
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Michela Schiavon | + 2132 word(s) | 2132 | 2021-04-22 09:37:11 | | | |
| 2 | Bruce Ren | -21 word(s) | 2111 | 2021-05-08 04:54:08 | | |
Video Upload Options
Humic substances (HS) are dominant components of soil organic matter and are recognized as natural, effective growth promoters to be used in sustainable agriculture. In recent years, many efforts have been made to get insights on the relationship between HS chemical structure and their biological activity in plants using combinatory approaches. Relevant results highlight the existence of key functional groups in HS that might trigger positive local and systemic physiological responses via a complex network of hormone-like signaling pathways. The biological activity of HS finely relies on their dosage, origin, molecular size, degree of hydrophobicity and aromaticity, and spatial distribution of hydrophilic and hydrophobic domains. The molecular size of HS also impacts their mode of action in plants, as low molecular size HS can enter the root cells and directly elicit intracellular signals, while high molecular size HS bind to external cell receptors to induce molecular responses. Main targets of HS in plants are nutrient transporters, plasma membrane H+-ATPases, hormone routes, genes/enzymes involved in nitrogen assimilation, cell division, and development.
1. Introduction
For many years, soil scientists have endeavored to define the chemical features and the molecular structure of humic substances (HS), and discover how they can modify the growth and development of plants. A few studies came out with the hypothesis that HS might act in plants through two distinct mechanisms, of which one is indirect and achieved via amelioration of soil chemical, physical and biological properties, while the other implies a more direct effect of HS active components on the regulation of growth processes, nutrient transport systems, and primary and secondary metabolism [1][2].
The biological activity of HS in soil and plants, which is responsible for plant growth promotion, becomes relevant in the context of sustainable agriculture that claims for solutions to address major issues of environmental pollution and economic costs related to fertilizer inputs, while preventing crop yield and quality trade-off. The use of nitrogen-based fertilizers is one of the most energy consuming processes in agricultural practices and its burst is associated to detrimental environmental consequences and significant releases of reactive N species (except N2) in the atmosphere. Because only a limited amount of nutrients in fertilizers can be promptly used by plants (e.g., only 30–50% of applied N fertilizers), attention is paid to low-impact agriculture approaches aimed to increase plant nutrient use acquisition and efficiency.
Among these strategies, the use of biostimulants is relevant, of which HS category is part [1][2][3]. Biostimulants by definition are substances that promote plant growth, nutrition and metabolism through modes of action that are challenging to decipher, but definitely different from those related to fertilizers [4]. They are supplied to plants at very low dosage in order to induce beneficial effects, thereby they cannot provide any nutritional substance to plants directly [5][6][7]. Rather, they stimulate the capacity of plants to better acquire nutrients and use them for primary and secondary metabolism, and biomass production. They also aid plants to overcome stress conditions by eliciting the upregulation of enzymatic and non-enzymatic antioxidant systems [8][9][10][11].
2. Structure of Humic Substances
While studying complex molecules, the first analytical approach is generally aimed to identify their molecular composition and, if necessary, the sequences of the individual components and which type of chemical bonds is implied. However, this method is not applicable to HS, whose bonds are more difficult to break down and the structural units are highly diversified and do not assemble in a regular sequence as in the typical bio-macromolecules (e.g., proteins, nucleic acids).
So far, the study of HS composition has been carried out under the action of strong oxidants (alkaline solution) or heat to determine the single structural units [12][13][14]. Nevertheless, the reactions obtained with either alkaline extraction or heat are extremely reactive, leading to the production of many artifacts that make the molecular recognition process further complicated. For this reason, linking the degradation products to their parent compounds is a very intricate issue.
Alkaline extraction, first used by Achard [15], remains; however, the most common method for detecting the solubility of HS from soil, according to the International Humic Substances Society (IHSS) [16]. This type of extraction ensures maximal yields of organic material, since most of the organic matter is intimately bound to mineral colloids (Figure 1). Other extraction procedures using organic solvents [17] do not provide similar efficiency because the associations between mineral and organic colloids are of high structural complexity and binding strength [18][19].
