
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | David Katerere | + 1445 word(s) | 1445 | 2021-05-08 04:40:09 | | | |
| 2 | Lindsay Dong | Meta information modification | 1445 | 2021-05-10 12:01:25 | | |
Video Upload Options
Plants that exhibit foaming properties when agitated in aqueous solutions are commonly referred to as soapy plants, and they are used in different communities for washing, bathing, and hair shampooing. The frothing ability of these plants is attributed to saponins which are also well-documented to possess antimicrobial attributes.
1. Introduction
Most medicinal and health-promoting properties of plants are due to secondary metabolites which exist in forms and concentrations which vary with species of plants and plant parts. Geographical, environmental, and climatic conditions also affect the types and amounts of phytochemicals [1]. Some of the most common classes of phytochemicals are terpenoids, saponins, tannins, flavonoids, and alkaloids [2][3].
Modern toilet soaps and detergents trace their origin to the ancient use of plants, commonly referred to as soapy plants, which possess foaming ability when they are agitated in water. The soapy properties of these plants are attributed to the presence of saponins and their scientific nomenclature arose as a result of this; for example, the genus Saponaria is made up of saponin-rich plants commonly called soapworts [4]. However, there are other genera such as Sapindaceae [5][6], Aceraceae [7][8], and Hippocastanaceae [9][10], whose saponin concentrations are also high. Saponins are identified by their ability to rupture erythrocytes or form colloidal solutions that can produce a stable lather when they are shaken in the presence of water [11].
Various studies have been published that reported the use of plants for oral hygiene purposes [12][13]. Moreover, there is a surge of sites on the internet that focus on formulation of plant-based shampoos, and this is probably due to the increase in the demand for commercial shampoo [14]. However, there is very little literature on the use of plants for hand hygiene. This highlights the fact that this is a neglected area of plant utilization. It may be that there is an assumption that hand and toilet soap are accessible to everyone in the world. This is not the case because the UN reported that about three billion people (which is 40% of the world’s population) survive without basic handwashing facilities with soap and water available in their homes [15]. Thus, a focus on the application of ethnobotany to hand and toilet hygiene is a unique approach on the role plants can play in enhancing community health. This paper tries to integrate what is known about saponins, their utility as soaps and how they can be applied in the community, either directly by communities which have no access to commercial soap, or by companies or non-governmental organizations formulating soaps using local resources.
A possible strategy to increase soap availability and affordability would be the use of the floristic heritage in many poor and rural communities. Soapy plants present natural sources of soaps with putative antimicrobial and disinfectant properties [16] which can be used in their natural forms as extracts or formulated into finished low-cost products. In order to do this, we saw the need for an in-depth review of literature and synthesis of knowledge on saponin-rich plants generally and Southern African flora in particular.
2. Saponin-Rich Plants
As shown in Figure 1, saponins are high molecular weight organic molecules, which form glycone (saccharide) and aglycone (non-saccharide) moieties upon hydrolysis [17][18]. The glycone part of saponins consists of one or two sugar moieties. The saccharide moiety is usually in the form of either pentoses or hexoses and is water soluble. It has been noted that arabinose, xylose, glucose, ribose, glucuronic acid, and rhamnose are the most commonly occurring saccharide moieties in saponins [18][19]. The part which is non-soluble in water, also referred to as the sapogenin or genin, is characterized by 27 to 30 carbon atoms [14] and is either a triterpene or a steroid [18]. Depending on the type of aglycone that they have, saponins are classified into triterpenoid or steroid saponins, the former of which is more common in the plant kingdom [2].
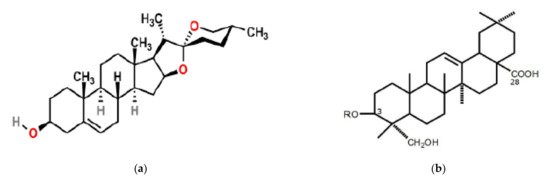
Figure 1. Possible structures of steroid saponins, with a steroid aglycone (a) (source [19]) and a triterpenoid aglycone (b) (source: [20]).
Saponins are present in different plants, in different quantities ranging from low to high. Table 1 gives information on plants that were found to have various concentrations of saponins, through different quantification techniques, by different researchers around the world. It is also worth noting that a 40 mg/ g concentration of saponins in plants was the benchmark for inclusion in this review.
