Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Thomas P. West | + 1665 word(s) | 1665 | 2021-04-25 06:14:09 | | | |
| 2 | Lily Guo | Meta information modification | 1665 | 2021-05-08 10:22:29 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
West, T. Gellan. Encyclopedia. Available online: https://encyclopedia.pub/entry/9401 (accessed on 14 January 2026).
West T. Gellan. Encyclopedia. Available at: https://encyclopedia.pub/entry/9401. Accessed January 14, 2026.
West, Thomas. "Gellan" Encyclopedia, https://encyclopedia.pub/entry/9401 (accessed January 14, 2026).
West, T. (2021, May 07). Gellan. In Encyclopedia. https://encyclopedia.pub/entry/9401
West, Thomas. "Gellan." Encyclopedia. Web. 07 May, 2021.
Copy Citation
Gellan is a water-soluble gum that structurally exists as a tetrasaccharide comprised of 20% glucuronic acid, 60% glucose and 20% rhamnose, for which various food, non-food and biomedical applications have been reported.
gellan
polysaccharide
water-soluble
bacteria
1. Introduction
The purpose of this review is to provide both background about the microbial polysaccharide gellan and, more specifically, to examine bacterial gellan synthesis on dairy and plant-based processing coproducts. The anionic heteropolysaccharide gellan is known to be synthesized by Sphingomonas elodea strain ATCC 31461 [1][2]. Although originally classified as Pseudomonas elodea, this strain was reclassified as a species of Sphingomonas [3][4]. Structurally, the water-soluble gum gellan exists as a tetrasaccharide composed of 20% glucuronic acid, 60% glucose and 20% rhamnose [5][6][7]. The native form of gellan has been shown to contain acetyl and l-glyceryl groups. These substituents need to be removed by an alkaline heat treatment to produce a gel. The degree of deacetylation of gellan can be directly correlated to its gel-forming ability [8]. The alkaline treatment of the native biopolymer results in a tetrasaccharide sequence that is anionic (Figure 1). As the deacylated biopolymer cools, a double helix forms from the disordered coils which results in gelation. Gelation of the polysaccharide is enhanced when the pH of the polysaccharide becomes more acidic. An acidic pH diminishes the negative charge of the polysaccharide molecule that results in greater repulsion within the helix. The addition of monovalent cations to a gellan solution increases its rate of gelation [9][10][11][12][13][14].
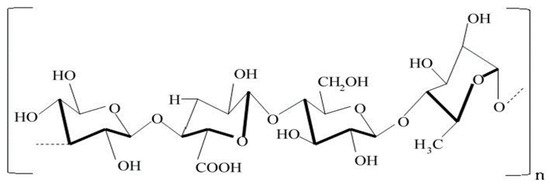
Figure 1. The structure of gellan involves tetrasaccharide repeating units consisting of two molecules of d-glucose as well as one molecule each of l-rhamnose and d-glucuronic acid.
When the monovalent cations bind to the helices, they bind to interact with the carboxylate groups on the polysaccharide to diminish repulsion within the helices. The binding of the monovalent ions to the helices increases with ionic size. Gellan behaves like a normal polymer solution when low monovalent cation concentrations are present. As the monovalent cation concentration is increased, a self-supporting gel is formed. The addition of divalent cations to a gellan solution also increases its rate of gelation [9][10][11][12][13][14][15][16][17]. The divalent cations bind between the helices to cause gelation. High concentrations of divalent cations results in a reduction in gel strength. It has been shown that the concentration of divalent cations needs to be equivalent to the carboxylate group content of the gellan to achieve maximum gellan strength. It has been noted that to achieve maximum gel strength, a higher concentration of monovalent cations needs to be added to the polymer solution than the divalent cation concentration. The binding properties of gellan allowed a colorimetric assay to be developed using the dye toluidine blue O where the gellan assay was found to be linear up to a concentration of 0.7 g/L [18].