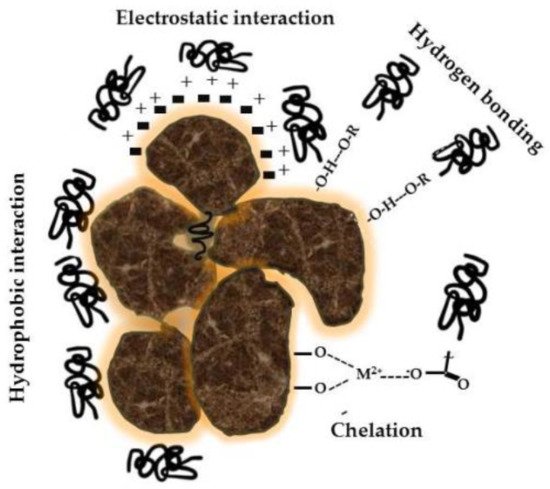
Figure 1. Associations between mineral colloids and humic substances are characterized by a variety of interactions and chemical bonds that make these structures stable in soils.
In the early 21st century, a few researchers began to dismiss the terminology associated with HS [20] and renamed humic substances as the fraction of organic matter that remains structurally unknown [21][22][23]. This was due to concerns about the effectiveness of alkaline extraction and the chemical alterations caused by this procedure on the HS structure [24]. In addition, some studies rejected the hypothesis of any apparent relationship between the biological function of soil organic material and its alkali-extracted fractions by postulating that alkali extracts do not exhibit the same properties that they acquire during the humification process [21][22]. Conversely, comparing the 13 C NMR spectra of the extracted material with those of the native soil substances, Weber et al. [25] concluded that alkaline extraction does not alter the HS structure. Also, the same classes of substances were detected in a soil and in its derived-humic fractions and humin using pyrolysis-field ionization mass spectroscopy [18].
The debate is still ongoing, as highlighted by several remarks in response to these criticisms [19][26][27]. In this context, the sticking point is the absence of any chemical structure to be used as a reference control [28].
The HS elemental composition has been extensively studied and well documented [16][17]. Briefly, the content of various elements (C, H, N, O) of the IHSS standard and reference fulvic and humic acids ranged as follows: C from 50 to 60%, N from 0.7 to 5.1%, H from 3.5 to 4.8%, and O from 31.6 to 45.5% [29]. Overall, the average elemental composition of HS from various sources is reasonably consistent in the literature [17][30][31].
Typically, HS bear functional groups (Figure 2) that contain oxygen (O), primarily in carbonyl (−C=O), carboxyl (C(=O)OH) attached to an R group, and hydroxyl (-OH) groups in alcohols and phenols; nitrogen (N) sets in functional groups of amines and amides, while sulfur (S−) in sulfhydryl groups. The various functions of HS are specially allocated to the carboxylic and phenolic groups, which are responsible for the weak acidity properties [29]. The concentrations of carboxyl and phenolic groups are commonly determined by direct titration, and usually range from 3.8 to 6.7 mmol g−1 and from 1.0 to 2.2 mmol g−1, respectively [29]. The pKa of most acidic groups ranges from 5 to 7.
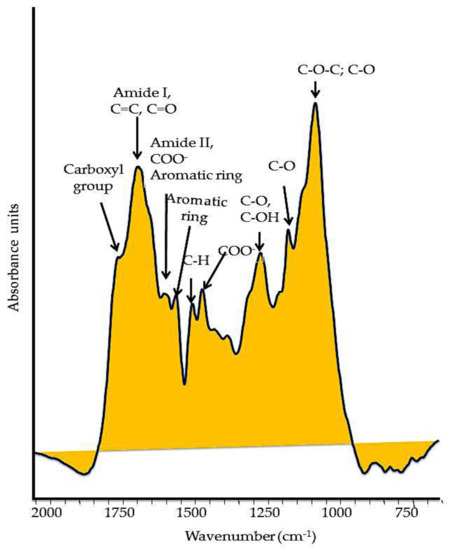
Figure 2. Typical FT–IR spectrum of a soil humic substance. The main oxygenated functional groups are reported in the spectrum.