Table 1. Plants which are rich in saponins as part of their phytochemical components.
| Plant Name and Family | Common Name(s) | Geographical Location | Plant Part Used | Approximate Saponin Amounts (mg/g) | Type of Extract | References |
|---|---|---|---|---|---|---|
| Adoxaceae | ||||||
| Viburnum cotinifolium D. Don | Smoke-tree leaved virbunum | Atlas Mountains (Northwest Africa) | Leaves | 45.30 | Aqueous ethanol | [21] |
| Aizoaceae | ||||||
| Carpobrotus edulis (L.) N. E. Br. | Sour fig, ice plant | South Africa | Leaves, stems | 45.00 | Ethanol | [22][23] |
| Amaranthaceae | ||||||
| Amaranthus hybridus L. | Pigweed | Southern Africa | Stem, leaves | 184.00 | Not stated | [24] |
| Spinacia oleracea L. | Spinach | Lesotho, Highveld of Southern Africa | Leaves | 52.70 | Methanol | [25] |
| Anacardiceaceae | ||||||
| Mangifera indica L. | Mango | Southern Africa | Ripe peels | 214.15 | Methanol | [26] |
| Unripe peels | 159.50 | |||||
| Annonaceae | ||||||
| Annona squamosa L. | Sugar apple | Madagascar, Malawi, Mozambique | Fruit | 63.88 | Aqueous | [27] |
| Monodora myristica (Gaertn) Dunal | African nutmeg | Western and Eastern Africa | Seeds | 120.40 | Not stated | [28] |
Apart from foaming properties, saponins are typically identified by their ability to exhibit hemolytic activity, which also renders them the ability to disrupt microbial cells. Thus, plants that exhibit high hemolytic activity are more likely to have more saponins.
As highlighted in Table 1, saponin-containing plants fall under a variety of taxa, though Fabaceae and Lamiaceae families were the most common in this review. We also noted that the quantities of saponins in plants still vary, even within families. There is evidence that environmental conditions, tissue type, age, physiological state, and genetic profiles of plants also impact on the concentration of phytochemicals present within a plant [18]. Saponin content in different parts of a single plant may also vary [29]. The results that are shown in Table 1 suggest that researchers use different solvents for extracting saponins, and this is more likely to affect the amount of quantifiable saponins that are extracted.
3. Use of Saponin-Rich Plants
3.1. Traditional Uses
Some plants have been documented to be used traditionally as natural soaps and shampoos as shown in Table 2. Only a small sub-set of saponin-rich plant species are traditionally utilized. Table 2 shows saponin-rich plants that are being traditionally utilized, as compared to a larger number of saponin-rich plants, as shown by Table 1, that are not being used for their soapy characteristics. This may be because of lack of knowledge about, and access to, the plants.
Table 2. Plants that have been, and are still, used as soaps and shampoos by various communities.
| Family and Scientific Name | English Common Name | Plant Part | Preparation and Use(s) | References |
|---|---|---|---|---|
| Aloaceae | [30] | |||
| Aloe maculata All | Soap Aloe | Leaves | The sap from the leaves is used as a soap for bathing and washing hair. | |
| Aloe Saponaria Mill | Soap Aloe | Leaves | The sap is used as soap for bathing. | |
| Caryophyllaceae | ||||
| Saponaria officinalis L. | Soapwort | Leaves | The leaves of the plant are added to pre-boiled water and left to simmer for about 5 min. | [31][32][33] |
| Fabaceae | ||||
| Acacia concinna Linn | Soap pod tree | Pods, bark | Roots that are boiled with water are used as soap. The dried and crushed bark forms a powder which is used as soap. | [34][35] |
| Albizia versicolor Welw. Ex Oliv | Large-leaved false thorn | Root, bark | [36] | |
| Malvaceae | ||||
| Sida rhombifolia L (Bhuinli) | Mallows. fanpetals | Tender shoot bark | The tender shoot bark is rubbed on the skin or hair to produce lather during bathing and shampooing. | [37] |
| Pedaliaceae | ||||
| Dicerocaryum eriocarpum (Decne.) Abels. | Devil’s thorn, boot protectors | Flowers | The flowers are soaked in water to produce soapy water. | [38] |
| Sesamum angolense Welw | Leaves | An infusion of the leaves is used as soap for bathing and shampooing. | [39] | |
| Quillajaceae | ||||
| Quillaja saponaria Mollina | Soap bark | Bark | The inner bark is reduced to powder and used as a soap. | [40] |
3.2. Topical Use
Saponins have been reported to be active ingredients in some patented dermatological products which are topically applied for haircare.
3.3. Antimicrobial Use
Numerous studies show evidence that saponins possess potent antiviral, [41][42][43][44][45][46], antibacterial, and antifungal activities [47][48][49][50].
