Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Damayanti Damayanti | + 3296 word(s) | 3296 | 2021-05-07 12:52:59 | | | |
| 2 | Catherine Yang | Meta information modification | 3296 | 2021-05-08 08:02:21 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Damayanti, D. The Polyethylene Terephthalate. Encyclopedia. Available online: https://encyclopedia.pub/entry/9403 (accessed on 08 February 2026).
Damayanti D. The Polyethylene Terephthalate. Encyclopedia. Available at: https://encyclopedia.pub/entry/9403. Accessed February 08, 2026.
Damayanti, Damayanti. "The Polyethylene Terephthalate" Encyclopedia, https://encyclopedia.pub/entry/9403 (accessed February 08, 2026).
Damayanti, D. (2021, May 08). The Polyethylene Terephthalate. In Encyclopedia. https://encyclopedia.pub/entry/9403
Damayanti, Damayanti. "The Polyethylene Terephthalate." Encyclopedia. Web. 08 May, 2021.
Copy Citation
The chemical recycling of PET is reviewed, such as pyrolysis, hydrolysis, methanolysis, glycolysis, ionic-liquid, phase-transfer catalysis and combination of glycolysis–hydrolysis, glycolysis–methanolysis and methanolysis–hydrolysis.
polyethylene terephthalate
pyrolysis
hydrolysis
1. Introduction
The most significant application of polymers in the last two decades is polyethylene terephthalate (PET), which is of excellent chemical and physical properties for many implementations, for instance, characteristic of the gas barrier, low diffusivity, excellent mechanical and thermo mechanical properties, highly inert material, clearness and fine process operation [1][2][3][4]. On the other hand, PET waste is already highlighting for humans and the environment. The global cumulative amount of plastic waste generated from 1950–2015 was approximately 6.3 billion tons, around 9% of which had been recycled, 12%was incinerated and 79% was accumulated in landfills or the natural environment [5]. Each year, the forecast PET waste about million metric tons transfers into the ocean and landfill. Currently, the recycling plastic methods are landfill, incineration and energy recovery and plastic recycling. The conventional landfill and incineration methods were concerned since the plastic component can be released into the ambient environment during processing. The landfill and incineration methods have hazardous substances released into the environment [6][7][8]. Most plastics are nondegradable, and take a long time to degrade, probably takes hundred years; however, nobody knows precisely when the plastic is degraded at the landfill. Based on the Environment Protection Agency, plastic recycling is divided into three-part, (i) be used directly, (ii) be undergone physical reprocessing, for instance, grinding, melting and reforming, and (iii) be undergone chemical processing when components are isolated and reprocessed for use in the chemical industry [9][10][11].
Several strategies can be applied to reduce the waste of PET until 2040. The approach to zero plastic pollution is divided into four critical types of interventions: reduce, substitute, recycle and dispose. Moreover, the eight actions can be implemented: (1) minimize the quantity of single used plastic (2) replaced the petroleum plastic with the other variant of materials and delivery systems (3) implementing design for recycling (4) raising the capacity of the collection (5) enlarge the capacity of sorting and mechanical recycling (6) increasing chemical conversion capacity (7) minimize post-collection environmental leakage (8) the trade of plastic become decrease slightly [12]. PET has a low modulus of synthetic fibers, its properties closeness to the other polymer, for instance, polyethylene, nylon and polyester [13][14]. The recycling process of PET can be conducted using mechanical and chemical processes. The primary purpose of recycling PET is to modify the polymer of PET into economically reusable forms. PET chemical recycling was broadly used in the chemical products such as polyester molding compound, varnishes, polymer plaster, topcoats of reinforced plastic, mortar and mineral filler, fiber, polyol for polyurethane elastomer, polyurethane with low flammability and foam [15]. The primary purpose of recycling PET to modify the polymer of PET into economically reusable forms; moreover, the critical point out of recycling PET is not only to reduce the cost of process production but also to maintaining an ecological balance is essential for sustainable to save our planet [16][17][18]. However, the cost of the chemical recycling of PET is higher than that of physical recycling of PET. For that reason, the innovation of technology to the chemical recycling of PET is needed.
2. The Chemical Recycling of PET
The conventional plastic stable carbon-based backbone makes the plastic resistant to depolymerization under different types of environmental conditions. Since plastic has good properties of materials, the production of plastic is increasing in the current decade. Furthermore, the plastic product’s significant application is short-term living, disposable packaging materials becoming waste after a single-use. Therefore, an enormous quantity of plastic waste is accumulating in the environment. Moreover, it already a severe problem of waste plastic because of poor biodegradability with detrimental effects to terrestrial and marine ecosystems and eventually on humans. On the other hand, plastic has many negative impacts, and the researchers try to develop some method/technology to recycling the PET [19][20][21].
Chemical recycling involves depolymerization, purification and then repolymerization. The best conceptual recycling of post-consumer waste is to modify the PET into their monomer as chemical raw material. The remarkable chemical properties of a polymer depend on designated use. PET is a partial–crystalline structure. The properties of the crystalline structure are high strength and tensile. There are so many technologies to recycle PET to obtain the recycled PET (rPET), for instance, packaging, textile and electronic compounds. Dias et al. studied the effect of thermal decomposition of rPET. The activation energy of rPET was 193 CV/Kj mol−1. The film absorption spectrum of rPET shows the features of 1722 cm−1 related to carbonyl esters (C=O), within 1024 and 1259 cm−1 regarding with ester (CO) bond, at 731 cm−1, p-substitution of the aromatic ring conjugated with the carbonyl at 2973 cm−1, stretching of the CH bond [22]. The alcoholysis of PET was heated with superheated methanol vapors. The principal product distributions are the combination of DMT, phthalate derivatives and alcohols. The complicated product distribution makes the separation process of the alcoholysis process more expensive. Furthermore, the catalyst should be deactivated to prevent transesterification of DMT with EG into diethylene glycol terephthalate and PET [23].