The elemental composition, and consequently the functional groups, are strongly influenced by the pedo-climatic conditions [17], as well as by anthropogenic activities. In a comparative study by Plaza and Senesi [32], HS fractions extracted from soils that received different organic fertilizers (animal manures, composts, sewage sludge, and olive oil mill wastewaters) exhibited elemental compositions, E4/E6 ratio, fluorescence spectra, FT-IR spectra, 13C NMR spectra, organic free radical concentrations, intermediate between each amendment and native HS fractions from untreated soils. An indication of these results is that the HS fractions were susceptible to recent soil management with organic fertilizers. Recently, Pospíšilová et al. [33] studied the effect of biochar, compost, and digestate on HS structure. The authors concluded that the structural modifications detected in soil HS depended on the chemical characteristics of the amending materials. The electron paramagnetic resonance (EPR) spectra revealed that the fertilization with different organic materials led to changes in the HS magnetic properties due to the variable concentration and structure of radicals, while FT-IR spectra identified structural differences in HS mostly related to aliphatic and aromatic groups.
The study carried out by Hatcher et al. [34] using 13C-NMR showed that 35–40% of the humic structures is made up of single ring aromatic units. The authors assumed that fused aromatic structures are a trivial component of humic substances. Moreover, by examining the HS spectra, other C functional groups could be recognized that are associated with distinct molecular structures: alkyl C (aliphatic hydrocarbons, lipids), O-alkyl C (sugar-like), and carboxyl (peptide-like and organic acids).
The carbon-14 analysis of different humic fractions extracted from the same soil revealed that the older fractions gained higher proportions of aromatic and carboxylic C [35]. A similar process can be observed during the time sequence of coalification from peat to lignite and up to hard coals. During the coalification process, there is loss of moisture, volatile compounds, and consequently the concentration of C and aromatic macromolecules increases [25][36].
Recently, in agreement with elemental analysis, quantitative solid-state 13C NMR spectra has demonstrated that HS standards by IHSS contain a large fraction (28% and 33%) of polycondensed rings not bound to H or O and, oxygen-bonded non-protonated carbons, such as aryl ketone. Other constituents like C in alkyl, and -COOH groups are additionally present [37].
The aromatic nature of HS can be deemed as an indicator of stability against chemical and biological degradation [9]. In particular, the stability of HS seems to be associated with the formation of a complex and heterogeneous molecular network providing certain recalcitrance. In this context, the HS stabilization also occurs by adsorption of functional groups on clay mineral surfaces and through physical protection, within the pores of soil clay particles resulting in limited accessibility of microbes and enzymes [38][39]. Thus, a deeper comprehension of organo-mineral interactions is importantly advisable, since it may yield new approaches for soil carbon sequestration through HS stabilization.
Phenolic compounds have traditionally been considered as the main “building blocks” of humic substances [17]. In particular, phenolic acids, i.e., chemical compounds with an aromatic core and phenolic and carboxylic functions (Figure 3A), are valued at up to 35% in HS [40].
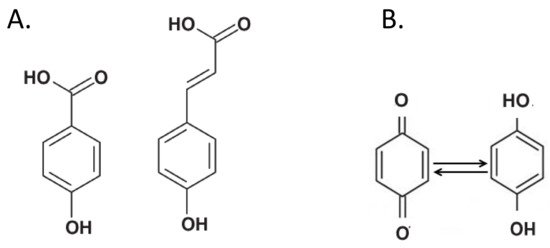
Figure 3. (A) Typical chemical structure of phenolic acids. These compounds are considered major components of soil humic substances. (B) Quinones (left) are groups that accept electrons and are reduced to hydroquinones (right).
Research on HS has confirmed the role of dihydroxyaromatic acids as structural building blocks functioning in metal complexation [30][40][41].