In the United States, the source of commercial gellan production is C.P. Kelco (Atlanta, GA, USA). There are a number of commercial applications reported for this polysaccharide gum. With respect to food applications, gellan is used in confectionery jellies, fabricated foods, pie fillings and puddings, bakery icings and frostings, dairy products, fruit, milk-based and carbonated beverages as well as film or coatings for food adhesion [19][20][21][22][23]. With respect to non-food applications, gellan was originally proposed as a replacement for agar in microbial growth media since agar is the most economical polysaccharide gum available to be used as a solidifying agent that produces opaque gels [24][25][26]. Gellan has found greater use as a substitute for agar in plant tissue culture. The higher clarity of the gels at lower gellan concentrations is a marked advantage over agar. Another advantage of gellan is that it contains less impurities compared to agar. The use of gellan gels in plant tissue cultures allows tissue development to be observed more clearly than using agar. Gellan also has a number of pharmaceutical uses for drug delivery and enzyme immobilization [27][28][29][30][31][32][33][34]. With its non-toxicity, rapid gelation, ability to retain water and its biodegradability, gellan is used in oral formulations for drug delivery in capsules, beads and tablets. In opthamalic formulations, it is used in in situ gelling solutions to deliver anti-glaucoma and anti-conjunctivitis drugs [35]. Gellan is employed in nasal formulations as an in situ nasal gel to deliver a variety of pharmaceuticals. Another biomedical application of gellan is its use in wound healing where its properties have promise to serve as a carrier in tissue engineering [36][37][38][39][40].
2. Effect of Culture Conditions on Gellan Production by Sphingomonas elodea
2.1. Effect of Carbon Source
Relative to the carbon source, glucose-grown cells of S. paucimobilis strain ATCC 31461 were first shown to synthesize gellan [1][14]. A model developed to optimize gellan production was devised using ATCC 31461 in a simplified 3% glucose-containing medium [41]. It has been shown that maximum yield and productivity for ATCC 31461 were determined in a medium containing 1% glucose when no nitrogen source was present [42]. The strain ATCC 31461 was also shown to synthesize gellan on sucrose, mannose, galactose, fructose or maltose as a carbon source after 72 h of growth at 30 °C [43]. It was also found that cellular biomass production by ATCC 31461 did not correlate with gellan synthesis [43]. A prior study examining the effect of carbon source concentration of glucose and sucrose noted that polysaccharide production was maximal after 52 h in a pH 7.0-buffered medium containing 3% glucose or 4% sucrose at 30 °C [44]. With respect to the optimal incubation temperature for ATCC 31461 cells grown in a glucose-containing medium, it was shown to be 30–31 °C [45]. Similarly, cellular productivity was maximum for gellan production at 31 °C after 72 h of growth in batch cultures [45]. A two-stage culture strategy was developed to improve gellan production by ATCC 31461 using sucrose as a carbon source. This strategy was based on the findings that lower sucrose concentrations and higher temperatures favored bacterial cell growth but not gellan synthesis while low cell growth was observed when higher sucrose concentrations and lower temperatures were used which favored increased gellan production. The initial stage of the process involved pulse fed batch feeding of the culture during the first 24 h. ATCC 31461 cells were cultured in a pulse fed-batch mode with an initial sucrose concentration 10 g/L at 33 °C while the second stage involved incubating the batch culture at 28 °C to promote gellan production [46]. The influence of the initial pH of a glucose-containing culture medium on gellan synthesis by ATCC 31461 was analyzed. When the initial pH of the culture medium was 6.8 to 7.4, it was observed that gellan formation was greatest after 72 h of growth at 30 °C. Biomass production by the ATCC 31461 after 72 h was highest when the initial pH of the glucose-containing medium was adjusted to 7.8 [47]. Lastly, the effect of adding the glucose analog 2-deoxy-d-glucose to ATCC 31461 cultures was examined. When 50 μg/L 2-deoxy-d-glucose was added to the ATCC 31461 culture medium after 24 h, it was observed that the highest gellan concentration (20.78 g/L) was produced. The activity of UDP-glucose pyrophosphorylase activity was inhibited while the glucosyltransferase activity was elevated. It was thought that the glucose analog greatly inhibited glycolysis while activating the biosynthetic pathway for gellan in ATCC 31461 cells [48].