Some of the organic bases, e.g., 1,5,7-triazabicyclo[4.4.0]dec-5-ene(TBD), 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) and 1,5-diazabicyclo[4.3.0]non-5-ene (DBN), can be applied to depolymerization of PET to small molecules of monomer. The glycolysis process’s operation conditions are at high pressure and temperatures up to 1.5–2 MPa and 250 °C, respectively. TBD was used as excess to convert PET to BHET, with a yield of up to 78% [24]. Furthermore, the PET chemical recycling based on environmentally friendly was developed by Pulido et al. In contrast, the separation process could occur from traditional methods such as distillation, crystallization. The range of ideal pore size rPET membrane was 35–100 nm with non-solvent-induced phase separation in ethanol or methanol. In addition, the resistances of membranes could be elevated the acid and oxidative media, for example, dimethylformamide at 100 °C [25]. The other advantage of recycling waste PET is used to active electrochemical material for energy storage. Some chemical processes are applied, such as the dissolution of rPET, fiberization through electrospinning and carbonization by a furnace [26].
Chemical recycling includes various methods such as glycolysis, methanolysis, hydrolysis, ammonolysis and aminolysis, which are usually carried out at high temperatures and in the presence of catalysts. Figure 1 shows that the possible reaction to recycling terephthalate [27]. The technologies of chemical recycling can be categories into two parts which are (1) the technologies that the polymers in the PET are dissolved as a long chain of the polymer and (2) the other methods are crack the chemical bonds within atoms in the polymer chains. In condensation polymers, for instance, polyesters and polyamides (PAs), the standard approach is to crack the ester or amide long chain. In contrast, in polyolefins (polyethylene and polypropylene), one has to break the relatively stable carbon chain [28].
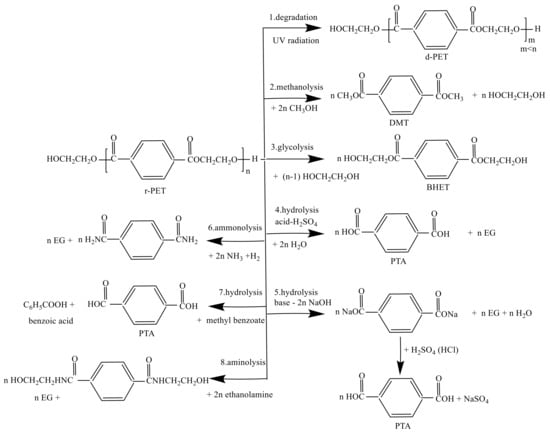
Figure 1. The 8 possible reactions of recycling PET including methanolysis, glycolysis, hydrolysis, ammonolysis and aminolysis.
The main objective of the chemical recycling of PET is to degrade the PET completely into several monomers, e.g., TPA, DMT, BHET and EG. Paszun and Spychaj [23] already present the advantages and disadvantages of the chemical recycling methods of PET. The depolymerization of PET is taking place to reverse the chemical reaction of the PET formation route. Furthermore, the degradation of PET can be degraded into its monomer or other chemical substances [29]. Moreover, the implementation of chemical recycling was derived with four groups used as (1) refinery raw materials, (2) fuel production, (3) petrochemical and (4) chemical upcycling [30]. PET is very vulnerable to chemical degradation. The eight possible reactions to recycling PET are shown in Figure 1. Table 1 lists the top industrial rPET production and their location for chemical recycling.
Table 1. Industrial rPET production and their locations.
| Company | Reaction | Country/Region | Ref |
|---|---|---|---|
| FENC | hydrolysis | Taiwan | [31] |
| Gr3n | hydrolysis | Switzerland | [32] |
| JEPLAN (PRT) | glycolysis | Japan | [33] |
| Garbo | glycolysis | Italy | [34] |
| IFPEN | glycolysis | France | [35] |
| Ioniqa | glycolysis | Netherlands | [36] |
| PerPETual | glycolysis | UK | [37] |
| Poseidon Plastics | glycolysis | UK | [38] |
| Eastman | methanolysis | USA | [39] |
| Loop industries | methanolysis | Canada | [40] |
| DePoly | Switzerland | [41] | |
| Carbios | enzyme | French | [42] |
| Agiylx | pyrolysis | USA | [43] |
| Pyrowave | microwave radiation | USA | [43] |
3. The Recent Development of PET Chemical Recycling
3.1. PET Recycling Using Microwave Irradiation
The PET recycling by microwave irradiation takes more attention from many researchers. The recycling process by microwave method allows relatives to take a short reaction time with much higher energy to heat consumption than the conventional heating process. Nevertheless, the benefit of microwave irradiation of PET by glycolysis process is still cost-prohibitive due to the energy consumptions. The waste PET and catalysts are directly put together into the microwave reactor. Utilizing a mixed catalytic system in a microwave absorber, the glycolytic depolymerization process of PET through microwave irradiation would optimize energy efficiency [44].