A characteristic property of phenols is related to their reducing capacities or electron-donating capacities (EDCs) [30][39][42][43]. In a recent research, the EDCs of HS were investigated by electrospray ionization (ESI) coupled with Fourier transform ion cyclotron resonance mass spectrometry (FT-ICR-MS), total phenolic content and mediated electrochemical oxidation (MEO) analysis [44]. A strong linear correlation was found between EDCs, total phenols, and the proportion of polyphenolic formulas by ESI-FT-ICR-MS, containing medium oxygen content (0.4 ≤ O/C ≤ 0.67). The study confirmed that the major electron-donating capacities were due to the presence of phenolics, particularly polyphenolic compounds.
Quinones are electron-accepting groups of phenol origin that are first reduced to semiquinones, and then to hydroquinones (Figure 3B), i.e., compounds of higher stability [43]. The quinones can perform a redox cycle with their semi-quinone radicals and cause the formation of reactive oxygen species (ROS). The semiquinone-type free radical concentration (SFRC) in humus was used to assess the soil C stability. The SFRC was estimated by electron spin resonance (ESR) spectroscopy and correlated with indexes estimated by ultraviolet-visible (E4/E6), fluorescence intensity (FI), 13C NMR and FT-IR spectroscopies of HS [45][46]. More recently, the low-molecular-weight HS fractions were found to exhibit great reducing capacity due to the presence of a large amount of quinones [47].
The structure of HS is operationally defined in (i) humic acids (HA), which are the fraction soluble in alkali, but insoluble during subsequent acidification, and (ii) fulvic acids (FA), which are soluble in both alkali and acids [17]. At alkaline pH, phenolic and carboxyl groups are extensively deprotonated, and the repulsion forces favor the dispersion of HS because intramolecular hydrogen bonds are completely disrupted [48][49][50]. Rheological results confirmed the extending configuration of the HS at alkaline pH and its ability to increase repulsive forces in suspension, promoting their dispersion [51]. As the pH decreases, functional groups are protonated and repulsion effects decrease, driving the molecule to dispose on a coiled structure, which is followed by intermolecular aggregation [18][30][31][48][49][50]. The coiled configuration leads to complete expulsion of the water molecules surrounding the HS surface and, as a consequence, the HS becomes insoluble and precipitate [18][30][31][51]. This effect is also observed by treating HS with weak acids, which cause the decrease of HS apparent molecular size or disintegration as weak non-covalent interactions, such as van der Waals, π-π, and CH-π, are disrupted [48][52]. Such processes may be mimicking the activity of root exudates, containing low molecular weight organic acids, and influence the molecular size and solubility of HS in the soil [53][54]. In addition, the carboxyl groups also contribute to determining HS solubility and biological reactivity [55][56].
References
- Canellas, L.P.; Oliveras, F.L. Physiological responses to humic substances as plant growth promoter. Chem. Biol. Technol. Agric. 2014, 1, 3.
- Nardi, S.; Ertani, A.; Francioso, O. Soil–root cross—Talking: The role of humic substances. J. Plant Nutr. Soil Sci. 2017, 180, 5–13.
- Jung, H.; Kwon, S.; Kim, J.-H.; Jeon, J.-R. Which traits of humic substances are investigated to improve their agronomical value? Molecules 2021, 26, 760.
- Regulation EU (2019/1009) of the European Parliament of the of the Council of 5 June 2019 laying down rules on the making available on the market of EU fertilising products and amending Regulations EC No 1069/2009 and EC No 1107/2009 and repealing Regulation EC No 2003/2003. Off. J. Eur. Union 2019, 25, 6.
- Nardi, S.; Pizzeghello, D.; Schiavon, M.; Ertani, A. Plant biostimulants: Physiological responses induced by protein hydrolyzed-based products and humic substances in plant metabolism. Sci. Agric. 2016, 73, 18–23.
- Yakhin, O.I.; Lubyanov, A.A.; Yakhin, I.A.; Brown, P.H. Biostimulants in plant science: A global perspective. Front. Plant Sci. 2017, 7, 2049.
- Rouphael, Y.; Colla, G. Synergistic biostimulatory action: Designing the next generation of plant biostimulants for sustainable agriculture. Front. Plant Sci. 2018, 9, 1655.
