Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Damayanti Damayanti and Version 2 by Catherine Yang.
The chemical recycling of PET is reviewed, such as pyrolysis, hydrolysis, methanolysis, glycolysis, ionic-liquid, phase-transfer catalysis and combination of glycolysis–hydrolysis, glycolysis–methanolysis and methanolysis–hydrolysis.
- polyethylene terephthalate
- pyrolysis
- hydrolysis
1. Introduction
The most significant application of polymers in the last two decades is polyethylene terephthalate (PET), which is of excellent chemical and physical properties for many implementations, for instance, characteristic of the gas barrier, low diffusivity, excellent mechanical and thermo mechanical properties, highly inert material, clearness and fine process operation [1][2][3][4][1,2,3,4]. On the other hand, PET waste is already highlighting for humans and the environment. The global cumulative amount of plastic waste generated from 1950–2015 was approximately 6.3 billion tons, around 9% of which had been recycled, 12%was incinerated and 79% was accumulated in landfills or the natural environment [5]. Each year, the forecast PET waste about million metric tons transfers into the ocean and landfill. Currently, the recycling plastic methods are landfill, incineration and energy recovery and plastic recycling. The conventional landfill and incineration methods were concerned since the plastic component can be released into the ambient environment during processing. The landfill and incineration methods have hazardous substances released into the environment [6][7][8][6,7,8]. Most plastics are nondegradable, and take a long time to degrade, probably takes hundred years; however, nobody knows precisely when the plastic is degraded at the landfill. Based on the Environment Protection Agency, plastic recycling is divided into three-part, (i) be used directly, (ii) be undergone physical reprocessing, for instance, grinding, melting and reforming, and (iii) be undergone chemical processing when components are isolated and reprocessed for use in the chemical industry [9][10][11][9,10,11].
Several strategies can be applied to reduce the waste of PET until 2040. The approach to zero plastic pollution is divided into four critical types of interventions: reduce, substitute, recycle and dispose. Moreover, the eight actions can be implemented: (1) minimize the quantity of single used plastic (2) replaced the petroleum plastic with the other variant of materials and delivery systems (3) implementing design for recycling (4) raising the capacity of the collection (5) enlarge the capacity of sorting and mechanical recycling (6) increasing chemical conversion capacity (7) minimize post-collection environmental leakage (8) the trade of plastic become decrease slightly [12]. PET has a low modulus of synthetic fibers, its properties closeness to the other polymer, for instance, polyethylene, nylon and polyester [13][14][13,14]. The recycling process of PET can be conducted using mechanical and chemical processes. The primary purpose of recycling PET is to modify the polymer of PET into economically reusable forms. PET chemical recycling was broadly used in the chemical products such as polyester molding compound, varnishes, polymer plaster, topcoats of reinforced plastic, mortar and mineral filler, fiber, polyol for polyurethane elastomer, polyurethane with low flammability and foam [15]. The primary purpose of recycling PET to modify the polymer of PET into economically reusable forms; moreover, the critical point out of recycling PET is not only to reduce the cost of process production but also to maintaining an ecological balance is essential for sustainable to save our planet [16][17][18][16,17,18]. However, the cost of the chemical recycling of PET is higher than that of physical recycling of PET. For that reason, the innovation of technology to the chemical recycling of PET is needed.
