Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Eleonora Russo | + 4425 word(s) | 4425 | 2021-05-06 11:50:17 | | | |
| 2 | Lily Guo | Meta information modification | 4425 | 2021-05-08 08:53:51 | | | | |
| 3 | Lily Guo | Meta information modification | 4425 | 2021-05-08 08:55:06 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Russo, E. Hydrogels in Hand Sanitizers. Encyclopedia. Available online: https://encyclopedia.pub/entry/9383 (accessed on 07 February 2026).
Russo E. Hydrogels in Hand Sanitizers. Encyclopedia. Available at: https://encyclopedia.pub/entry/9383. Accessed February 07, 2026.
Russo, Eleonora. "Hydrogels in Hand Sanitizers" Encyclopedia, https://encyclopedia.pub/entry/9383 (accessed February 07, 2026).
Russo, E. (2021, May 07). Hydrogels in Hand Sanitizers. In Encyclopedia. https://encyclopedia.pub/entry/9383
Russo, Eleonora. "Hydrogels in Hand Sanitizers." Encyclopedia. Web. 07 May, 2021.
Copy Citation
Hand hygiene can be considered a strategic key useful in the containment of infections such as COVID-19 both at home and in communities because it can dramatically reduce the widespread outbreak of infections.
Hydrogels
Hand Sanitizers
disinfectants
COVID-19
1. Introduction
It has been estimated that there are not less than 10,000 organisms per cm2 of normal skin, pathogenic transient flora included [1], and hands are regarded as one of the principal sites responsible for transmitting infections, such as pandemic ones [2][3][4]. Therefore, hand hygiene and disinfection can be considered strategic keys in the containment of several infections, such as COVID-19, both at home and in communities because they can dramatically reduce the widespread outbreak of pathogens and they can also prevent the transmission of them to food [5]. Hand sanitization includes (1) handwashing, in particular using a common soap in the presence of water; (2) handwashing, using a detergent (possibly antiseptic ones) with water; and (3) hand sanitization using alcoholic hand rubs [6].
The Centers for Disease Control and Prevention (CDC) recommends washing hands with soap and water for at least 20 s. Rinse-off detergents are considered better performing than hand rub sanitizers in the removal of certain pathogens such as Norovirus, Cryptosporidium, and Clostridioides difficile [7][8], but when they are not available, or when repeated hand washing alters the skin’s natural barrier [9], “instant” hand sanitizers are recommended [10]. The main goal of these topic sanitizers (antiseptic handrub or handrub products) is to remove or reduce the level of transient bacteria and viruses. In particular, an “instant” hand sanitizer is intended to be applied to dry hands, rubbed thoroughly over the fingers and hand surfaces for at least 30 s, and completely air-dried. They are formulated as foam, gel, or liquid preparations [11][12][13] and they can be classified as alcohol-based rubs (ABR) or alcohol-free rubs (AFR), according to the active, antiseptic ingredients used. Their application can be considered more versatile, convenient, quick, and less irritating [14][15] when compared with the use of rinse-off detergents. ABRs generally contain alcohol, water, and other ingredients (in particular humectants and emollients); hands are their target to quickly destroy microorganisms and suppress their growth, in a broad germicidal spectrum. Nevertheless, their effect on pathogens seems short-lived and they do not have a strong activity against protozoa, bacterial spores, and some non-enveloped viruses [4].
The “WHO Guidelines on Hand Hygiene in Health Care: First Global Patient Safety Challenge Clean Care Is Safer Care” provides a useful scientific review on hand hygiene argument and suggests the best procedures in health care. The WHO describes a sanitizing hand rub as: “An alcohol-containing preparation (liquid, gel or foam) designed for application to the hands to inactivate microorganisms and/or temporarily suppress their growth. Such preparations may contain one or more types of alcohol, other active ingredients with excipients, and humectants” [16]. The Centers for Disease Control and Prevention (CDC) and the World Health Organization recommend the use of ABR formulations containing 60 to 95% alcohol as the best practice for hand sanitization, but only when hands are not noticeably dirty [17]. ABRs are considered a better performing tool in minimizing hand contamination, especially when compared to soap and water [14]; however, the activity against non-enveloped viruses is still debated [18][19][20][21][22][23][24], particularly for formulations containing < 75% alcohol [25][26][27]. Moreover, only a few researches refer to skin toxicity due to high alcohol content [28]. To help countries in the adoption of alcoholic hand rubs as the best practice for hand hygiene and sanitization, the WHO has identified two simple formulations for local preparation, when commercial products may be unavailable [29]. These formulations (Table 1) are reported in the “Guide to Local Production: WHO-recommended Handrub Formulations”. The choice of the selected ingredients is due to three main factors: low cost, availability, and microbicidal activity [21]. These formulations are recommended for local production, recommending a maximum of 50 L per lot in order to ensure safe production and storage.
