakita, M. Diagnostic and prognostic usefulness of N1,N8-diacetylspermidine and N1,N12-diacetylspermine in urine as novel markers of malignancy. J. Cancer Res. Clin. Oncol. 1997, 123, 539–545.
- Tsoi, T.H.; Chan, C.F.; Chan, W.L.; Chiu, K.F.; Wong, W.T.; Ng, C.F.; Wong, K.L. Urinary Polyamines: A Pilot Study on Their Roles as Prostate Cancer Detection Biomarkers. PLoS ONE 2016, 11, e0162217.
- Chiu, P.K.; Fung, Y.H.; Teoh, J.Y.; Chan, C.H.; Lo, K.L.; Li, K.M.; Tse, R.T.; Leung, C.H.; Wong, Y.P.; Roobol, M.J.; et al. Urine spermine and multivariable Spermine Risk Score predict high-grade prostate cancer. Prostate Cancer Prostatic Dis. 2021.
- van der Graaf, M.; Schipper, R.G.; Oosterhof, G.O.; Schalken, J.A.; Verhofstad, A.A.; Heerschap, A. Proton MR spectroscopy of prostatic tissue focused on the detection of spermine, a possible biomarker of malignant behavior in prostate cancer. MAGMA 2000, 10, 153–159.
- Swanson, M.G.; Vigneron, D.B.; Tabatabai, Z.L.; Males, R.G.; Schmitt, L.; Carroll, P.R.; James, J.K.; Hurd, R.E.; Kurhanewicz, J. Proton HR-MAS spectroscopy and quantitative pathologic analysis of MRI/3D-MRSI-targeted postsurgical prostate tissues. Magn. Reson. Med. 2003, 50, 944–954.
- Swanson, M.G.; Zektzer, A.S.; Tabatabai, Z.L.; Simko, J.; Jarso, S.; Keshari, K.R.; Schmitt, L.; Carroll, P.R.; Shinohara, K.; Vigneron, D.B.; et al. Quantitative analysis of prostate metabolites using 1H HR-MAS spectroscopy. Magn. Reson. Med. 2006, 55, 1257–1264.
- Maxeiner, A.; Adkins, C.B.; Zhang, Y.; Taupitz, M.; Halpern, E.F.; McDougal, W.S.; Wu, C.L.; Cheng, L.L. Retrospective analysis of prostate cancer recurrence potential with tissue metabolomic profiles. Prostate 2010, 70, 710–717.
- Nagarajan, R.; Gomez, A.M.; Raman, S.S.; Margolis, D.J.; McClure, T.; Thomas, M.A. Correlation of endorectal 2D JPRESS findings with pathological Gleason scores in prostate cancer patients. NMR Biomed. 2010, 23, 257–261.
- Garcia-Martin, M.L.; Adrados, M.; Ortega, M.P.; Fernandez Gonzalez, I.; Lopez-Larrubia, P.; Viano, J.; Garcia-Segura, J.M. Quantitative (1) H MR spectroscopic imaging of the prostate gland using LCModel and a dedicated basis-set: Correlation with histologic findings. Magn. Reson. Med. 2011, 65, 329–339.
- Giskeodegard, G.F.; Bertilsson, H.; Selnaes, K.M.; Wright, A.J.; Bathen, T.F.; Viset, T.; Halgunset, J.; Angelsen, A.; Gribbestad, I.S.; Tessem, M.B. Spermine and citrate as metabolic biomarkers for assessing prostate cancer aggressiveness. PLoS ONE 2013, 8, e62375.
- Selnaes, K.M.; Gribbestad, I.S.; Bertilsson, H.; Wright, A.; Angelsen, A.; Heerschap, A.; Tessem, M.B. Spatially matched in vivo and ex vivo MR metabolic profiles of prostate cancer—Investigation of a correlation with Gleason score. NMR Biomed. 2013, 26, 600–606.
- Basharat, M.; Payne, G.S.; Morgan, V.A.; Parker, C.; Dearnaley, D.; deSouza, N.M. TE = 32 ms vs TE = 100 ms echo-time (1)H-magnetic resonance spectroscopy in prostate cancer: Tumor metabolite depiction and absolute concentrations in tumors and adjacent tissues. J. Magn. Reson. Imaging 2015, 42, 1086–1093.