2. The Chemical Recycling of PET
The conventional plastic stable carbon-based backbone makes the plastic resistant to depolymerization under different types of environmental conditions. Since plastic has good properties of materials, the production of plastic is increasing in the current decade. Furthermore, the plastic product’s significant application is short-term living, disposable packaging materials becoming waste after a single-use. Therefore, an enormous quantity of plastic waste is accumulating in the environment. Moreover, it already a severe problem of waste plastic because of poor biodegradability with detrimental effects to terrestrial and marine ecosystems and eventually on humans. On the other hand, plastic has many negative impacts, and the researchers try to develop some method/technology to recycling the PET [19][20][21][47,48,49]. Chemical recycling involves depolymerization, purification and then repolymerization. The best conceptual recycling of post-consumer waste is to modify the PET into their monomer as chemical raw material. The remarkable chemical properties of a polymer depend on designated use. PET is a partial–crystalline structure. The properties of the crystalline structure are high strength and tensile. There are so many technologies to recycle PET to obtain the recycled PET (rPET), for instance, packaging, textile and electronic compounds. Dias et al. studied the effect of thermal decomposition of rPET. The activation energy of rPET was 193 CV/Kj mol−1. The film absorption spectrum of rPET shows the features of 1722 cm−1 related to carbonyl esters (C=O), within 1024 and 1259 cm−1 regarding with ester (CO) bond, at 731 cm−1, p-substitution of the aromatic ring conjugated with the carbonyl at 2973 cm−1, stretching of the CH bond [22][50]. The alcoholysis of PET was heated with superheated methanol vapors. The principal product distributions are the combination of DMT, phthalate derivatives and alcohols. The complicated product distribution makes the separation process of the alcoholysis process more expensive. Furthermore, the catalyst should be deactivated to prevent transesterification of DMT with EG into diethylene glycol terephthalate and PET [23][51]. Some of the organic bases, e.g., 1,5,7-triazabicyclo[4.4.0]dec-5-ene(TBD), 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) and 1,5-diazabicyclo[4.3.0]non-5-ene (DBN), can be applied to depolymerization of PET to small molecules of monomer. The glycolysis process’s operation conditions are at high pressure and temperatures up to 1.5–2 MPa and 250 °C, respectively. TBD was used as excess to convert PET to BHET, with a yield of up to 78% [24][52]. Furthermore, the PET chemical recycling based on environmentally friendly was developed by Pulido et al. In contrast, the separation process could occur from traditional methods such as distillation, crystallization. The range of ideal pore size rPET membrane was 35–100 nm with non-solvent-induced phase separation in ethanol or methanol. In addition, the resistances of membranes could be elevated the acid and oxidative media, for example, dimethylformamide at 100 °C [25][53]. The other advantage of recycling waste PET is used to active electrochemical material for energy storage. Some chemical processes are applied, such as the dissolution of rPET, fiberization through electrospinning and carbonization by a furnace [26][54]. Chemical recycling includes various methods such as glycolysis, methanolysis, hydrolysis, ammonolysis and aminolysis, which are usually carried out at high temperatures and in the presence of catalysts. Figure 14 shows that the possible reaction to recycling terephthalate [27][55]. The technologies of chemical recycling can be categories into two parts which are (1) the technologies that the polymers in the PET are dissolved as a long chain of the polymer and (2) the other methods are crack the chemical bonds within atoms in the polymer chains. In condensation polymers, for instance, polyesters and polyamides (PAs), the standard approach is to crack the ester or amide long chain. In contrast, in polyolefins (polyethylene and polypropylene), one has to break the relatively stable carbon chain [28][56].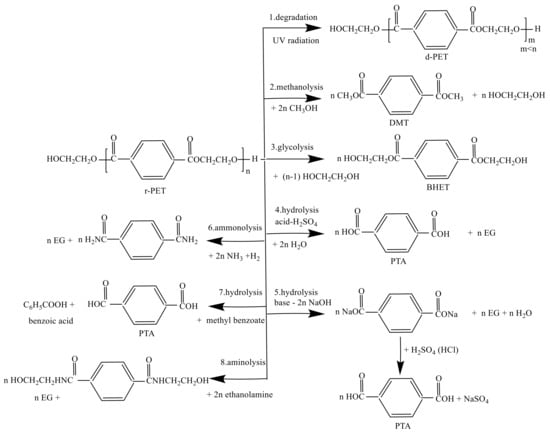
Figure 14. The 8 possible reactions of recycling PET including methanolysis, glycolysis, hydrolysis, ammonolysis and aminolysis.
Table 1.
Industrial rPET production and their locations.
| Company | Reaction | Country/Region | Ref | |
|---|---|---|---|---|
| FENC | hydrolysis | Taiwan | [31] | [58] |
| Gr3n | hydrolysis | Switzerland | [32] | [59] |
| JEPLAN (PRT) | glycolysis | Japan | [33] | [60] |
| Garbo | glycolysis | Italy | [34] | [61] |
| IFPEN | glycolysis | France | [35] | [62] |
| Ioniqa | glycolysis | Netherlands | [36] | [63] |
| PerPETual | glycolysis | UK | [37] | [64] |
| Poseidon Plastics | glycolysis | UK | [38] | [65] |
| Eastman | methanolysis | USA | [39] | [66] |
| Loop industries | methanolysis | Canada | [40] | [67] |
| DePoly | Switzerland | [41] | [68] | |
| Carbios | enzyme | French | [42] | [69] |
| Agiylx | pyrolysis | USA | [43] | [45] |
| Pyrowave | microwave radiation | USA | [43] | [45] |
3. The Recent Development of PET Chemical Recycling
3.1. PET Recycling Using Microwave Irradiation
The PET recycling by microwave irradiation takes more attention from many researchers. The recycling process by microwave method allows relatives to take a short reaction time with much higher energy to heat consumption than the conventional heating process. Nevertheless, the benefit of microwave irradiation of PET by glycolysis process is still cost-prohibitive due to the energy consumptions. The waste PET and catalysts are directly put together into the microwave reactor. Utilizing a mixed catalytic system in a microwave absorber, the glycolytic depolymerization process of PET through microwave irradiation would optimize energy efficiency [44][115].