Table 1. Liquid formulations recommended by the WHO.
| Ethanol 96%: 80% v/v Hydrogen peroxide 3%: 1.45% v/v Glycerol 98%: 0.125% v/v Water |
| 2 Isopropyl alcohol 99.8%: 75% v/v Hydrogen peroxide 3%: 1.45% v/v Glycerol 98%: 0.125% v/v Water |
The denatured alcohol works as the topical antiseptic or antimicrobial agent; hydrogen peroxide is included to inactivate contaminating bacterial spores in the final solution, but it cannot be considered an antiseptic ingredient. Glycerin is useful as the humectant agent; it affects the viscosity of the final product and provides a minimal level of moisturization to the skin, but an excessive amount of glycerin can reduce the germicidal activity of isopropanol and ethanol, as cited by a footnote of the World Health Organization (glycerin mixed with alcohols forms an azeotrope that can affect their activity. As an alternative, PEG-10 dimethicone and PEG-7 glyceryl cocoate can be use as refatting agents [30]). Water performs as a solvent and vehicle to help deliver the final product to the skin. The addition of perfumes or dyes is not recommended.
Alcohol-free products (AFR) contain chemicals (biocides) with antiseptic properties, often used at low concentrations, and can be considered relatively safer than ABR, especially for children, also being non-flammable [31][32][33]. However, they are less preferred by the health organizations [34][35] for fighting COVID-19 because of their lower efficacy and because they are not broad-spectrum agents [36]. Their antimicrobial action can be affected by different variables, such as other ingredients in the formulation components, dilution, the presence of an organic load, etc.
These liquid formulations present some difficulties to handle, potentially leading to the delivery of insufficient doses of active agents on the hands and to an overall reduction in hygiene compliance [37][38][39]. In a recent study on ABRs [40], researchers investigated how many elements such as skin health, education, and user acceptance of ABRs might affect healthcare workers’ hand sanitization during and after application. The results show that despite the benefits that liquid products give (clean sensation, smooth and moisturized feel), the difficult handling and applying of the products cancel out the advantages of such formulations. Even if the WHO has recommended and described the preparation of two liquid hydro-alcoholic hand rub formulations, in the consumer market, hydrogel sanitizers are becoming increasingly popular. In fact, viscosity plays a significant role in many key aspects of a hand sanitizer gel’s functionality. Efficiency, performance, and customer perception are closely linked to viscosity values. The literature reports only a few papers that highlight the role of hydrogels in hand disinfection, but these semi-solid preparations present numerous advantages over liquid forms, not only for their ability to disinfect, but also for the ease with which they can be dispensed and used on-the-go. Hydrogels can be considered more desirable than liquid forms thanks to fast absorption and drying, a pleasant hand feel, absence of stickiness, mild smell, and clean and cold sensation during application. Coldness can also help in monitoring the complete hand covering. Hydrogels, when compared to liquid-based preparations, are easier to have at hand and more practical to deliver on-the-spot, because of their simplicity of delivery and low risk of leakage. Moreover, they can reduce the alcoholic evaporation rate, allowing a better spreadability and a deeper penetration through contaminating organisms. On the other side, they can present negative features such as skin dehydration after prologued use and a stinging sensation for contact to broken skin. As regards adverse reactions, the most commonly reported ones are allergic and irritant contact dermatitis [36]. The main problem regards the depletion of the skin lipophilic defense, in particular after a repeated and prolonged exposure to fat-dissolving alcohols [41][42]. In a study carried out on a selected group of nurses, the compliance of a number of sanitizing formulations was investigated. It emerged that all the nurses chose liquids as the least favorite format, mainly for the difficulties in application, for the low covering, low doses, and unpleasant, uncontrolled dripping. Liquid bowls were also more difficult to handle than gel and foam dispensers [16][40].