2.2. Influence of Nitrogen Source
The effect of a complex nitrogen source substituting for ammonium nitrate in a glucose-containing culture medium of ATCC 31461 was explored [43]. The complex nitrogen source tested was soytone (hydrolyzed soy protein), corn steep liquor, corn steep solids, ethanol stillage, peptone or tryptone [43]. In all cases, the complex nitrogen source promoted higher gellan production by ATCC 31461 in the glucose-containing medium compared to the medium containing glucose as the carbon source and ammonium nitrate as the nitrogen source [43]. Gellan production by ATCC 31461 was found to be highest in a medium containing glucose and soytone. It was also observed that soytone stimulated ATCC 31461 biomass production in the glucose-containing medium [43]. Having determined that the nitrogen source soytone enhanced gellan production by ATCC 31461, yeast extract supplementation of the glucose-containing medium was studied to learn whether the yeast extract concentration in the medium could further promote gellan production [49]. It was determined that only if the concentration of yeast extract in the glucose-containing medium was 0.1% did the polysaccharide level produced by ATCC 31461 increase significantly.
2.3. Effect of Aeration and Surfactants
The effect of aeration on gellan and cell biomass biosynthesis by ATCC 31461 was investigated. Biomass production was higher than gellan production by the bacterium as aeration and dissolved oxygen tension was elevated [50]. It was observed that 100% dissolved oxygen tension caused the gellan yield to increase to 23 g/L, with the polysaccharide being synthesized having increased viscosity and molecular weight. Another study found that high dissolved oxygen tension was not required for elevated gellan synthesis. Instead, it appeared that maximum gellan synthesis by the sphingomonad occurred when oxygen limitation preceded the initiation of gellan formation. It was also concluded that maximum gellan production by the bacterium did not correlate with maximum cellular biomass production [51]. It has been reported that treatment with 2–4 mM hydrogen peroxide of sphingomonad cells over a period of 12 h resulted in 36% greater gellan production than the untreated cells. In addition, cell growth was noted to be inhibited by the presence of hydrogen peroxide. The study found that oxidative stress placed upon the bacterial cells resulted in the enzymes UDP-glucose pyrophosphorylase activity and glucosyltransferase activity being elevated [52]. The increase in these enzyme activities likely accounts for the enhanced bacterial gellan production. The addition of the non-ionic surfactants Tween 80, Tween 40 and Triton X-100 to the medium of ATCC 31461 cultures were studied to determine whether they affected gellan synthesis [53]. It was observed that supplementation of each surfactant enhanced polysaccharide production by ATCC 31461 cells. A 1.2-fold increase in gellan synthesis by ATCC 31461 was noted when 0.75 g/L Triton X-100 was present in the culture medium compared to gellan production by ATCC 31461 cells grown in unsupplemented culture medium. Surfactant supplementation of the bacterial cultures also resulted in a high viscosity polysaccharide being synthesized. The same study found that the gellan yield (14.62 g/L) was highest in a 5 L laboratory fermentor incubated at 29.6 °C in a culture medium buffered at pH 6 containing 1.0 g/L Triton X-100 [53]. Gellan production by ATCC 31461 in the fermentor could be elevated by 1.9-fold with an aeration speed of 1000 rpm and a 100% dissolved oxygen tension similar to what was observed in an earlier study [50][53].
References
- Kang, K.S.; Veeder, G.T.; Mirrasoul, P.J.; Kaneko, T.; Cottrell, I.W. Agar-like polysaccharide produced by a Pseudomonas species: Production and basic properties. Appl. Environ. Microbiol. 1982, 43, 1086–1091.