The advantages of the chemical recycling of PET by microwave irradiation are to supple the energy of chemical reactions, increase the reaction rate, decrease the reaction time and increase the product’s yield. It can reach even though under milder reaction conditions. Milan et al. studied the degradation of PET by microwave with the solvolysis process. The first step of the PET process was mixed up with a microwave absorbing activator under atmospheric pressure and then the PET was melted. It continued with solvolysis processes such as acidic or basic hydrolysis, alcoholysis or glycolysis in the presence of a catalyst under continuing microwave radiation with atmospheric pressure. The product were TPA, salts or esters and EG [45].
The effect of 1-buthyl-3-methylimidazolium bromide liquid with PET glycolysis process under microwave was studied by Alnaqbi et al. The temperature and reaction time of glycolysis were 170–175 °C and 1.75–2 h, respectively. The major liquid product was BHET with the conversion and yield up to 100% and 64 wt% [46]. The combination of hydrolysis and alcoholysis with microwave irradiation can be achieved by an acidic catalyst, such as montmorillonites K10 and KSF, ion exchangers, zeolites, phosphoric acid supported on alumina or silica, copper(II), iron(III), Zinc(II), aluminum (III), antimony(III), bismuth(III) chlorides or acetates, respectively. Or it can be performed by homogeneous catalysts, such as p-toluene sulphonic, formic, acetic, benzoic, terephthalic or sulfuric acid, respectively. Liu et al explored related to catalytic hydrolysis under microwave assisted with Brønsted acidic ionic liquids for instances, 1-hexyl-3-methylimidazolium hydrogen sulfate ([hexanemim][HSO4]), 1-Ethyl-3-methylimidazolium hydrogen sulfate([Emim][HSO4]), N-methylimidazolium hydrogensulfate([Hmim][HSO4]), 1-butyl-3-methylimidazolium hydrogen sulfate([Bmim][HSO4]) [45][47]. The hydrolysis of PET under high pressure up to 2.8 – 3.0 Mpa investigated by Ikenaga et al with the range temperature reaction 235 – 237 °C and added 1.0 %wt as a catalyst to the major product was TPA [48].
Gr3n company technology offers a new and revolutionary approach to the chemical treatment in water systems using a microwave-assisted technology able to treat rPET in a closed-loop cycle process. The core of the technology is DEMETO (depolymerization by microwave technology), a patented technology, able to depolymerize continuously, a wide range of PET manufacturers (e.g., color bottles, food containers, polyester textile), which reduces the reaction time from 180 to 10 min [32].
3.2. PET Recycling Using Ionic Liquid
The ionic liquids have been developed significantly as a catalyst for PET depolymerization by the glycolysis process since the first invention in 2009. The first publication of ionic liquids was published by Zhang et al. that the halometallate catalyst based on ionic liquids for depolymerization of PET in the presence of EG. Furthermore, acidic ionic liquids’ application as dual-purpose catalysts simultaneously performs as a Lewis acid and nucleophile. It shows higher activity and selectivity than those used simple metal salts or solely organic ionic liquids [49][50][51]. Furthermore, the PET recycling by ionic liquid has several advantages: the extensive range broad of compound selection combined with anion and cation, non–volatility, thermal, electrochemical and low flammability [52][53][54][55].
The degradation of PET in supercritical ethanol with ionic liquid 1-butyl-3-methylimidazolium tetrafluoroborate ([Bmim][BF4]) was studied by Nenes et al. It indicated an up-and-coming method to degradation of PET in a sustained way [50][51][56]. Furthermore, Al-Sabagh et al. studied that the effect of ionic liquid-coordinated ferrous acetate complex immobilized on bentonite as a novel separable catalyst for PET glycolysis under mild conditions. The PET conversion up to 100% and the yield of BHET up to 44% with the reaction temperature at 190 °C [57].
3.3. PET Recycling Using Phase–Transfer Catalysis
One of the essential methods in organic synthesis is phase-transfer catalysis. In the manufacture of fine chemical, it will be easier that can be used two immiscible phases, for instance, liquid-liquid and solid-liquid, in the presence of a chain transfer agent, which can be a quaternary ammonium salt or crown ether [58][59][60][61]. The effect of quaternary ammonium salt as a phase-transfer catalyst for the microwave depolymerization of PET waste bottles had been studied by Khalaf et al. The PET was depolymerized to TPA and EG by hydrolysis process using 10% NaOH and ammonium salt as phase-transfer catalyst under microwave irradiation, which PET conversion was up to 99% [62]. López-Fonseca et al. presented that tributylhexadecylphosphonium bromide was found to be the most effective catalyst to conduct the PET alkaline hydrolysis (>90% conversion) at 80 °C and 1.5 h [63]. Kosmidis et al. presented the kinetics of PET alkaline hydrolysis by phase-transfer catalysis [64].
3.4. PET Recycling Using Nautral or Biomass-Based Catalyst
Stanica-Ezeanu and Matei obtained a high yield of rTPA from PET depolymerization by neutral hydrolysis in marine water [65]. Lalhmangalihzuala et al. studied the glycolysis of PET at 190 °C using biomass-waste orange peel ash as a catalyst that obtained the 79% BHET after 1.5 h [66]. Rorrer et al. investigated the combination between rPET and biomass became long life time composite materials with the properties over petroleum materials based. The rPET would be reacted with the monomer of diols from biomass. Then, the deconstruction of PET took place in this process, and the process continued with bio-derivable olefinic acid. The outcome from this process was unsaturated polyester (UPE). Furthermore, it continued with the diluent process of reactive bio-derivable, and the results were fiberglass reinforced plastic, and it could save energy up to 57% [67]
3.5. PET Recycling Using an Enzymatic Catalyst
Enzymatic hydrolysis offers an interesting biotechnological route to PET degradation under mild conditions but with severe limitations such as the requirement for amorphous or low-crystallinity PET necessary for the proper activity of PETase enzymes [68][69]. The most advanced enzymatic PET degradation is carried out by Carbios company in French which collaborates with the most significant industrial enzyme production. Novozymes company in Danish is going to commercialize this PET enzymatic recycling process.