- Hansen, A.F.; Sandsmark, E.; Rye, M.B.; Wright, A.J.; Bertilsson, H.; Richardsen, E.; Viset, T.; Bofin, A.M.; Angelsen, A.; Selnaes, K.M.; et al. Presence of TMPRSS2-ERG is associated with alterations of the metabolic profile in human prostate cancer. Oncotarget 2016, 7, 42071–42085.
- Shukla-Dave, A.; Castillo-Martin, M.; Chen, M.; Lobo, J.; Gladoun, N.; Collazo-Lorduy, A.; Khan, F.M.; Ponomarev, V.; Yi, Z.; Zhang, W.; et al. Ornithine Decarboxylase Is Sufficient for Prostate Tumorigenesis via Androgen Receptor Signaling. Am. J. Pathol. 2016, 186, 3131–3145.
- Braadland, P.R.; Giskeodegard, G.; Sandsmark, E.; Bertilsson, H.; Euceda, L.R.; Hansen, A.F.; Guldvik, I.J.; Selnaes, K.M.; Grytli, H.H.; Katz, B.; et al. Ex vivo metabolic fingerprinting identifies biomarkers predictive of prostate cancer recurrence following radical prostatectomy. Br. J. Cancer 2017, 117, 1656–1664.
- Lynch, M.J.; Nicholson, J.K. Proton MRS of human prostatic fluid: Correlations between citrate, spermine, and myo-inositol levels and changes with disease. Prostate 1997, 30, 248–255.
- Serkova, N.J.; Gamito, E.J.; Jones, R.H.; O'Donnell, C.; Brown, J.L.; Green, S.; Sullivan, H.; Hedlund, T.; Crawford, E.D. The metabolites citrate, myo-inositol, and spermine are potential age-independent markers of prostate cancer in human expressed prostatic secretions. Prostate 2008, 68, 620–628.
- Cipolla, B.; Moulinoux, J.P.; Quemener, V.; Havouis, R.; Martin, L.A.; Guille, F.; Lobel, B. Erythrocyte polyamine levels in human prostatic carcinoma. J. Urol. 1990, 144, 1164–1166.
- Cipolla, B.; Guille, F.; Moulinoux, J.P.; Quemener, V.; Staerman, F.; Corbel, L.; Lobel, B. Polyamines and prostatic carcinoma: Clinical and therapeutic implications. Eur. Urol. 1993, 24, 124–131.
- Cipolla, B.; Guille, F.; Moulinoux, J.P.; Bansard, J.Y.; Roth, S.; Staerman, F.; Corbel, L.; Quemener, V.; Lobel, B. Erythrocyte polyamines and prognosis in stage D2 prostatic carcinoma patients. J. Urol. 1994, 151, 629–633.
- Cipolla, B.G.; Ziade, J.; Bansard, J.Y.; Moulinoux, J.P.; Staerman, F.; Quemener, V.; Lobel, B.; Guille, F. Pretherapeutic erythrocyte polyamine spermine levels discriminate high risk relapsing patients with M1 prostate carcinoma. Cancer 1996, 78, 1055–1065.
- Schipper, R.G.; Romijn, J.C.; Cuijpers, V.M.; Verhofstad, A.A. Polyamines and prostatic cancer. Biochem. Soc. Trans. 2003, 31, 375–380.
- Kobus, T.; Wright, A.J.; Weiland, E.; Heerschap, A.; Scheenen, T.W. Metabolite ratios in 1H MR spectroscopic imaging of the prostate. Magn. Reson. Med. 2015, 73, 1–12.
- Klomp, D.W.; Bitz, A.K.; Heerschap, A.; Scheenen, T.W. Proton spectroscopic imaging of the human prostate at 7 T. NMR Biomed. 2009, 22, 495–501.
- Xu, Z.; Wang, Y.; Xiao, Z.G.; Zou, C.; Zhang, X.; Wang, Z.; Wu, D.; Yu, S.; Chan, F.L. Nuclear receptor ERRalpha and transcription factor ERG form a reciprocal loop in the regulation of TMPRSS2:ERG fusion gene in prostate cancer. Oncogene 2018, 37, 6259–6274.
- Cohen, R.J.; McNeal, J.E.; Edgar, S.G.; Robertson, T.; Dawkins, H.J. Characterization of cytoplasmic secretory granules (PSG), in prostatic epithelium and their transformation-induced loss in dysplasia and adenocarcinoma. Hum. Pathol. 1998, 29, 1488–1494.