- Calderín-García, A.; Azevedo-Santos, L.; de Souza, L.G.A.; Huertas-Tavares, O.C.; Zonta, E.; Gomes, E.T.M.; Garcia-Mina, J.M.; Louro Berbera, R.L. Vermicompost humic acids modulate the accumulation and metabolism of ROS in rice plants. J. Plant Physiol. 2016, 192, 56–63.
- Calderin-Garcia, A.; de Souza, L.G.A.; Pereira, M.G.; Castro, R.N.; Garcia-Mina, J.M.; Zonta, E.; Lisboa, F.J.G.; Berbara, R.L.L. Structure-property-function relationship in humic substances to explain the biological activity in plants. Sci. Rep. 2016, 6, 20798.
- Canellas, L.P.; da Irineu, L.E.S.; Olivares, F.L.; Piccolo, A. Plant chemical priming by humic acids. Chem. Biol. Technol. Agric. 2020, 7, 12.
- Yoon, H.Y.; Jeong, H.J.; Yung Cha, J.; Choi, M.; Soon Jang, K.; Kim, W.Y.; Kim, M.G.; Jeon, J.R. Structural variation of humic-like substances and its impact on plant stimulation: Implication for structure-function relationship of soil organic matters. Sci. Total Environ. 2020, 725, 138409.
- Schnitzer, M.; Khan, S.U. Soil Organic Matter; Elsevier Scientific Pub. Co.: New York, NY, USA, 1978.
- Hayes, M.H.B.; Swift, R.S. The chemistry of soil organic colloids. In The Chemistry of Soil Constituents; Greenland, D.J., Hayes, M.H.B., Eds.; Wiley: Hoboken, NJ, USA, 1978; pp. 175–320.
- Clapp, C.E.; Hayes, M.H.B.; Simpson, A.J.; Kingery, W.L. The chemistry of soil organic matter. In Chemical Processes in Soils; Tabatabai, A., Sparks, D.L., Eds.; Book Series No. 8; Soil Science Society of America: Madison, WI, USA, 2005; pp. 1–150.
- Achard, F.K. Chemische Untersuchung des Torfs. Crell’s Chem. Ann. 1786, 2, 391–403.
- IHSS. What Are Humic Substances? International Humic Substances Society. 2019. Available online: (accessed on 4 January 2019).
- Stevenson, F.J. Humus Chemistry: Genesis, Composition, Reactions; John Wiley & Sons: Hoboken, NJ, USA, 1994.
- Schnitzer, M.; Monreal, C.M. Quo vadis soil organic matter research? A biological link to the chemistry of humification. Adv. Agron. 2011, 113, 143–217.
- Hayes, M.H.B.; Swift, R.S. Vindication of humic substances as a key component of organic matter in soil and water. Adv. Agron. 2020, 163, 1–37.
- Lützow, M.; Kögel-Knabner, I.; Ekschmitt, K.; Matzner, E.; Guggenberger, G.; Marschner, B.; Flessa, H. Stabilization of organic matter in temperate soils: Mechanisms and their relevance under different soil conditions—A review. Eur. J. Soil Sci. 2006, 57, 426–445.
- Hedges, J.I.; Eglinton, G.; Hatcher, P.G.; Kirchman, D.L.; Arnosti, C.; Derenne, S.; Evershed, R.P.; Kogel-Knabner, I.; de Leeuw, J.W.; Littke, R.; et al. The molecularly-uncharacterized component of nonliving organic matter in natural environments. Org. Geochem. 2000, 31, 945–958.
- Baldock, J.A.; Broos, K. Soil organic matter. In Handbook of Soil Sciences; CRC Press: Boca Raton, FL, USA, 2011; pp. 11–52.
- Kleber, M.; Lehmann, J. Humic substances extracted by alkali are invalid proxies for the dynamics and functions of organic matter in terrestrial and aquatic ecosystems. J. Environ. Qual. 2019, 48, 207–216.
- Lehmann, J.; Kleber, M. The contentious nature of soil organic matter. Nature 2015, 528, 60–68.
- Weber, J.; Chen, Y.; Jamroz, E.; Miano, T. Preface: Humic substances in the environment. J. Soils Sediments 2018, 18, 2665–2667.