The advantages of the chemical recycling of PET by microwave irradiation are to supple the energy of chemical reactions, increase the reaction rate, decrease the reaction time and increase the product’s yield. It can reach even though under milder reaction conditions. Milan et al. studied the degradation of PET by microwave with the solvolysis process. The first step of the PET process was mixed up with a microwave absorbing activator under atmospheric pressure and then the PET was melted. It continued with solvolysis processes such as acidic or basic hydrolysis, alcoholysis or glycolysis in the presence of a catalyst under continuing microwave radiation with atmospheric pressure. The product were TPA, salts or esters and EG [45][116].
The effect of 1-buthyl-3-methylimidazolium bromide liquid with PET glycolysis process under microwave was studied by Alnaqbi et al. The temperature and reaction time of glycolysis were 170–175 °C and 1.75–2 h, respectively. The major liquid product was BHET with the conversion and yield up to 100% and 64 wt% [46][117]. The combination of hydrolysis and alcoholysis with microwave irradiation can be achieved by an acidic catalyst, such as montmorillonites K10 and KSF, ion exchangers, zeolites, phosphoric acid supported on alumina or silica, copper(II), iron(III), Zinc(II), aluminum (III), antimony(III), bismuth(III) chlorides or acetates, respectively. Or it can be performed by homogeneous catalysts, such as p-toluene sulphonic, formic, acetic, benzoic, terephthalic or sulfuric acid, respectively. Liu et al explored related to catalytic hydrolysis under microwave assisted with Brønsted acidic ionic liquids for instances, 1-hexyl-3-methylimidazolium hydrogen sulfate ([hexanemim][HSO4]), 1-Ethyl-3-methylimidazolium hydrogen sulfate([Emim][HSO4]), N-methylimidazolium hydrogensulfate([Hmim][HSO4]), 1-butyl-3-methylimidazolium hydrogen sulfate([Bmim][HSO4]) [45][47][116,118]. The hydrolysis of PET under high pressure up to 2.8 – 3.0 Mpa investigated by Ikenaga et al with the range temperature reaction 235 – 237 °C and added 1.0 %wt as a catalyst to the major product was TPA [48][119].
Gr3n company technology offers a new and revolutionary approach to the chemical treatment in water systems using a microwave-assisted technology able to treat rPET in a closed-loop cycle process. The core of the technology is DEMETO (depolymerization by microwave technology), a patented technology, able to depolymerize continuously, a wide range of PET manufacturers (e.g., color bottles, food containers, polyester textile), which reduces the reaction time from 180 to 10 min [32][59].
3.2. PET Recycling Using Ionic Liquid
The ionic liquids have been developed significantly as a catalyst for PET depolymerization by the glycolysis process since the first invention in 2009. The first publication of ionic liquids was published by Zhang et al. that the halometallate catalyst based on ionic liquids for depolymerization of PET in the presence of EG. Furthermore, acidic ionic liquids’ application as dual-purpose catalysts simultaneously performs as a Lewis acid and nucleophile. It shows higher activity and selectivity than those used simple metal salts or solely organic ionic liquids [49][50][51][120,121,122]. Furthermore, the PET recycling by ionic liquid has several advantages: the extensive range broad of compound selection combined with anion and cation, non–volatility, thermal, electrochemical and low flammability [52][53][54][55][123,124,125,126].
The degradation of PET in supercritical ethanol with ionic liquid 1-butyl-3-methylimidazolium tetrafluoroborate ([Bmim][BF4]) was studied by Nenes et al. It indicated an up-and-coming method to degradation of PET in a sustained way [50][51][56][121,122,127]. Furthermore, Al-Sabagh et al. studied that the effect of ionic liquid-coordinated ferrous acetate complex immobilized on bentonite as a novel separable catalyst for PET glycolysis under mild conditions. The PET conversion up to 100% and the yield of BHET up to 44% with the reaction temperature at 190 °C [57][128].