3.4. Activity against Viruses
An in vivo experimental study on saponin extracts from Quillaja saponaria Mollina demonstrated antiviral activity of the extracts against rotavirus in mice [44]. Triterpenoid saponins from P. anserine inactivated the Hepatis B virus by inhibiting the replication of its DNA.
3.5. Activity against Bacteria
Saponin extracts that were prepared from Sorghum bicolor L Moench were tested for their antimicrobial activity against Escherichia coli, Candida albicans, and Staphylococcus aureus. The results showed inhibition of S. aureus [47].
3.6. Activity against Fungi
Saponins are active against various types of fungi, including commercially important yeasts. Another piece of research revealed the antifungal properties against Saccaromyces cerevisiae by saponins from M. sativa L. and Medicago arborea L. [50]. Much more evidence on the antifungal activity of saponins has been reported from various studies [51][52][53][54].
References
- Applequist, W.L.; Brinckmann, J.A.; Cunningham, A.B.; Hart, R.E.; Heinrich, M.; Katerere, D.R.; van Andel, T. Scientists’ warning on climate change and medicinal plants. Planta Med. 2020, 86, 10–18.
- Alara, O.R.; Abdurahman, N.H.; Ukaegbu, C.I.; AzharI, N.H.; Kabbashi, N.A. Metabolic profiling of flavonoids, saponins, alkaloids, and terpenoids in the extract from Vernonia cinerea leaf using LC-Q-TOF-MS. J. Liq. Chromatogr. Relat. Technol. 2018, 41, 722–731.
- Saxena, M.; Saxena, J.; Nema, R.; Singh, D.; Gupta, A. Phytochemistry of medicinal plants. J. Pharmacogn. Phytochem. 2013, 1, 168–182.
- Osbourn, A. Saponins and plant defence—A soap story. Trends Plant Sci. 1996, 1, 4–9.
- Qasim, M.; Islam, W.; Ashraf, H.J.; Ali, I.; Wang, L. Saponins in Insect Pest Control. Co Evol. Second. Metab. 2020, 897–924.
- Voutquenne, L. Saponins and hemolytic activity. Saponins and glycosides from five species of Sapindaceae. Ann. Pharm. Fr. 2001, 59, 407–414.
- Glénsk, M.; Włodarczyk, M.; Bassarello, C.; PIzza, C.; Stefanowicz, P.; Świtalska, M. A major saponin from leaves extract of Acer velutinum. Z. Nat. B 2009, 64, 1081–1086.
- Kurimoto, S.I.; Sasaki, Y.F.; Suyama, Y.; Tanaka, N.; Kashiwada, Y.; Nakamura, T. Acylated triterpene saponins from the stem bark of cernikoense (Aceraceae). Chem. Pharm. Bull. 2016, 64, 924–929.
- Cheng, J.T.; Chen, S.T.; Guo, C.; Jiao, M.J.; Cui, W.J.; Wang, S.H.; Deng, Z.; Chen, C.; Chen, S.; Zhang, J. Triterpenoid Saponins from the seeds of Aesculus chinensis and their cytotoxicities. Nat. Prod. Bioprospect. 2018, 8, 47–56.
- Karatoprak, G.Ş. Horse Chestnut. In Nonvitamin and Nonmineral Nutritional Supplements; Elsevier: Amsterdam, The Netherlands, 2019.
- Sparg, S.; Light, M.; van Staden, J. Biological activities and distribution of plant saponins. J. Ethnopharmacol. 2004, 94, 219–243.
- Bodiba, D.; Szuman, K.M.; Lall, N. The role of medicinal plants in oral care. In Medicinal Plants for Holistic Health and Well-Being; Academic Press: Cambridge, MA, USA, 2018; pp. 183–212.
- Muhammad, S.; Lawal, M.T. Oral hygiene and the use of plants. Sci. Res. Essays 2010, 5, 1788–1795.
- Peace with the Wild. Available online: (accessed on 16 January 2021).
- United Nations. Available online: (accessed on 21 January 2021).
- Kregiel, D.; Berlowska, J.; Witonska, I.; Antolak, H.; Proestos, C.; Babic, M.; Babic, L.; Zhang, B. Saponin-based, biological-active surfactants from plants. Appl. Charact. Surfactants 2017, 6, 184–205.
- Hostettmann, K.; Marston, A. Saponins; Cambridge University Press: Cambridge, UK, 2005.
- Desai, S.D.; Desai, D.G.; Kaur, H. Saponins and their biological activities. Pharma Times 2009, 41, 13–16.