Taking into account all these statements, the aim of this review is to highlight the properties and advantages of hydrogels in regard to hand sanitizers, with particular attention to alcohol-based hydrogels that can be considered the best performing and most active topic infection preventive tools [43]; having different compositions, sanitizing hydrogels need a deep study for their correct formulation together with an appropriate labelling, dispenser, and closure so as to achieve a proper dose/amount of the sanitizer for an efficient disinfection on each use [44]. For a better comprehension of all these concepts, the review will deal with different aspects related to the sanitizing approach such as the main biological differences between bacteria and viruses, the principal ingredients and products useful for their deactivation, the most important properties and characterizations of hydrogels, more information regarding carbomers and cellulose derivatives, and a brief overview on the current international regulation.
2. Sanitizing Hydrogels: Properties and Characterization
Hydrogels are three-dimensional, hydrophilic cross-linked polymeric networks extensively swollen with water (or biological fluids) [45]. Several parameters, such as the cross-linking degree of the polymer and its hydrophilicity [46][47][48], can significantly affect their properties. Hydrophilic polymers show the ability to swell in water and to hold more than 10% water within the gel’s network. This property depends on the presence of different functional groups on the polymeric chain, such as carboxylic (-COOH), hydroxylic (-OH), amidic (-CONH), and sulphonic (-SO3H) ones [49]. Hydrogel texture can be influenced by modifications in the structure and functionality of the polymer, in changes of its concentration and in the use of different cross-linkers. Moreover, new hydrogels have been studied and realized in different fields of engineering (environmental, biomedical), biotechnology, and many other contexts [50]. The growing interest in the topic can be easily checked by a quick search for the term “hydrogel” in the PubMed database that shows a significant exponential trend in the number of published papers regarding this item (Figure 1).
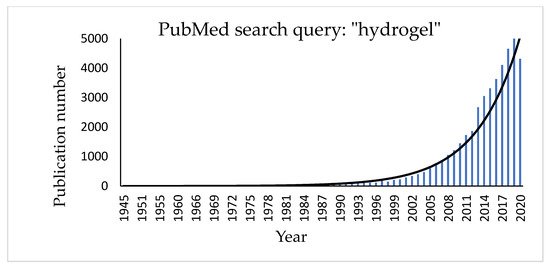
Figure 1. Histogram showing the increasing number of publications for the term “hydrogel” in the PubMed database.
The first hydrogels reported in the literature were described by Wichterle and Lím [51] who used poly (hydroxyethyl methacrylate) (PHEMA) hydrogel for soft disposable contact lenses. There are several advantages of a hydrogel in such an application: they are elastic, biocompatible, maintain the natural eye humidity, and allow oxygen diffusion from the outside.
Hydrogels can be ranked as natural and synthetic according to the nature of their polymers, configuration, electrical network charge, crosslinking, and physical appearance. Natural hydrogels such as proteins and polysaccharides have recently been replaced by synthetic polymers, due to the great advantages regarding, for example, biocompatibility and strength [52]. Synthetic hydrogels are obtained starting from homopolymers or copolymers by several preparation techniques such as bulk, solution, and suspension, by chemical or physical cross-linking pathways [53][54][55][56]. The “three-dimensional polymerization” occurs starting from a hydrophilic monomer with a crosslinking agent by direct or indirect crosslinking. Chemically cross-linked hydrogels are the most favorable since they have a good mechanical strength. They present covalent junctions between the polymeric chains, added by the cross-linking method [57]. In addition, the polymerization can be facilitated by employing specific initiators (ammonium peroxodisulphate, benzoyl peroxide or 2,2-azo-isobutyronitrile) or by UV and gamma radiations with electron beam. Another technique is presented by suspension polymerization or inverse-suspension polymerization which consists of dispersing a monomer in a hydrocarbon phase to give a W/O process with the addition of a suspending agent with a low hydrophilic–lipophilic balance (HLB) [58].
The most significant properties of hydrogels regard swelling, mechanical and rheological properties, biodegradability, and biocompatibility. The phenomenon of hydrogel swelling is the behavior that is observed when, in deep contact with water, the polymeric material relaxes its network system and expands towards a certain state of solvation [59].