- Anson, A.; Fisher, P.J.; Kennedy, A.F.D.; Sutherland, I.W. A bacterium yielding a polysaccharide with unusual properties. J. Appl. Bacteriol. 1987, 62, 147–150.
- Pollock, T.J. Gellan-related polysaccharides and the genus Sphingomonas. J. Gen. Microbiol. 1993, 139, 1939–1945.
- West, T.P. Pyrimidine nucleoside catabolism in Sphingomonas paucimobilis: Role of cytidine deaminase and uridine phosphorylase. Microbiol. Res. 1995, 150, 149–152.
- O’Neil, M.A.; Silvendran, R.R.; Morris, J. Structure of extracellular gelling polysaccharide produced by Pseudomonas elodea. Carbohydr. Res. 1983, 124, 123–133.
- Jansson, P.-E.; Lindberg, B.; Sandford, P.A. Structural studies of gellan gum, an extracellular polysaccharide elaborated by Pseudomonas elodea. Carbohydr. Res. 1983, 124, 135–139.
- Chandrasekaran, R.; Millane, R.P.; Arnott, S.; Atkins, E.D.T. The crystal structure of gellan. Carbohydr. Res. 1988, 175, 1–15.
- Campana, S.; Ganter, J.; Milas, M.; Rinaudo, M. On the solution properties of bacterial polysaccharides of the gellan family. Carbohydr. Res. 1992, 231, 31–38.
- Crescenzi, V.; Dentini, M.; Dea, I.C.M. The influence of side-chains on the dilute-solution properties of three structurally related bacterial anionic polysaccharides. Carbohydr. Res. 1987, 160, 283–302.
- Chandrasekaran, R.; Puigjaner, L.C.; Joyce, K.L.; Arnott, S. Cation interactions in gellan: An X-ray study of the potassium salt. Carbohydr. Res. 1988, 181, 23–40.
- Chandrasekaran, R.; Radha, A.; Thailambal, V.G. Roles of potassium ions, acetyl and l-glyceryl groups in native gellan double helix: An X-ray study. Carbohydr. Res. 1992, 224, 1–17.
- Tang, J.; Lelievre, J.; Tung, M.A.; Zeng, Y. Polymer and ion concentration effects on gellan strength and strain. J. Food Sci. 1994, 59, 216–220.
- Tang, J.; Tung, M.A.; Zeng, Y. Mechanical properties of gellan gels in relation to divalent cations. J. Food Sci. 1995, 60, 748–752.
- Manna, B.; Gambhir, A.; Ghosh, P. Production and reheological characteristics of the microbial gum gellan. Lett. Appl. Microbiol. 1996, 23, 141–145.
- Kirchmajer, D.M.; Steinhoff, B.; Warren, H.; Clark, R.; in het Panhuis, M. Enhanced gelation properties of purified gellan gum. Carbohydr. Res. 2014, 388, 125–129.
- Sharma, S.; Bhattacharya, S. Flow behaviour of gellan sol with selected cations. J. Food Sci. Technol. 2015, 52, 1233–1237.
- Vilela, J.A.P.; da Cunha, R.L. Emulsions stabilized by high acyl gellan and KCl. Food Res. Int. 2017, 91, 47–54.
- West, T.P.; Strohfus, B.; Santiago, M.F. A colorimetric assay for gellan elaborated by Sphingomonas paucimobilis ATCC 31461. World J. Microbiol. Biotechnol. 2000, 16, 529–531.
- Giavasis, I.; Harvey, L.M.; McNeil, B. Gellan gum. Crit. Rev. Biotechnol. 2000, 20, 177–211.
- Danalache, F.; Carvalho, C.; Alves, V.D.; Moldão-Martins, M.; Mata, P. Optimisation of gellan gum edible coating for ready-to-eat mango (Mangifera indica L.) bars. Int. J. Biol. Macromol. 2016, 84, 43–53.