- Cohen, R.J.; Fujiwara, K.; Holland, J.W.; McNeal, J.E. Polyamines in prostatic epithelial cells and adenocarcinoma; the effects of androgen blockade. Prostate 2001, 49, 278–284.
- Lima, A.R.; Bastos Mde, L.; Carvalho, M.; Guedes de Pinho, P. Biomarker Discovery in Human Prostate Cancer: An Update in Metabolomics Studies. Transl. Oncol. 2016, 9, 357–370.
- Moulinoux, J.P.; Le Calve, M.; Quemener, V.; Quash, G. In vitro studies on the entry of polyamines into normal red blood cells. Biochimie 1984, 66, 385–393.
- Martinez-Outschoorn, U.E.; Peiris-Pages, M.; Pestell, R.G.; Sotgia, F.; Lisanti, M.P. Cancer metabolism: A therapeutic perspective. Nat. Rev. Clin. Oncol. 2017, 14, 11–31.
- Bettuzzi, S.; Davalli, P.; Astancolle, S.; Carani, C.; Madeo, B.; Tampieri, A.; Corti, A. Tumor progression is accompanied by significant changes in the levels of expression of polyamine metabolism regulatory genes and clusterin (sulfated glycoprotein 2) in human prostate cancer specimens. Cancer Res. 2000, 60, 28–34.
- Dunzendorfer, U.; Relyea, N.M.; Kleinert, E.; Balis, M.E.; Whitmore, W.F., Jr. Antigrowth effect of some inhibitors of polyamine synthesis on transplantable prostate cancer. Oncology 1983, 40, 57–62.
- Herr, H.W.; Kleinert, E.L.; Relyea, N.M.; Whitmore, W.F., Jr. Potentiation of methylglyoxal-bis-guanylhydrazone by alpha-difluoromethylornithine in rat prostate cancer. Cancer 1984, 53, 1294–1298.
- Delworth, M.; Nishioka, K.; Pettaway, C.; Gutman, M.; Killion, J.; Voneschenbach, A.; Fidler, I. Systemic administration of 4-amidinoindanon-1-(2′-amidino)-hydrazone, a new inhibitor of s-adenosylmethionine decarboxylase, produces cytostasis of human prostate-cancer in athymic nude-mice. Int. J. Oncol. 1995, 6, 293–299.
- Heston, W.D.; Kadmon, D.; Lazan, D.W.; Fair, W.R. Copenhagen rat prostatic tumor ornithine decarboxylase activity (ODC) and the effect of the ODC inhibitor alpha-difluoromethylornithine. The Prostate 1982, 3, 383–389.
- Kadmon, D. Chemoprevention in prostate cancer: The role of difluoromethylornithine (DFMO). J. Cell. Biochem. 1992, 50 (Suppl. 16H), 122–127.
- Devens, B.H.; Weeks, R.S.; Burns, M.R.; Carlson, C.L.; Brawer, M.K. Polyamine depletion therapy in prostate cancer. Prostate Cancer Prostatic Dis. 2000, 3, 275–279.
- Herr, H.W.; Warrel, R.P.; Burchenal, J.H. Phase I trial of alpha-difluoromethyl ornithine (DFMO) and methylglyoxal bis (guanylhydrazone) (MGBG) in patients with advanced prostatic cancer. Urology 1986, 28, 508–511.
- Horn, Y.; Schechter, P.J.; Marton, L.J. Phase I-II clinical trial with alpha-difluoromethylornithine--an inhibitor of polyamine biosynthesis. Eur. J. Cancer Clin. Oncol. 1987, 23, 1103–1107.
- Carbone, P.P.; Douglas, J.A.; Larson, P.O.; Verma, A.K.; Blair, I.A.; Pomplun, M.; Tutsch, K.D. Phase I chemoprevention study of piroxicam and alpha-difluoromethylornithine. Cancer Epidemiol. Biomark. Prev. 1998, 7, 907–912.
- Wallace, H.M.; Niiranen, K. Polyamine analogues—An update. Amino Acids 2007, 33, 261–265.
- Gupta, S.; Ahmad, N.; Marengo, S.R.; MacLennan, G.T.; Greenberg, N.M.; Mukhtar, H. Chemoprevention of prostate carcinogenesis by alpha-difluoromethylornithine in TRAMP mice. Cancer Res. 2000, 60, 5125–5133.