The most important factors affecting the swelling properties of hydrogels are represented by the nature of solvents, the solvent–polymer interaction parameters and the network density [60]. Several studies regarding swelling have been carried on by immersion of the dried hydrogel into water and subsequently by removing and weighing it (after drying the medium excess from the surface). For the percentage of swelling ratio, the Rs of hydrogels can be defined by Equation (1):

where Ws is the weight of the swollen hydrogel and Wd is the original weight of the hydrogel before immersion in water. The Rs values were dramatically affected by the crosslinking degree: increasing this parameter decreases the Rs value, while with a low cross-linking degree, a higher hydrodynamic free volume of the network is observed because it has to store a greater amount of water, increasing the matrix swelling. The mobility and relaxation of the polymeric chains are prevented by an increase of the cross-linking degree, which prevent water mobility and consequently decrease the Rs values [61]. Water retention, Wr, can be obtained from Equation (2):
 where Wt represents the complexive mass of the hydrogels, at a defined time interval, Ws and Wd represent the hydrogel weight in the swollen and dried state, respectively [62]. Another theory that explains the swelling behavior of a hydrogel is the one proposed by Flory–Rehner, using Gibbs free energy, about equilibrium swelling theory [63]. This theory is based on the following equation:
where Wt represents the complexive mass of the hydrogels, at a defined time interval, Ws and Wd represent the hydrogel weight in the swollen and dried state, respectively [62]. Another theory that explains the swelling behavior of a hydrogel is the one proposed by Flory–Rehner, using Gibbs free energy, about equilibrium swelling theory [63]. This theory is based on the following equation:

where ΔG total represents the complexive free energy of the polymeric network, ΔGmix represents the free energy contributions deriving from the enthalpy of mixing, and ΔGel represents the free energy contribution derived from the elastic retractile network forces [64].
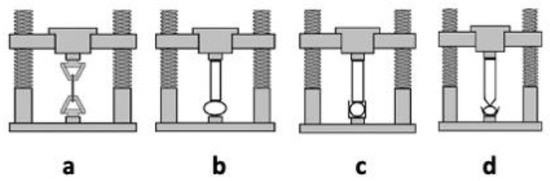
Hydrogels present mechanical properties that can be considered significant parameters for several biomedical applications in particular in drug delivery and tissue engineering [62]. A hydrogel should preserve its texture, for a given time, in order to deliver a drug at a required target; this behavior can be affected by the type and concentration of the crosslinking agent. The crosslinking degree ensures the hydrogels’ stable mechanical and elastic properties: an optimal crosslinking degree must be obtained to have a relatively strong and yet elastic hydrogel [65]; an increase of this value leads to a stronger hydrogel, even if the higher crosslinking degree decreases the percentage of hydrogel elongation, creating a more brittle structure. Different techniques, such as tension, compression (either confined or unconfined), and indentation testing (Figure 2) can be applied to measure the mechanical properties of hydrogels. During the tensile test (a), the sample is placed between two clamps; the two ends, thus secured, are detached by applying a force until breaking [66]. The tensile test takes advantage of a dynamometer with a load cell, obtaining stress-strain curves useful to obtain several mechanical properties (i.e., Young’s modulus, yield strength, and ultimate tensile strength).

Figure 2. Testing methods applied to assess mechanical properties of hydrogels: tensile testing (a), unconfined compression testing (b), confined compression test (c), and indentation testing (d).
The compression tests are carried out in an unconfined model (b) or confined one (c and d). In the first model (b), two plates compress the hydrogel, which is placed between the two punches. In the second test (confined compression, c), the hydrogel is confined inside a sample holder and compressed by an upper punch. In the indentation testing (d), the hydrogels are serrated by a probe, of defined shape, that penetrates the thickness for a given depression, then measuring the specific force needed to lead to this indentation [67].