- El Aziz, M.; Ashour, A.; Melad, A. A review on saponins from medicinal plants: Chemistry, isolation, and determination. J. Nanomed. Res. 2019, 8, 6–12.
- Abed El Aziz, M.M.; Ashour, A.S.; Madbouly, H.A.; Melad, A.S.; El Kerikshi, K. Investigations on green preparation of heavy metal saponin complexes. J. Water Environ. Nanotechnol. 2017, 2, 103–111.
- Khan, A.M.; Qureshi, R.A.; Ullah, F.; Gilani, S.A.; Nosheen, A.; Sahreen, S.; Laghari, M.K.; Laghari, M.Y.; Hussain, I.; Murad, W. Phytochemical analysis of selected medicinal plants of Margalla Hills and surroundings. J. Med. Plants Res. 2011, 5, 6055–6060.
- Mudimba, T.N.; Nguta, J.M. Traditional uses, phytochemistry and pharmaco-logical activity of Carpobrotus edulis: A global perspective. J. Phytopharm. 2019, 8, 111–116.
- Omoruyi, B.E.; Bradley, G.; Afolayan, A.J. Antioxidant and phytochemical properties of Carpobrotus edulis (L.) bolus leaf used for the management of common infections in HIV/AIDS patients in Eastern Cape Province. BMC Complementary Altern. Med. 2012, 12, 1–9.
- Okoye, E. Qualitative and quantitative phytochemical analysis and antimicrobial screening of solvent extracts of Amaranthus hybridus (stem and leaves). Chem. Res. J. 2018, 3, 9–13.
- Soni, A.; Sosa, S. Phytochemical analysis and free radical scavenging potential of herbal and medicinal plant extracts. J. Pharmacogn. Phytochem. 2013, 2, 22–29.
- Falusi, V.; Adesina, I.; Aladejimokun, A.; Elehinafe, T. Phytochemical Screening and Antibacterial Activity of Methanolic Extracts of Ripe and Unripe Peels of Mango (Mangifera indica L.). J. Appl. Life Sci. Int. 2017, 14, 1–7.
- Madhu, M.; Sailaja, V.; Satyadev, T.; Satyanarayana, M. Quantitative phytochemical analysis of selected medicinal plant species by using various organic solvents. J. Pharmacogn. Phytochem. 2016, 5, 25–29.
- Nkwocha, C.C.; Nworah, F.N.; Okagu Innocent, U.; Nwagwe, O.R. Proximate and Phytochemical Analysis of Monodora myristica (African Nutmeg) from Nsukka, Enugu State, Nigeria. J. Food Nutr. Res. 2018, 6, 597–601.
- Oboh, G. Effect of blanching on the antioxidant properties of some tropical green leafy vegetables. LWT Food Sci. Technol. 2005, 38, 513–517.
- Sedaghathoor, S.; Kojeidi, M.I.; Poormassalegoo, A. Study on the effect of brassinolide and salicylic acid on vegetative and physiological traits of Aloe maculata All. in different substrates in a pot experiment. J. Appl. Res. Med. Aromat. Plants 2017, 6, 111–118.
- Jia, Z.; Koike, K.; Nikaido, T. Major triterpenoid saponins from Saponaria officinalis. J. Nat. Prod. 1998, 61, 1368–1373.
- Mitich, L.W. Bouncingbet–The soap weed. Weed Technol. 1990, 4, 221–223.
- Herbal Academy. Natural Soapwort Shampoo and Body Wash. 2015. Available online: (accessed on 4 January 2021).
- Rakesh, M.R.; Ashok, K.; Kumar, S.A.; Amitabh, T. Formulation of herbal shampoos from Asparagus racemosus, Acacia concinna, Sapindus mukorossi. Int. J. Pharm Sci. Rev. Res. 2010, 4, 39–44.
- Gaikwad, D.; Undale, K.; Kalel, R.; Patil, D. Acacia concinna pods: A natural and new bioreductant for palladium nanoparticles and its application to Suzuki–Miyaura coupling. J. Iran. Chem. Soc. 2019, 16, 2135–2141.
- Long, C. Swaziland’s Flora- siSwati Names and Uses. 2005. Available online: (accessed on 28 December 2020).
- Mehta, P.; Bhatt, K. Traditional Soap and Detergent Yielding Plants of Uttaranchal. 2007. Available online: (accessed on 2 January 2021).
- Odiyo, J.; Bassey, O.; Ochieng, A.; Chimuka, L. Coagulation efficiency of Dicerocaryum eriocarpum (DE) plant. Water SA 2017, 43, 1–6.