The decomposition of PET recycling by lipase enzyme as a valuable biocatalyst comprised of (i) hydrolysis of BHET and (ii) esterification of the resulted products (mono (2-hydroxyethyl)terephthalic acid, MHET and TPA) using dimethyl carbonate [70]. The degradation of PET nanoparticles prepared from PET films as substrate by TfCut2, a polyester hydrolase from Thermobifida fusca, was studied in the presence of EG, TPA, BHET and MHET. The initial reaction rates were determined and kinetically analyzed using a Michaelis–Menten reaction kinetics model [71].
PET biodegradation currently one of the popular methods to degrade PET. This process’s advantages are environmentally friendly and low cost, e.g., 3 Kg enzyme can degrade 1000 Kg of PET with the cost of up to 63 €. Meanwhile, the byproduct of that process can be applied to different applications. However, biodegradation’s reaction time took longer than that by chemical and mechanical techniques [6][72][73]. Several kinds of bacteria and fungi were discovered to depolymerize PET into short chains of oligomer and monomers (BHET and MHET) [74]. The Ideonella sakaiensis bacteria was discovered by Yoshida et al., This kind of bacteria proved to degrade PET effectively [75]. In addition, Esterase is a member of the enzyme that could be cut the ester bond (short-chain alkyl ester). That kind of bacteria could be discovered at surface modification of PET. Hence, the initial degradation of PET took place by bacillus and nocardia via esterases [76].
3.6. PET Recycling Using Methanolysis–Hydrolysis
The first stage in the methanolysis–hydrolysis process is reacted with superheated methanol vapor to get DMT, monomethyl terephthalate, EG oligomeric products. The next step is to remove residue with mainly containing oligomers with fractional distillation after TPA’s precipitation. The reaction was completed after 15 min, which leads to a significant decrease in the time required for the degradation of the polymer process. On the other hand, conventional hydrolysis needs to take 45 min. The Mitsubishi heavy industries Ltd. established the latest technology of methanolysis–hydrolysis with plant scale. Figure 2. shows that the process of diagram recycling PET by methanolysis–hydrolysis of Mitsubishi process. The PET begins with collecting the waste of PET, and the polyethylene goes to the shredding unit. The function of the shredding unit is to get a small size of particles of PET. The PET through the depolymerization unit with added methanol and the following processes is divided into two processes: DMT purification process and EG/methanol purification. The next step of DMT purification must be passed to the hydrolysis unit to become a TPA product, and it will become the raw material of PET [77][78][79][80][81].
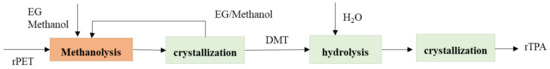
Figure 2. The process of PET recycling with methanolysis–crystallization-hydrolysis.
3.7. PET Recycling Using Glycolysis–Methanolysis
The recycling of PET with a combination of glycolysis–methanolysis starts from dissolving PET in a mixture of EG, TPA, DMT and oligomers. The reactant will be reacted at the superheated methanol condition with the reaction temperature range of 250–290 °C to get a large amount of DMT. Furthermore, the hybrid process technology to recycle by glycolysis–methanolysis is developed to get the high yield of product and production rate. The different types of reaction parameters were designed with the methanolysis process, which had a better role than the glycolysis process to increase the product yield with the ratio of EG and PET = 0.52 and the temperature reaction 240 °C [82][83].
3.8. PET Recycling Using Glycolysis–Hydrolysis
The combined application of glycolysis–hydrolysis had been discovered in literature since 1986. The patent claimed that the recycling of PET by extruder with increasing reaction temperature up to 280 °C with the slightest amount of EG before hydrolysis, the molecular weight would be reduced from 30,000 to 9000–1000. Furthermore, it depends on the quantity of EG. Moreover, the reaction time would reduce from 45 to 12–15 min to get EG and TPA. The high reaction of temperature and a large excess of reactants was considered to get many conversions and achieve low–intermediate molecular weight. It considers that hydrolysis and glycolysis are reversible reactions, and the equilibrium of polymerization with the reverse reaction of polyesterifications must be changed [84][85][86]. Figure 3 shows the possible reaction PET by the glycolysis–hydrolysis process. Optimizing the economic process with a capacity of a plant of 8000 t/year, the unitary cost of producing 1 kg of BHET and TPA are 1.99 €/kg BHET and 1.02 €/kgTPA, respectively [87].
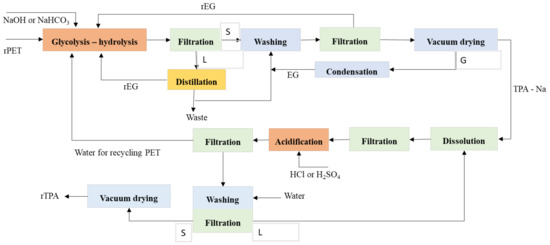
Figure 3. The possible reaction of PET by glycolysis–hydrolysis with the separation process.