Rheology is useful to investigate different mechanical properties such as the mechanical strength and flow of hydrogels and can therefore be considered a basic tool for the characterization of industrially significant properties. Moreover, rheological measurements can provide information related to the internal structure of soft materials, according to their response as regards dynamic behavior. They are useful tools for studying bulk phase transitions, in particular solution-to-gel (solgel) transitions, which can be induced by significant changes of pH, concentration, and temperature [68]. The physical structure and rheological properties are significant parameters to be considered for strategic hydrogel applications in biomedical contexts; in this case, the rheological behavior of the studied material is measured by a rheometer whose several available shapes can ensure well-defined conditions of flow for a rheological experiment. “Concentric cylinders” (Couette), “cone-and-plate”, and “parallel disks” are the most commonly applied instruments [69]. Rheology techniques can also be applied for characterizing gelation behavior such as the crosslinking degree and structural properties (homogeneity/heterogeneity) [70]. In the case of hydrogels used for hand sanitization, it is very important to have the correct viscosity that allows the formulation to be dispensed in the appropriate dose and a good spreading coefficient that guarantees the complete covering of the skin. A reasonably high viscosity is relevant for the spreadability of skin formulations. However, it is still not well defined how increasing viscosities from fluid to semi-solid formulations will affect skin penetration. As regards the impact of rheological formulation properties on skin penetration, scientific conclusions are controversial. A recent work reported that the optimal viscosity values for a good hand sanitizer gel are 47,000 to 150,000 mPa.s [71], meeting the standards set by Zatz and Kushla [72].
3. Natural and Synthetic Polymers in Sanitizing Hydrogels
A great variety of natural and synthetic polymeric compounds, commonly used in the pharmaceutical, cosmetic and food fields, are available to obtain hydroalcoholic and non-alcoholic sanitizing hydrogels. Each of them is available in several grades, presenting different thickening behavior, rheological properties, solubility, and classification (pharmaceutical, cosmetic, or food grade). Thickening properties can change according to several parameters such as pH, presence of electrolytes, and the addition of excipients. In order to provide a sort of general guide for selecting thickeners in the development of hydrogel hand sanitizers, in Table 1, we have reported the most common synthetic and natural polymers available on the market useful as gelling agents in AFR and ABR, accompanied by the most significant data that can influence their rheological behavior (as reported by suppliers) such as dosage range, eventual maximum alcoholic amount (in the case of ABR) and pH range. As regards “electrolyte tolerance”, we could only give approximative levels (low, good, and very good) as found in several technical data sheets collected, and it must be said that despite the same term, the numerical meaning can be very different from one company to another.
Table 1. Most common natural and synthetic polymers useful as rheologic modifiers in hydroalcoholic and non-alcoholic sanitizing hydrogels.
| Chemical Name (INCI) | Trade Name (Supplier) |
Dosage Range (%) |
Max EtOH Amount (% v/v) |
pH Range | Electrolyte Tolerance |
|---|---|---|---|---|---|
| Carbomer | CARBOPOL ULTREZ 10 (Lubrizol) |
0.1 to 0.5 | 60 to 95 (according to neutralizer) |
5 to 9 | low |
| CARBOPOL 980 (Lubrizol) ASHLAND 980 Carbomer (Ashland) |
0.1 to 0.5 | 60 to 80 (according to neutralizer) |
5 to 10 | low | |
| TEGO Carbomer 140 (Evonik) |