- Olk, D.C.; Bloom, P.R.; Perdue, E.M.; McKnight, D.M.; Chen, Y.; Farenhorst, A.; Senesi, N.; Chin, Y.P.; Schmitt-Kopplin, P.; Hertkorn, N.; et al. Environmental and Agricultural Relevance of Humic Fractions Extracted by Alkali from Soils and Natural Waters. J. Environ. Qual. 2019, 48, 217–232.
- Dou, S.; Shan, J.; Song, X.; Rui Cao, R.; Wu, M.; Li, C.; Guan, S. Are humic substances soil microbial residues or unique synthesized compounds? A perspective on their distinctiveness. Pedosphere 2020, 30, 159–167.
- Calderin García, A.; van Tol de Castro, T.A.; Santos, L.A.; Tavares, O.C.H.; Castro, R.N.; Berbara, R.L.L.; García-Mina, J.M. Structure-property-function relationship of humic substances in modulating the root growth of plants: A review. J. Environ. Qual. 2019, 48, 1622–1632.
- Ritchie, J.D.; Perdue, E.M. Analytical constraints on acidic functional groups in humic substances. Org. Geochem. 2008, 39, 783–799.
- de Melo, B.A.; Lopes Motta, F.; Andrade Santana, M.H. Humic acids: Structural properties and multiple functionalities for novel technological developments. Mat. Sci. Eng. C 2016, 62, 967–974.
- Olaetxea, M.; de Hita, D.; Calderin Garcia, A.; Fuentesa, M.; Baigorri, R.; Mora, V.; Garnica, M.; Urrutia, O.; Erro, J.; Zamarreño, A.M.; et al. Hypothetical framework integrating the main mechanisms involved in the promoting action of rhizospheric humic substances on plant root- and shoot growth. Appl. Soil Ecol. 2018, 123, 521–537.
- Plaza, C.; Senesi, N. The effect of organic matter amendment on native soil humic substances. In Biophysico-Chemical Processes Involving Natural Nonliving Organic Matter in Environmental Systems; Senesi, N., Xing, B., Huang, P.M., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2009; pp. 147–181.
- Pospíšilová, L.; Horáková, E.; Fišera, M.; Jerzykiewicz, M.; Menšík, L. Effect of selected organic materials on soil humic acids chemical properties. Environ.Res. 2020, 187, 109663.
- Hatcher, P.G.; Schnitzer, M.; Dennis, L.W.; Maciel, G.E. Aromaticity of Humic Substances. Soil Sci. Soc. Am. 1981, 45, 1089–1094.
- Legorreta Padilla, F.J. The Impact of Maize and Soybean Cropping Systems on Carbon and Nitrogen Dynamics in Soil Organic Matter; The University of Nebraska-Lincoln, ProQuest Dissertations Publishing: Ann Arbor, MI, USA, 2005.
- Wang, C.F.; Fan, X.; Zhang, F.; Wang, S.Z.; Zhao, Y.P.; Zhao, X.Y.; Zhao, W.; Zhu, T.G.; Lu, J.L.; Wei, X.Y. Characterization of humic acids extracted from a lignite and interpretation for the mass spectra. RSC Adv. 2017, 7, 20677–20684.
- Cao, X.; Schmidt Rohr, K. Abundant nonprotonated aromatic and oxygen-bonded carbons make humic substances distinct from biopolymers. Environ. Sci. Technol. Lett. 2018, 5, 476–480.
- von Lützow, M.; Kögel-Knabner, I.; Ekschmitt, K.; Flessa, H.; Guggenberger, G.; Matzner, E.; Marschner, B. Som fractionation methods: Relevance to functional pools and to stabilization mechanisms. Soil Biol. Biochem. 2007, 39, 2183–2207.
- Lipczynska-Kochany, E. Humic substances, their microbial interactions and effects on biological transformations of organic pollutants in water and soil: A review. Chemosphere 2018, 202, 420–437.