3.9. PET Recycling Using Steam Hydrolysis
The recycling of PET using steam hydrolysis is carried out under high temperature and pressure. This process can be applied to a continuous process with the temperature range of was 200–300 °C under high-pressure steam up to 15 atm. The super-high steam is conducted at the bottom of the hydrolysis zone following the condensation process [88]. Rosen et al. studied the steam hydrolysis of PET with a mechanical process. The PET waste was crushing or grinding. Furthermore, it removed the impurities of waste PET by water. It was heated by increasing the temperature range between 221–316 °C to hydrolyze the solution and then obtained crude TPA in a cooling process [89]. PET waste was decomposed to main product TPA (90%) in sub- and supercritical water at short reaction times (1–30 min) in a batch reactor at temperatures from 250 to 400 °C by Čolnik et al. [90], which by-product was benzoic acid, 1,4-dioxane, acetaldehyde, isophthalic acid and CO2.
3.10. PET Recycling Using Solid-State Hydrolysis
Strukil studied the solid-state PET hydrolysis by mechanochemical milling and vapor-assisted aging [68]. Mechanochemical PET hydrolysis does not depend on the properties of plastic substrate such as crystallinity and uses low energy. Benzaria et al. presented that the PET hydrolysis was conducted in the reactor-mixer-extruder communicate with a sealed heating chamber at a temperature between 50 °C and 200 °C for a time sufficient to complete the saponification, for example, 5 to 30 min [91].
References
- Ubeda, S.; Aznar, M.; Nerín, C. Determination of oligomers in virgin and recycled polyethylene terephthalate (PET) samples by UPLC-MS-QTOF. Anal. Bioanal. Chem. 2018, 410, 2377–2384.
- Welle, F. Food Law Compliance of Poly(ethylene Terephthalate) (PET) Food Packaging Materials. In Food Additives and Packaging; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2014; Volume 1162, pp. 167–195.
- Li, B.; Wang, Z.-W.; Lin, Q.-B.; Hu, C.-Y. Study of the Migration of Stabilizer and Plasticizer from Polyethylene Terephthalate into Food Simulants. J. Chromatogr. Sci. 2016, 54, 939–951.
- Begley, T.H.; Biles, J.E.; Cunningham, C.; Piringer, O. Migration of a UV stabilizer from polyethylene terephthalate (PET) into food simulants. Food Addit. Contam. 2004, 21, 1007–1014.
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782.
- Hopewell, J.; Dvorak, R.; Kosior, E. Plastics recycling: Challenges and opportunities. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2115–2126.
- Williams, P.T. Waste Treatment and Disposal; John Wiley & Sons: Hoboken, NJ, USA, 2005.
- Le, D.K.; Ng, G.N.; Koh, H.W.; Zhang, X.; Thai, Q.B.; Phan-Thien, N.; Duong, H.M. Methyltrimethoxysilane-coated recycled polyethylene terephthalate aerogels for oil spill cleaning applications. Mater. Chem. Phys. 2020, 239, 122064.
- Administration, F.D. Points to Consider for the Use of Recycled Plastics: Food Packaging, Chemistry Considerations; FDA Division of Food Chemistry and Technology Publication: Washington, DC, USA, 1992; Volume 410.
- Nikles, D.E.; Farahat, M.S. New Motivation for the Depolymerization Products Derived from Poly(Ethylene Terephthalate) (PET) Waste: A Review. Macromol. Mater. Eng. 2005, 290, 13–30.
- Jankauskaite, V.; Macijauskas, G.; Lygaitis, R. Polyethylene terephthalate waste recycling and application possibilities: A review. Mater. Sci. (Medzg.) 2008, 14, 119–127.
- Lau, W.W.Y.; Shiran, Y.; Bailey, R.M.; Cook, E.; Stuchtey, M.R.; Koskella, J.; Velis, C.A.; Godfrey, L.; Boucher, J.; Murphy, M.B.; et al. Evaluating scenarios toward zero plastic pollution. Science 2020, 369, 1455–1461.
- Chowdhury, S.; Maniar, A.T.; Suganya, O. Polyethylene Terephthalate (PET) Waste as Building Solution. Int. J. Chem. Environ. Biol. Sci. 2013, 1, 2320–4087.
- Alaloul, W.S.; John, V.O.; Musarat, M.A. Mechanical and Thermal Properties of Interlocking Bricks Utilizing Wasted Polyethylene Terephthalate. Int. J. Concr. Struct. Mater. 2020, 14, 1–11.
- Jankauskaite, V. Recycled Polyethylene Terephthalate Waste for Different Application Solutions. Environ. Res. Eng. Manag. 2016, 72, 5–7.
- Sanches, R.A.; Takamune, K.; Guimarães, B.; Alonso, R.; Baruque-Ramos, J.; de Held, M.S.B.; Marcicano, J.P.P. Wearbility Analysis of knited fabrics produced with colored organic cotton. Bamboo rayon, corn, recycled pet/cotton and recycled pet/polyester. Am. Int. J. Contemp. Res. 2014, 4, 28–37.
- Shen, L.; Worrell, E.; Patel, M.K. Open-loop recycling: A LCA case study of PET bottle-to-fibre recycling. Resour. Conserv. Recycl. 2010, 55, 34–52.
- Kumartasli, S.; Avinc, O. Important Step in Sustainability: Polyethylene Terephthalate Recycling and the Recent Developments; Springer International Publishing: Cham, Switzerland, 2020; pp. 1–19.