- Tomadonia, B.; Moreira, M.R.; Pereda, M.; Ponce, A.G. Gellan-based coatings incorporated with natural antimicrobials in fresh-cut strawberries: Microbiological and sensory evaluation through refrigerated storage. LWT-Food Sci. Technol. 2018, 97, 384–389.
- Torresa, O.; Yamada, A.; Rigby, N.M.; Hanawa, T.; Kawano, Y.; Sarkar, A. Gellan gum: A new member in the dysphagia thickener family. Biotribiology 2019, 17, 8–18.
- Shinsho, A.; Brenner, T.; Descallar, F.B.; Tashiro, Y.; Ando, N.; Zhou, Y.; Ogawa, H.; Matsukawa, S. The thickening properties of native gellan gum are due to freeze drying–induced aggregation. Food Hydrocoll. 2020, 109, 105997.
- Linn, C.C.; Cassida, L.E. Gelrite as a gelling agent in media for the growth of thermophilic microorganisms. Appl. Environ. Microbiol. 1984, 47, 427–429.
- Rygaard, A.M.; Thøgersen, M.S.; Nielsen, K.F.; Gram, L.; Bentzon-Tilia, M. Effects of gelling agent and extracellular signaling molecules on the culturability of marine bacteria. Appl. Environ. Microbiol. 2017, 83, e00243-17.
- McGuffey, J.C.; Leon, D.; Dhanji, E.Z.; Mishler, D.M.; Barrick, J.E. Bacterial production of gellan gum as a do-it-yourself alternative to agar. J. Microbiol. Biol. Educ. 2018, 19, 1530.
- Crescenzi, V.; Dentini, M.; Segatori, M.; Tiblandi, C.; Callegaro, L.; Benedetti, L. Synthesis and preliminary characterisation of new esters of the bacterial polysaccharide gellan. Carbohydr. Res. 1992, 231, 73–81.
- Osmałek, T.; Froelich, A.; Tasarek, S. Application of gellan gum in pharmacy and medicine. Int. J. Pharm. 2014, 466, 328–340.
- Mahdi, M.H.; Conway, B.R.; Smith, A.M. Development of mucoadhesive sprayable gellan gum fluid gels. Int. J. Pharm. 2015, 488, 12–19.
- Wahba, M.I. Processed gellan gum beads as covalent immobilization carriers. Biocatal. Agric. Biotechnol. 2018, 14, 270–278.
- Zia, Z.M.; Tabasum, S.; Khan, M.F.; Akram, N.; Akhter, N.; Noreen, A.; Zuber, M. Recent trends on gellan gum blends with natural and synthetic polymers: A review. Int. J. Biol. Macromol. 2018, 109, 1068–1087.
- Kuhn, K.R.; e Silva, F.G.D.; Netto, F.M.; da Cunha, R.L. Production of whey protein isolate—Gellan microbeads for encapsulation and release of flaxseed bioactive compounds. J. Food Eng. 2019, 247, 104–114.
- Palumbo, F.S.; Federico, S.; Pitarresi, G.; Fiorica, C.; Giammona, G. Gellan gum-based delivery systems of therapeutic agents and cells. Carbohydr. Polym. 2020, 229, 115430.
- Monier, M.; Shafik, A.L.; El-Mekabaty, A. Designing and investigation of photo-active gellan gum for the efficient immobilization of catalase by entrapment. Int. J. Biol. Macromol. 2020, 161, 539–549.
- Sun, J.; Zhou, Z. A novel ocular delivery of brinzolamide based on gellan gum: In vitro and in vivo evaluation. Drug Des. Dev. Ther. 2018, 12, 383–389.
- Moxon, S.R.; Smith, A.M. Controlling the rheology of gellan gum hydrogels in cell culture conditions. Int. J. Biol. Macromol. 2016, 84, 79–86.
- Sebri, N.J.M.; Amin, K.A.M. Gellan gum/ibuprofen hydrogel for dressing application: Mechanical properties, release activity and biocompatibility studies. Int. J. Appl. Chem. 2016, 12, 483–498.