3.3. PET Recycling Using Phase–Transfer Catalysis
One of the essential methods in organic synthesis is phase-transfer catalysis. In the manufacture of fine chemical, it will be easier that can be used two immiscible phases, for instance, liquid-liquid and solid-liquid, in the presence of a chain transfer agent, which can be a quaternary ammonium salt or crown ether [58][59][60][61][129,130,131,132]. The effect of quaternary ammonium salt as a phase-transfer catalyst for the microwave depolymerization of PET waste bottles had been studied by Khalaf et al. The PET was depolymerized to TPA and EG by hydrolysis process using 10% NaOH and ammonium salt as phase-transfer catalyst under microwave irradiation, which PET conversion was up to 99% [62][133]. López-Fonseca et al. presented that tributylhexadecylphosphonium bromide was found to be the most effective catalyst to conduct the PET alkaline hydrolysis (>90% conversion) at 80 °C and 1.5 h [63][134]. Kosmidis et al. presented the kinetics of PET alkaline hydrolysis by phase-transfer catalysis [64][135].
3.4. PET Recycling Using Nautral or Biomass-Based Catalyst
Stanica-Ezeanu and Matei obtained a high yield of rTPA from PET depolymerization by neutral hydrolysis in marine water [65][85]. Lalhmangalihzuala et al. studied the glycolysis of PET at 190 °C using biomass-waste orange peel ash as a catalyst that obtained the 79% BHET after 1.5 h [66][136]. Rorrer et al. investigated the combination between rPET and biomass became long life time composite materials with the properties over petroleum materials based. The rPET would be reacted with the monomer of diols from biomass. Then, the deconstruction of PET took place in this process, and the process continued with bio-derivable olefinic acid. The outcome from this process was unsaturated polyester (UPE). Furthermore, it continued with the diluent process of reactive bio-derivable, and the results were fiberglass reinforced plastic, and it could save energy up to 57% [67][137]
3.5. PET Recycling Using an Enzymatic Catalyst
Enzymatic hydrolysis offers an interesting biotechnological route to PET degradation under mild conditions but with severe limitations such as the requirement for amorphous or low-crystallinity PET necessary for the proper activity of PETase enzymes [68][69][138,139]. The most advanced enzymatic PET degradation is carried out by Carbios company in French which collaborates with the most significant industrial enzyme production. Novozymes company in Danish is going to commercialize this PET enzymatic recycling process.
The decomposition of PET recycling by lipase enzyme as a valuable biocatalyst comprised of (i) hydrolysis of BHET and (ii) esterification of the resulted products (mono (2-hydroxyethyl)terephthalic acid, MHET and TPA) using dimethyl carbonate [70][140]. The degradation of PET nanoparticles prepared from PET films as substrate by TfCut2, a polyester hydrolase from Thermobifida fusca, was studied in the presence of EG, TPA, BHET and MHET. The initial reaction rates were determined and kinetically analyzed using a Michaelis–Menten reaction kinetics model [71][141].
PET biodegradation currently one of the popular methods to degrade PET. This process’s advantages are environmentally friendly and low cost, e.g., 3 Kg enzyme can degrade 1000 Kg of PET with the cost of up to 63 €. Meanwhile, the byproduct of that process can be applied to different applications. However, biodegradation’s reaction time took longer than that by chemical and mechanical techniques [6][72][73][6,24,142]. Several kinds of bacteria and fungi were discovered to depolymerize PET into short chains of oligomer and monomers (BHET and MHET) [74][143]. The Ideonella sakaiensis bacteria was discovered by Yoshida et al., This kind of bacteria proved to degrade PET effectively [75][144]. In addition, Esterase is a member of the enzyme that could be cut the ester bond (short-chain alkyl ester). That kind of bacteria could be discovered at surface modification of PET. Hence, the initial degradation of PET took place by bacillus and nocardia via esterases [76][145].
3.6. PET Recycling Using Methanolysis–Hydrolysis
The first stage in the methanolysis–hydrolysis process is reacted with superheated methanol vapor to get DMT, monomethyl terephthalate, EG oligomeric products. The next step is to remove residue with mainly containing oligomers with fractional distillation after TPA’s precipitation. The reaction was completed after 15 min, which leads to a significant decrease in the time required for the degradation of the polymer process. On the other hand, conventional hydrolysis needs to take 45 min. The Mitsubishi heavy industries Ltd. established the latest technology of methanolysis–hydrolysis with plant scale. Figure 27. shows that the process of diagram recycling PET by methanolysis–hydrolysis of Mitsubishi process. The PET begins with collecting the waste of PET, and the polyethylene goes to the shredding unit. The function of the shredding unit is to get a small size of particles of PET. The PET through the depolymerization unit with added methanol and the following processes is divided into two processes: DMT purification process and EG/methanol purification. The next step of DMT purification must be passed to the hydrolysis unit to become a TPA product, and it will become the raw material of PET [77][78][79][80][81][162,163,164,165,166].