- Kalita, N.K.; Kalamdhad, A.; Katiyar, V. Recent trends and advances in the biodegradation of conventional plastics. In Advances in Sustainable Polymers; Springer: Berlin/Heidelberg, Germany, 2020; pp. 389–404.
- Zimmermann, W. Biocatalytic recycling of polyethylene terephthalate plastic. Philos. Trans. R. Soc. A 2020, 378.
- Thompson, R.C.; Moore, C.J.; Vom Saal, F.S.; Swan, S.H. Plastics, the environment and human health: Current consensus and future trends. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2153–2166.
- Dias, D.S.; Crespi, M.S.; Ribeiro, C.A.; Kobelnik, M. Evaluation of the thermal decomposition of blends prepared with poly(3-hydroxybutyrate) (PHB) and recyclable ethylene poly-terephthalate (RPET). J. Therm. Anal. Calorim. 2021, 143, 3447–3457.
- Raheem, A.B.; Noor, Z.Z.; Hassan, A.; Hamid, M.K.A.; Samsudin, S.A.; Sabeen, A.H. Current developments in chemical recycling of post-consumer polyethylene terephthalate wastes for new materials production: A review. J. Clean. Prod. 2019, 225, 1052–1064.
- Myren, T.H.T.; Stinson, T.A.; Mast, Z.J.; Huntzinger, C.G.; Luca, O.R. Chemical and Electrochemical Recycling of End-Use Poly(ethylene terephthalate) (PET) Plastics in Batch, Microwave and Electrochemical Reactors. Molecules 2020, 25, 2742.
- Pulido, B.A.; Habboub, O.S.; Aristizabal, S.L.; Szekely, G.; Nunes, S.P. Recycled Poly(ethylene terephthalate) for High Temperature Solvent Resistant Membranes. ACS Appl. Polym. Mater. 2019, 1, 2379–2387.
- Mirjalili, A.; Dong, B.; Pena, P.; Ozkan, C.S.; Ozkan, M. Upcycling of polyethylene terephthalate plastic waste to microporous carbon structure for energy storage. Energy Storage 2020, 2, e201.
- Spychaj, T.; Fabrycy, E.; Spychaj, S.; Kacperski, M. Aminolysis and aminoglycolysis of waste poly(ethylene terephthalate). J. Mater. Cycles Waste Manag. 2001, 3, 24–31.
- Pohjakallio, M.; Vuorinen, T. Chemical routes for recycling—dissolving, catalytic, and thermochemical technologies. In Plastic Waste and Recycling; Elsevier: Amsterdam, The Netherlands, 2020; pp. 359–384.
- Rahimi, A.; García, J.M. Chemical recycling of waste plastics for new materials production. Nat. Rev. Chem. 2017, 1, 0046.
- Meys, R.; Frick, F.; Westhues, S.; Sternberg, A.; Klankermayer, J.; Bardow, A. Towards a circular economy for plastic packaging wastes–the environmental potential of chemical recycling. Resour. Conserv. Recycl. 2020, 162, 105010.
- FENC. New Process Recycles Any PET Waste. Available online: (accessed on 4 March 2021).
- gr3n. Technological Breakthough. Available online: (accessed on 4 March 2021).
- JEPLAN. BRING Technology™. Available online: (accessed on 4 March 2021).
- Garbo. CHEMPET PROJECT. Available online: (accessed on 4 March 2021).
- Ifp. Contribution of Chemistry to Plastics Recycling. Available online: (accessed on 4 March 2021).
- Ioniqa. Ioniqa’s Circular Solution. Available online: (accessed on 4 March 2021).
- Perpetual. Technology. Available online: (accessed on 4 March 2021).
- Poseidon Plastics. Bringing an End to Single Use Plastic. Available online: (accessed on 4 March 2021).
- Eastman. Eastman Offers Innovative Recycling Technology for Polyesters. Available online: (accessed on 4 March 2021).
- Loop Indystries. Revolutionary Technology. Available online: (accessed on 4 March 2021).
- DePoly. Simple Sustainable Recycling. Available online: (accessed on 4 March 2021).
- Carbios. Biorecycling. Available online: (accessed on 4 March 2021).
- Tullo, A.H. Plastic Has a Problem; Is Chemical Recycling the Solution? Available online: (accessed on 24 March 2021).
- Parrott, M. Chemical Recycling of Polyethylene Terephthalate by Microwave Irradiation. U.S. Patent 2749736, 23 March 2020.
- Hajek, M.; Sobek, J.; Brustman, J. Method for the Chemical Depolymerization of Waste Polyethylene Terephthalate. U.S. Patent 20100133088A1, 3 June 2010.
- Alnaqbi, M.A.; Mohsin, M.A.; Busheer, R.M.; Haik, Y. Microwave assisted glycolysis of poly(ethylene terephthalate) catalyzed by 1-butyl-3-methylimidazolium bromide ionic liquid. J. Appl. Polym. Sci. 2015, 132.
- Liu, N.; Ma, Y.S.; Shu, K.W.; Wu, B.; Zhang, D. Catalysis investigation of PET Depolymerization with Brnsted acidic ionic liquid under microwave irradiation. Adv. Mater. Res. 2014, 893, 23–26.
- Ikenaga, K.; Inoue, T.; Kusakabe, K. Hydrolysis of PET by combining direct microwave heating with high pressure. Procedia Eng. 2016, 148, 314–318.