0.05 to 1.0 | 60 to 95 | 3 to 10 | low | |
| CARBOPOL 940 (Lubrizol) ASHLAND 940 Carbomer (Ashland) |
0.1 to 0.5 | 60 to 95 (according to neutralizer) |
5 to 10 | low | |
| Acrylates / C10–30 Alkyl Acrylate Crosspolymer | CARBOPOL ULTREZ 21 (Lubrizol) |
0.1 to 0.5 | 60 to 95 (according to neutralizer |
5 to 10 | low |
| CARBOPOL ULTREZ 20 | 0.1 to 0.6 | 60 to 95 (according to neutralizer) |
4 to 11 (lower viscosity) |
low | |
| TEGO® Carbomer 341ER (Evonik) |
0.05 to 1.0 | 60 to 95 | 4 to 11 (lower viscosity) |
low | |
| Cellulose gum (CMC) | AQUALON (BLANOSE) (Ashland) |
1.0 to 2.0 | 60 | 3 to 12 | low |
| Hydroxyethylcellulose (HEC) | NATROSOL 250 HHR CS (Ashland) |
0.2 to 2.5 | 65 | 3 to 12 | good |
| TYLOSE HS (Shin-Etsu) |
0.5 to 2.0 | 62 | 3 to 12 | good | |
| Hdroxypropylmethyl cellulose (HPMC) | BENECEL E10M (Ashland) TYLOPURE DG (Shin-Etsu) |
0.2 to 2.0 | 70 | 5 to 8 | good |
| Hydroxypropyl Guar | JAGUAR HP 120COS (Solvay) |
1 to 1.5 | 70 | 4 to 8 | very good |
| Ammonium Acryloyl dimethyltaurate/ Beheneth-25 Methacrylate Crosspolymer (pre-neutralized) |
ARISTOFLEX HMB (Clariant) |
0.5 to 1.0 | 70 | 2.5 to 8 | low |
| Ammonium Acryloyl dimethyltaurate/VP Copoymer (Pre neutralized) |
ARISTOFLEX AVC (Clariant) |
0.5 to 1.0 | 70 | 4 to 8 | low |
| Sodium Acryloyldimethyltaurate/ VP Crosspolymer (Pre neutralized) |
ARISTOFLEX AVS (Clariant) |
0.5 to 1.2 | 70 | 4 to 11 | low |
| Polyacrylates Crosspolymer-11 (pre-neutralized) |
ARISTOFLEX VELVET (Clariant) |
0.5 to 1.5 | 70 | 3 to 8 | low |
| Sodium Polyacryloyl dimethyltaurate |
ARISTOFLEX SILK (Clariant) |
1 to 1.5 | 70 | 2 to 11 | good |
| Polyacrylamide—C13–14-isoparaffin—laureth 7 (Pre-neutralized) |
SEPIGEL 305 (Seppic) |
0.5 to 5.0 | 70 | 3 to 12 | very low |
| Polyacrylate 13—polyisobutene—polysorbate 20 (Pre-neutralized) |
SEPIPLUS 400 (Seppic) |
0.1 to 2.2 | 65 | 3 to 12 | good |
| Hydroxyethyl acrylate—sodium acryloyldimethyl taurate copolymer (Pre-neutralized) |
SEPINOV EMT10 (Seppic) |
0.5 to 3.0 | 65 | 3 to 12 | good |
| Polyacrylate crosspolymer—6 (Pre-neutralized) |
SEPIMAX ZEN (Seppic) |
0.8 to 2.0 | 70 | 2 to 8 | very good |
The performance of the classes of polymers reported in Table 1 is well-known as regards aqueous media, but their behavior in hydroalcoholic solvents has not yet been deeply investigated. For this reason, and with the aim of giving useful indications for increased ABR production and development, especially in this pandemic emergency, in Table 2, we have reported a collection of examples regarding alcohol-based hydrogel formulations. The polymer and alcoholic amount and eventual addition of excipients are reported as suggested in the suppliers’ brochures, accompanied by the most meaningful data related to the obtained hydrogel in terms of viscosity and transparency.
Table 2. Hydrogel ABRs: most common commercial polymers, corresponding polymeric dose, suggested ethanolic percentage, clarity and viscosity of the obtained hydrogel are reported.
| Polymer Trade Name |
Polymer Amount (%) |
EtOH Amount (% v/v) |
Notes (Additives) |
Hydrogel Aspect * |
Hydrogel Viscosity (mPa.s) ** |
|---|---|---|---|---|---|
| CARBOPOL ULTREZ 10 (Lubrizol) |
0.5 | 70 | 0.35% aminomethyl propanol (neutralizer) |
Clear | 3500 to 4500 |
| ASHLAND 980 Carbomer (Ashland) |
0.35 | 73 | 0.15% aminomethyl propanol (neutralizer) 1.5% glycerin |
Clear | 15,000 to 25,000 |
| TEGO® Carbomer 341 ER (Evonik) |
0.3 | 70 | 0.5% tetrahydroxy propyl Ethylenediamine, (neutralizer) 3% glycerin |
Clear | 4350 |
| CARBOPOL 940 (Lubrizol) |
0.5 | 50 | triethanolamine up to pH 6 | Clear | 1200 |
| CARBOPOL ULTREZ 21 (Lubrizol) |
0.2 | 60 | 0.25%Triisopropanolamine (neutralizer) 0.5% propylen glycol |
Clear | 8000 to 12,000 |
| CARBOPOL ULTREZ 20 (Lubrizol) |
0.2 | 60 | 0.25%Triisopropanolamine (neutralizer) 0.5% propylen glycol |
Clear | 4000 to 6000 |
| NATROSOL 250 HHR CS (Ashland) |