- Lago, M.E.L.; da Silva, L.P.; Henriques, C.; Carvalho, A.F.; Reis, R.L.; Marques, A.P. Generation of gellan gum-based adipose-like microtissues. Bioengineering 2018, 5, 52.
- Manda, M.G.; da Silva, L.P.; Cerqueira, M.T.; Pereira, D.R.; Oliveira, M.B.; Mano, J.F.; Marques, A.P.; Oliveira, J.M.; Correlo, V.M.; Reis, R.L. Gellan gum-hydroxyapatite composite spongy-like hydrogels for bone tissue engineering. J. Biomed. Mater. Res. Part A 2018, 106A, 479–490.
- Muthukumar, T.; Song, J.E.; Khang, G. Biological role of gellan gum in improving scaffold drug delivery, cell adhesion properties for tissue engineering applications. Molecules 2019, 24, 4514.
- Wang, X.; Xu, P.; Yuan, Y.; Liu, C.; Zhang, D.; Yang, Z.; Yang, C.; Ma, C. Modeling for gellan gum production by Sphingomonas paucimobilis ATCC 31461 in a simplified medium. Appl. Environ. Microbiol. 2006, 72, 3367–3374.
- Lim, S.-M.; Wu, J.R.; Lee, J.-W.; Kim, S.-K. Optimization of culture condition for gellan production by Pseudomonas elodea ATCC 31461. Korean J. Life Sci. 2003, 13, 705–711.
- West, T.P.; Strohfus, B. Effect of complex nitrogen sources upon gellan production by Sphingomonas paucimobilis ATCC 31461. Microbios 1998, 94, 145–152.
- Kanari, B.; Banik, R.R.; Upadhyay, S.N. Effect of environmental factors and carbohydrate on gellan gum production. Appl. Biochem. Biotechnol. 2002, 102–103, 129–139.
- West, T.P. Effect of temperature on bacterial gellan production. World J. Microbiol. Biotechnol. 2003, 19, 649–652.
- Zhu, G.; Sheng, L.; Tong, Q. A new strategy to enhance gellan production by two-stage culture in Sphingomonas paucimobilis. Carbohydr. Polym. 2013, 98, 829–834.
- West, T.P.; Fullenkamp, N.A. Effect of culture medium pH on bacterial gellan production. Microbios 2001, 105, 133–140.
- Zhu, G.; Guo, N.; Yong, Y.; Xiong, Y.; Tong, Q. Effect of 2-deoxy-d-glucose on gellan gum biosynthesis by Sphingomonas paucimobilis. Bioprocess Biosyst. Eng. 2019, 42, 897–900.
- West, T.P.; Strohfus, B. Effect of yeast extract on gellan production by Sphingomonas paucimobilis ATCC 31461. Microbios 1999, 97, 85–93.
- Banik, R.M.; Santhiagu, A. Improvement in production and quality of gellan gum by Sphingomonas paucimobilis under high dissolved oxygen tension levels. Biotechnol. Lett. 2006, 28, 1347–1350.
- Giaviasis, I.; Robertson, I.; McNeil, B.; Harvey, L.M. Simultaneous and rapid monitoring of biomass and biopolymer production by Sphingomonas paucimobilis using Fourier transform-near infrared spectroscopy. Biotechnol. Lett. 2003, 25, 975–979.
- Zhu, G.; Sheng, L.; Tong, Q. Enhanced gellan gum production by hydrogen peroxide (H2O2) induced oxidative stresses in Sphingomonas paucimobilis. Bioprocess Biosyst. Eng. 2014, 37, 743–748.
- Arockiasamy, S.; Banik, R.M. Optimization of gellan gum production by Sphingomonas paucimobilis ATCC 31461 with nonionic surfactants using central composite design. J. Biosci. Bioeng. 2008, 105, 204–210.
More
Information
Subjects:
Chemistry, Applied
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.5K
Revisions:
2 times
(View History)
Update Date:
08 May 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