- Cano, I.; Martin, C.; Fernandes, J.A.; Lodge, R.W.; Dupont, J.; Casado-Carmona, F.A.; Lucena, R.; Cardenas, S.; Sans, V.; de Pedro, I. Paramagnetic ionic liquid-coated Fe3O4 nanoparticles—The next generation of magnetically recoverable nanocatalysts applied in the glycolysis of PET. Appl. Catal. B Environ. 2020, 260, 118110.
- Wang, H.; Liu, Y.; Li, Z.; Zhang, X.; Zhang, S.; Zhang, Y. Glycolysis of poly (ethylene terephthalate) catalyzed by ionic liquids. Eur. Polym. J. 2009, 45, 1535–1544.
- Wang, H.; Yan, R.; Li, Z.; Zhang, X.; Zhang, S. Fe-containing magnetic ionic liquid as an effective catalyst for the glycolysis of poly (ethylene terephthalate). Catal. Commun. 2010, 11, 763–767.
- Dupont, J.; de Souza, R.F.; Suarez, P. Ionic liquid (molten salt) phase organometallic catalysis. Chem. Rev. 2002, 102, 3667–3692.
- Kosmulski, M.; Gustafsson, J.; Rosenholm, J.B. Thermal stability of low temperature ionic liquids revisited. Thermochim. Acta 2004, 412, 47–53.
- Matsumoto, H.; Yanagida, M.; Tanimoto, K.; Nomura, M.; Kitagawa, Y.; Miyazaki, Y. Highly conductive room temperature molten salts based on small trimethylalkylammonium cations and bis (trifluoromethylsulfonyl) imide. Chem. Lett. 2000, 29, 922–923.
- Wang, H.; Li, Z.; Liu, Y.; Zhang, X.; Zhang, S. Degradation of poly (ethylene terephthalate) using ionic liquids. Green Chem. 2009, 11, 1568–1575.
- Nunes, C.S.; da Silva, M.J.V.; da Silva, D.C.; dos Reis Freitas, A.; Rosa, F.A.; Rubira, A.F.; Muniz, E.C. PET depolymerisation in supercritical ethanol catalysed by [Bmim][BF 4]. RSC Adv. 2014, 4, 20308–20316.
- Al-Sabagh, A.M.; Yehia, F.Z.; Eissa, A.M.F.; Moustafa, M.E.; Eshaq, G.; Rabie, A.M.; ElMetwally, A.E. Cu- and Zn-acetate-containing ionic liquids as catalysts for the glycolysis of poly(ethylene terephthalate). Polymer Degradation and Stability 2014, 110, 364–377.
- Yue, Q.F.; Wang, C.X.; Zhang, L.N.; Ni, Y.; Jin, Y.X. Glycolysis of poly (ethylene terephthalate)(PET) using basic ionic liquids as catalysts. Polym. Degrad. Stabil. 2011, 96, 399–403.
- Naik, S.D.; Doraiswamy, L.J.A.J. Phase transfer catalysis: Chemistry and engineering. Aiche J. 1998, 44, 612–646.
- Starks, C.; Liotta, C.M. Halpern in Phase-Transfer Catalysis: Fundamentals, Applications and Industrial Perspectives; Chapman & Hall: New York, NY, USA, 1994.
- Yang, H.-M.; Wu, H.-S. Interfacial mechanism and kinetics of phase-transfer catalysis. Catal. Rev. 2003, 45, 463–540.
- Khalaf, H.I.; Hasan, O.A. Effect of quaternary ammonium salt as a phase transfer catalyst for the microwave depolymerization of polyethylene terephthalate waste bottles. Chem. Eng. J. 2012, 192, 45–48.
- López-Fonseca, R.; González-Marcos, M.P.; González-Velasco, J.R.; Gutiérrez-Ortiz, J.I. Chemical recycling of PET by alkaline hydrolysis in the presence of quaternary phosphonium and ammonium salts as phase transfer catalysts. In Waste Management and the Environment IV; WIT Press: Southampton, UK, 2008; pp. 511–520.
- Kosmidis, V.A.; Achilias, D.S.; Karayannidis, G.P. Poly(ethylene terephthalate) Recycling and Recovery of Pure Terephthalic Acid. Kinetics of a Phase Transfer Catalyzed Alkaline Hydrolysis. Macromol. Mater. Eng. 2001, 286, 640–647.
- Stanica-Ezeanu, D.; Matei, D. Natural depolymerization of waste poly(ethylene terephthalate) by neutral hydrolysis in marine water. Sci. Rep. 2021, 11, 4431.
- Lalhmangaihzuala, S.; Laldinpuii, Z.; Lalmuanpuia, C.; Vanlaldinpuia, K. Glycolysis of Poly (Ethylene Terephthalate) Using Biomass-Waste Derived Recyclable Heterogeneous Catalyst. Polymers 2020, 13, 37.
- Rorrer, N.A.; Nicholson, S.; Carpenter, A.; Biddy, M.J.; Grundl, N.J.; Beckham, G.T. Combining Reclaimed PET with Bio-based Monomers Enables Plastics Upcycling. Joule 2019, 3, 1006–1027.
- Štrukil, V. Highly Efficient Solid-State Hydrolysis of Waste Polyethylene Terephthalate by Mechanochemical Milling and Vapor-Assisted Aging. ChemSusChem 2021, 14, 330–338.
- Kawai, F.; Kawabata, T.; Oda, M. Current knowledge on enzymatic PET degradation and its possible application to waste stream management and other fields. Appl. Microbiol. Biotechnol. 2019, 103, 4253–4268.