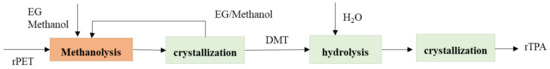

Figure 27.
The process of PET recycling with methanolysis–crystallization-hydrolysis.
3.7. PET Recycling Using Glycolysis–Methanolysis
The recycling of PET with a combination of glycolysis–methanolysis starts from dissolving PET in a mixture of EG, TPA, DMT and oligomers. The reactant will be reacted at the superheated methanol condition with the reaction temperature range of 250–290 °C to get a large amount of DMT. Furthermore, the hybrid process technology to recycle by glycolysis–methanolysis is developed to get the high yield of product and production rate. The different types of reaction parameters were designed with the methanolysis process, which had a better role than the glycolysis process to increase the product yield with the ratio of EG and PET = 0.52 and the temperature reaction 240 °C [82][83][167,168].
3.8. PET Recycling Using Glycolysis–Hydrolysis
The combined application of glycolysis–hydrolysis had been discovered in literature since 1986. The patent claimed that the recycling of PET by extruder with increasing reaction temperature up to 280 °C with the slightest amount of EG before hydrolysis, the molecular weight would be reduced from 30,000 to 9000–1000. Furthermore, it depends on the quantity of EG. Moreover, the reaction time would reduce from 45 to 12–15 min to get EG and TPA. The high reaction of temperature and a large excess of reactants was considered to get many conversions and achieve low–intermediate molecular weight. It considers that hydrolysis and glycolysis are reversible reactions, and the equilibrium of polymerization with the reverse reaction of polyesterifications must be changed [84][85][86][169,170,171]. Figure 38 shows the possible reaction PET by the glycolysis–hydrolysis process. Optimizing the economic process with a capacity of a plant of 8000 t/year, the unitary cost of producing 1 kg of BHET and TPA are 1.99 €/kg BHET and 1.02 €/kgTPA, respectively [87][172].
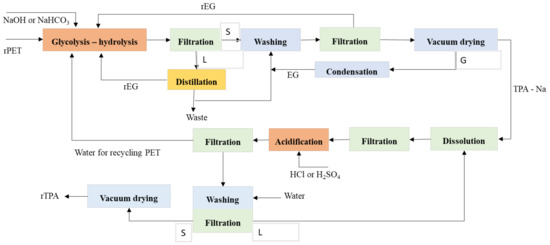

Figure 38.
The possible reaction of PET by glycolysis–hydrolysis with the separation process.
3.9. PET Recycling Using Steam Hydrolysis
The recycling of PET using steam hydrolysis is carried out under high temperature and pressure. This process can be applied to a continuous process with the temperature range of was 200–300 °C under high-pressure steam up to 15 atm. The super-high steam is conducted at the bottom of the hydrolysis zone following the condensation process [88][173]. Rosen et al. studied the steam hydrolysis of PET with a mechanical process. The PET waste was crushing or grinding. Furthermore, it removed the impurities of waste PET by water. It was heated by increasing the temperature range between 221–316 °C to hydrolyze the solution and then obtained crude TPA in a cooling process [89][174]. PET waste was decomposed to main product TPA (90%) in sub- and supercritical water at short reaction times (1–30 min) in a batch reactor at temperatures from 250 to 400 °C by Čolnik et al. [90][175], which by-product was benzoic acid, 1,4-dioxane, acetaldehyde, isophthalic acid and CO2.
3.10. PET Recycling Using Solid-State Hydrolysis
Strukil studied the solid-state PET hydrolysis by mechanochemical milling and vapor-assisted aging [68][138]. Mechanochemical PET hydrolysis does not depend on the properties of plastic substrate such as crystallinity and uses low energy. Benzaria et al. presented that the PET hydrolysis was conducted in the reactor-mixer-extruder communicate with a sealed heating chamber at a temperature between 50 °C and 200 °C for a time sufficient to complete the saponification, for example, 5 to 30 min [91][176].