- Simoneau, A.R.; Gerner, E.W.; Nagle, R.; Ziogas, A.; Fujikawa-Brooks, S.; Yerushalmi, H.; Ahlering, T.E.; Lieberman, R.; McLaren, C.E.; Anton-Culver, H.; et al. The effect of difluoromethylornithine on decreasing prostate size and polyamines in men: Results of a year-long phase IIb randomized placebo-controlled chemoprevention trial. Cancer Epidemiol. Biomark. Prev. 2008, 17, 292–299.
- Jeffers, L.; Church, D.; Basu, H.; Marton, L.; Wilding, G. Effects of the polyamine analogues BE-4-4-4-4, BE-3-7-3, and BE-3-3-3 on the proliferation of three prostate cancer cell lines. Cancer Chemother. Pharmacol. 1997, 40, 172–179.
- Zagaja, G.P.; Shrivastav, M.; Fleig, M.J.; Marton, L.J.; Rinker-Schaeffer, C.W.; Dolan, M.E. Effects of polyamine analogues on prostatic adenocarcinoma cells in vitro and in vivo. Cancer Chemother. Pharmacol. 1998, 41, 505–512.
- McCloskey, D.E.; Woster, P.M.; Casero, R.A., Jr.; Davidson, N.E. Effects of the polyamine analogues N1-ethyl-N11-((cyclopropyl)methyl)-4,8-diazaundecane and N1-ethylN-11-((cycloheptyl)methyl)-4,8-diazaundecane in human prostate cancer cells. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2000, 6, 17–23.
- Reddy, V.K.; Sarkar, A.; Valasinas, A.; Marton, L.J.; Basu, H.S.; Frydman, B. cis-Unsaturated analogues of 3,8,13,18,23-pentaazapentacosane (BE-4-4-4-4): Synthesis and growth inhibitory effects on human prostate cancer cell lines. J. Med. Chem. 2001, 44, 404–417.
- Valasinas, A.; Sarkar, A.; Reddy, V.K.; Marton, L.J.; Basu, H.S.; Frydman, B. Conformationally restricted analogues of 1N,14N-bisethylhomospermine (BE-4-4-4): Synthesis and growth inhibitory effects on human prostate cancer cells. J. Med. Chem. 2001, 44, 390–403.
- Frydman, B.; Blokhin, A.V.; Brummel, S.; Wilding, G.; Maxuitenko, Y.; Sarkar, A.; Bhattacharya, S.; Church, D.; Reddy, V.K.; Kink, J.A.; et al. Cyclopropane-containing polyamine analogues are efficient growth inhibitors of a human prostate tumor xenograft in nude mice. J. Med. Chem. 2003, 46, 4586–4600.
- Valasinas, A.; Reddy, V.K.; Blokhin, A.V.; Basu, H.S.; Bhattacharya, S.; Sarkar, A.; Marton, L.J.; Frydman, B. Long-chain polyamines (oligoamines) exhibit strong cytotoxicities against human prostate cancer cells. Bioorg. Med. Chem. 2003, 11, 4121–4131.
- Frydman, B.; Bhattacharya, S.; Sarkar, A.; Drandarov, K.; Chesnov, S.; Guggisberg, A.; Popaj, K.; Sergeyev, S.; Yurdakul, A.; Hesse, M.; et al. Macrocyclic polyamines deplete cellular ATP levels and inhibit cell growth in human prostate cancer cells. J. Med. Chem. 2004, 47, 1051–1059.
- Kee, K.; Vujcic, S.; Merali, S.; Diegelman, P.; Kisiel, N.; Powell, C.T.; Kramer, D.L.; Porter, C.W. Metabolic and antiproliferative consequences of activated polyamine catabolism in LNCaP prostate carcinoma cells. J. Biol. Chem. 2004, 279, 27050–27058.
- Kee, K.; Foster, B.A.; Merali, S.; Kramer, D.L.; Hensen, M.L.; Diegelman, P.; Kisiel, N.; Vujcic, S.; Mazurchuk, R.V.; Porter, C.W. Activated polyamine catabolism depletes acetyl-CoA pools and suppresses prostate tumor growth in TRAMP mice. J. Biol. Chem. 2004, 279, 40076–40083.