- Lehtonen, T.; Peuravuori, J.; Pihlaja, K. Degradative analysis of aquatic fulvic acid: CuO oxidation versus pyrolysis after tetramethylammonium hydroxide treatments in air and helium atmospheres. Anal. Chim. Acta 2004, 511, 349–356.
- Borges, F.; Guimaraes, C.; Lima, J.L.F.C.; Pintoc, I.; Reisc, S. Potentiometric studies on the complexation of copper(II) by phenolic acids as discrete ligand models of humic substances. Talanta 2005, 66, 670–673.
- Alberts, J.J.; Schindler, J.F.; Miller, R.W.; Nutter, E. Elemental mercury evolution mediated by humic acid. Science 1974, 184, 895–897.
- Aeschbacher, M.; Graf, C.; Schwarzenbach, R.P.; Sander, M. Antioxidant properties of humic substances. Environ. Sci. Technol. 2012, 46, 4916–4925.
- Lv, J.; Han, R.; Huang, Z.; Luo, L.; Cao, D.; Zhang, S. Relationship between molecular components and reducing capacities of humic substance. ACS Earth Space Chem. 2018, 2, 330–339.
- Canellas, L.P.; Baldotto, M.A.; Busato, J.G.; Marciano, C.R.; Menez, S.C.; Silva, N.M.; Rumjanek, V.M.; Velloso, A.C.X.; Simões, M.L.; Martin-Neto, L. Estoque e qualidade da matéria orgânica de um solo cultivado com cana-de-açúcar por longo tempo. R. Bras. Cienc. Solo 2007, 31, 331–340.
- Canellas, L.P.; Zandonadi, D.B.; Busato, J.G.; Baldotto, M.A.; Simões, M.L.; Martin-Neto, L.; Façanha, A.R.; Spaccini, R.; Piccolo, A. Bioactivity and chemical characteristics of humic acids from tropical soils sequence. Soil Sci. 2008, 173, 624–637.
- Yang, Z.; Kappler, A.; Jiang, J. Reducing capacities and distribution of redox-active functional groups in low molecular weight fractions of humic acids. Environ. Sci. Technol. 2016, 50, 12105–12113.
- Smejkalova, D.; Piccolo, A. Supramolecular assemblies by NMR diffusion ordered spectroscopy (DOSY-NMR). Environ. Sci. Technol. 2008, 42, 699–706.
- Corrado, G.; Sanchez-Cortes, S.; Francioso, O.; Garcia-Ramos, J.V. Surface-enhanced Raman and fluorescence joint analysis of soil humic acids. Anal. Chim. Acta 2008, 616, 69–77.
- Colombo, C.; Palumbo, G.; Angelico, R.; Goo-Cho, H.; Francioso, O.; Ertani, A.; Nardi, S. Spontaneous aggregation of humic acid observed with AFM at different pH. Chemosphere 2015, 138, 821–828.
- Souza, F.; Bragança, S.R. Extraction and characterization of humic acid from coal for the application as dispersant of ceramic powders. J. Mater. Res. Technol. 2018, 7, 254–260.
- Piccolo, A.; Conte, P. Molecular size of humic substances. Supramolecular associations versus macromolecular polymers. Adv. Environ. Res. 2000, 3, 508–521.
- Nardi, S.; Concheri, G.; Pizzeghello, D.; Sturaro, A.; Rella, R.; Parvoli, G. Soil organic matter mobilization by root exudates. Chemosphere 2000, 41, 653–658.
- Piccolo, A.; Conte, P.; Spaccini, R.; Chiarella, M. Effects of some dicarboxylic acids on the association of dissolved humic substances. Biol. Fertil. Soils 2003, 37, 255–259.
- Muscolo, A.; Sidari, M.; Francioso, O.; Tugnoli, V.; Nardi, S. The auxin-like activity of humic substances is related to membrane interactions in carrot cell cultures. J. Chem. Ecol. 2007, 33, 115–129.
- Muscolo, A.; Pizzeghello, D.; Francioso, O.; Sanchez Cortes, S.; Nardi, S. Effectiveness of humic substances and phenolic compounds in regulating plant-biological functionality. Agronomy 2020, 10, 1553.