- Ion, S.; Voicea, S.; Sora, C.; Gheorghita, G.; Tudorache, M.; Parvulescu, V.I. Sequential biocatalytic decomposition of BHET as valuable intermediator of PET recycling strategy. Catal. Today 2021, 366, 177–184.
- Barth, M.; Oeser, T.; Wei, R.; Then, J.; Schmidt, J.; Zimmermann, W. Effect of hydrolysis products on the enzymatic degradation of polyethylene terephthalate nanoparticles by a polyester hydrolase from Thermobifida fusca. Biochem. Eng. J. 2015, 93, 222–228.
- Al-Sabagh, A.M.; Yehia, F.Z.; Eshaq, G.; Rabie, A.M.; ElMetwally, A.E. Greener routes for recycling of polyethylene terephthalate. Egypt. J. Pet. 2016, 25, 53–64.
- Tournier, V.; Topham, C.; Gilles, A.; David, B.; Folgoas, C.; Moya-Leclair, E.; Kamionka, E.; Desrousseaux, M.-L.; Texier, H.; Gavalda, S. An engineered PET depolymerase to break down and recycle plastic bottles. Nature 2020, 580, 216–219.
- Wei, R.; Oeser, T.; Schmidt, J.; Meier, R.; Barth, M.; Then, J.; Zimmermann, W. Engineered bacterial polyester hydrolases efficiently degrade polyethylene terephthalate due to relieved product inhibition. Biotechnol. Bioeng. 2016, 113, 1658–1665.
- Yoshida, S.; Hiraga, K.; Takehana, T.; Taniguchi, I.; Yamaji, H.; Maeda, Y.; Toyohara, K.; Miyamoto, K.; Kimura, Y.; Oda, K. A bacterium that degrades and assimilates poly (ethylene terephthalate). Science 2016, 351, 1196–1199.
- Sharon, C.; Sharon, M. Studies on biodegradation of polyethylene terephthalate: A synthetic polymer. J. Microbiol. Biotechnol. Res. 2012, 2, 248–257.
- Aguado, J.; Serrano, D.P. Feedstock Recycling of Plastic Wastes; Royal Society of Chemistry: Cambridge, UK, 2007.
- Genta, M.; Yano, F.; Kondo, Y.; Matsubara, W.; Oomoto, S. Development of chemical recycling process for post-consumer PET bottles by methanolysis in supercritical methanol. Tech. Rev 2003, 40, 1–4.
- Goje, A.; Thakur, S.; Patil, T.M.; Mishra, S. Glycolytic aminolysis of poly (ethylene terephthalate) waste for recovery of value-added comonomer at atmospheric pressure. J. Appl. Polym. Sci. 2003, 90, 3437–3444.
- Thomas, S.; Rane, A.V.; Kanny, K.; Abitha, V.; Thomas, M.G. Recycling of Polyethylene Terephthalate Bottles; William Andrew: Norwich, NY, USA, 2018.
- Padhan, R.K.; Sreeram, A. Chemical depolymerization of PET bottles via combined chemolysis methods. In Recycling of Polyethylene Terephthalate Bottles; Elsevier: Amsterdam, The Netherlands, 2019; pp. 135–147.
- Rogers, M.E.; Long, T.E. Synthetic Methods in Step-Growth Polymers; John Wiley & Sons: Hoboken, NJ, USA, 2003.
- Kim, B.-K.; Kim, D.; Cho, Y.; Han, M. Chemical recycling of poly (ethylene terephthalate) using a new hybrid process. J. Chem. Eng. Jpn. 2008, 41, 923–928.
- George, N.; Kurian, T. Recent developments in the chemical recycling of postconsumer poly(ethylene terephthalate) waste. Ind. Eng. Chem. Res. 2014, 53, 14185–14198.
- Doerr, M.L. Depolymerization of Condensation Polymers Involving a Pre-Molecular Weight Reduction Step. U.S. Patent 4620032A, 28 October 1986.
- Güçlü, G.; Yalçınyuva, T.; Özgümüş, S.; Orbay, M. Simultaneous glycolysis and hydrolysis of polyethylene terephthalate and characterization of products by differential scanning calorimetry. Polymer 2003, 44, 7609–7616.
- Aguado, A.; Martínez, L.; Becerra, L.; Arieta-araunabeña, M.; Arnaiz, S.; Asueta, A.; Robertson, I. Chemical depolymerisation of PET complex waste: Hydrolysis vs. glycolysis. J. Mater. Cycles Waste Manag. 2013, 16, 201–210.
- Mandoki, J.W. Depolymerization of Condensation Polymers. U.S. Patent 4605762A, 12 August 1986.
- Rosen, B.I. Preparation of Purified Terephthalic Acid from Waste Polyethylene Terephthalate. U.S. Patent 5,095,145A, 10 March 1992.
- Čolnik, M.; Knez, Ž.; Škerget, M. Sub- and supercritical water for chemical recycling of polyethylene terephthalate waste. Chem. Eng. Sci. 2021, 233, 116389.
- Benzaria, J.; Durif-Varambon, B.; Dawans, F.; Gaillard, J.-B. Process for Recovery of Alkali Metal or Alkali Earth Metal Terephtalate and Alkylene Glycol from Alkylene. Polyterephtalates. Patent EP 0597751A1, 21 January 1998.
More
Information
Subjects:
Polymer Science
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
3.1K
Revisions:
2 times
(View History)
Update Date:
08 May 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