- Flora of Zimbabwe. Deinbollia xanthocarpa (Klotzsch) Radlk. 2018. Available online: (accessed on 14 November 2020).
- Van Setten, D.C.; van de Werken, G. Molecular structures of saponins from Quillaja saponaria Molina. In Saponins Used in Traditional and Modern Medicine; Springer: Berlin/Heidelberg, Germany, 1996.
- Yendo, A.C.A.; de Costa, F.; Cibulski, S.P.; Teixeira, T.F.; Colling, L.C.; Mastrogiovanni, M.; Soulé, S.; Roehe, P.M.; Gosmann, G.; Ferreira, F.A. A rabies vaccine adjuvanted with saponins from leaves of the soap tree (Quillaja brasiliensis) induces specific immune responses and protects against lethal challenge. Vaccine 2016, 34, 2305–2311.
- Hayashi, K.; Sagesaka, Y.M.; Suzuki, T.; Suzuki, Y. Inactivation of human type A and B influenza viruses by tea-seed saponins. Biosci. Biotechnol. Biochem. 2000, 64, 184–186.
- Lee, J.; Lim, S.; Kang, S.M.; Min, S.; Son, K.; Lee, H.S.; Park, E.M.; Ngo, H.T.; Tran, H.T.; Lim, Y.S. Saponin inhibits hepatitis C virus propagation by up-regulating suppressor of cytokine signaling 2. PLoS ONE 2012, 7, e39366.
- Tam, K.I.; Roner, M.R. Characterization of in vivo anti-rotavirus activities of saponin extracts from Quillaja saponaria Molina. Antivir. Res. 2011, 90, 231–241.
- Sindambiwe, J.; Calomme, M.; Geerts, S.; Pieters, L.; Vlietinck, A.; Vanden Berghe, D. Evaluation of biological activities of triterpenoid saponins from Maesa lanceolata. J. Nat. Prod. 1998, 61, 585–590.
- Zhao, Y.L.; Cai, G.M.; Hong, X.; Shan, L.M.; Xiao, X.H. Anti-hepatitis B virus activities of triterpenoid saponin compound from potentilla anserine L. Phytomedicine 2008, 15, 253–258.
- Oyekunle, M.; Aiyelaagbe, O.; Fafunso, M. Evaluation of the antimicrobial activity of saponins extract of Sorghum bicolor L. Moench. Afr. J. Biotechnol. 2006, 5, 2405–2407.
- Dong, S.; Yang, X.; Zhao, L.; Zhang, F.; Hou, Z.; Xue, P. Antibacterial activity and mechanism of action saponins from Chenopodium quinoa Willd. husks against foodborne pathogenic bacteria. Ind. Crop. Prod. 2020, 149, 112350.
- Sonfack, G.; Fossi, T.C.; Simo, I.K.; Bitchagno, G.T.M.; Nganou, B.K.; Çelik, İ.; Tene, M.; Funda Görkem, S.; Opatz, T.; Penlap Beng, V. Saponin with antibacterial activity from the roots of Albizia adianthifolia. Nat. Prod. Res. 2019, 1–9.
- Avato, P.; Bucci, R.; Tava, A.; Vitali, C.; Rosato, A.; Bialy, Z.; Jurzysta, M. Antimicrobial activity of saponins from Medicago sp.: Structure-activity relationship. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2006, 20, 454–457.
- Sautour, M.; Miyamoto, T.; Lacaille-Dubois, M.A. Steroidal saponins from Smilax medica and their antifungal activity. J. Nat. Prod. 2005, 68, 1489–1493.
- Tsuzuki, J.K.; Svidzinski, T.I.; Shinobu, C.S.; Silva, L.F.; Rodrigues-Filho, E.; Cortez, D.A.; Ferreira, I.C. Antifungal activity of the extracts and saponins from Sapindus saponaria L. An. Acad. Bras. Cienc. 2007, 79, 577–583.
- Escalante, A.M.; Santecchia, C.B.; López, S.N.; Gattuso, M.A.; Ravelo, A.G.; Delle, M.F.; Sierra, M.G.; Zacchino, S.A. Isolation of antifungal saponins from Phytolacca tetramera, an Argentinean species in critic risk. J. Ethnopharmacol. 2002, 82, 29–34.
- Chapagain, B.P.; Wiesman, Z.; Tsror, L. In vitro study of the antifungal activity of saponin-rich extracts against prevalent phytopathogenic fungi. Ind. Crop. Prod. 2007, 26, 109–115.