1.4 | 65 | - | Turbid | 14,700 |
| Tylose HS 100000 (Shin-Etsu) |
1.5 | 62 | triethanolamine up to pH 8.5 2% glycerin |
Turbid | 37,000 |
| Benecel E10M (Ashland) |
1.5 | 75 65 |
1.5% glycerin 2.0 % glycerin |
Clear | 4000 to 6000 1325 |
| TYLOPURE DG 4T (Shin-Etsu) |
2.0 | 65 75 85 |
3.0 % glycerin | Clear | 7768 6184 5352 |
| JAGUAR® HP 120 COS (Solvay) |
1.2 | 75 | citric acid (pH adjuster) | Clear | 3500 to 5000 |
| ARISTOFLEX® HMB (Clariant) |
1.0 | 62 | - | Clear | 20,000 |
| ARISTOFLEX® AVC (Clariant) |
1.0 | 65 75 |
- | Clear | 30,000 40,000 |
| ARISTOFLEX® VELVET (Clariant) |
0.45 to 0.5 | 70 to 80 | 2% glycerin | Clear | 2940 to 2100 |
| ARISTOFLEX® SILK (Clariant) |
1% | 60 | 1.5% glycerin | Clear | 14,000 |
| SEPIGEL 305 (Seppic) |
1.6 | 65 | 3% glycerin | Turbid | 8000 |
| 2.0 | 70 | 0.2% sepimax zen | Turbid | 8000 | |
| 2.2 | 65 | 1% SIMULSOL 1293 (solubilizing nonionic Surfactant—Seppic) |
Clear | 7148 | |
| 3 | 65 | - | Turbid | 35,000 | |
| SEPIPLUS 400 (Seppic) |
2.25 | 65 | - | Turbid | 46,000 |
| SEPINOV EMT10 (Seppic) |
0.80 | 65 | sprayable | Turbid | 580 |
| 1.50 | 65 | - | Turbid | 8300 | |
| SEPIMAX ZEN (Seppic) |
0.80 | 66 | 3% glycerin | Clear | 8900 |
3.1. Carbomers
Carbomers represent a series of polymers widely used in cosmetic and pharmaceutical products as rheological modifiers. They are cross-linked polyacrylic acid polymers with high molecular weight, show a very efficient thickening capability, and are considered powerful stabilizers at low concentrations in water and hydroalcoholic solutions (0.1 to 3% w/w). The most common classification groups them according to the cross-linker type: carbomer homopolymers (acrylic acid crosslinked with allyl pentaerythritol or allyl sucrose), carbomer copolymers (acrylic acid and C10-C30 alkyl acrylate crosslinked with allyl pentaerythritol), and carbomer interpolymers (homopolymeric or copolymeric carbomer containing a block copolymer of polyethylene glycol and a long chain alkyl acid ester) [73]. According to the cross-linking density (low, medium, or high) polymers with a specific ability of increasing the viscosity of aqueous systems are provided. Being acidic in their undissociated state, they need to be neutralized with a specific basic organic or inorganic compound to perform as thickening agents. Despite the large number of neutralizing agents useful for aqueous dispersions (such as sodium, ammonium, and potassium hydroxides, aminomethyl propanol, tetrahydroxypropyl ethylenediamine, triethanolamine, diisopropanolamine, and triisopropanolamine), when carbomers are used for hydroalcoholic hydrogels, the neutralizer has to be carefully chosen in order to prevent the polymer precipitation. The most common organic and inorganic bases are the following:
-
Inorganic bases, such as NaOH and KOH, specifically for hydro-alcoholic mixtures with a max content of 20% ethanol.
-
Triethanolamine is the most suitable neutralizing agent for formulations containing up to 50 to 60% ethanol.
Taking into account the ethanolic amount in ABR (60 to 95%), suppliers and productors of carbomers recommend specific neutralizers, in particular tetrahydroxypropyl ethylenediamine, aminomethyl propanol, and triisopropanolamine [74]. All carbomers can thicken hydroalcoholic systems, but several grades can offer different advantages in terms of aspect and performance, such as higher transparency, better efficiency, and ease of handling also leading to the optimization of the overall aesthetic characteristics of commercial hand sanitizing gels [75][76]. Carbomers have better thickening properties than cellulose derivatives, but the rheological behavior of carbomers in aqueous and hydroalcoholic media shows a reduction of hydrogel consistency in the presence of ethanol, in particular at a polymer concentration of 0.1% w/w and at low pH values (pH = 4) [77] (Table 1 and Table 2).
3.2. Cellulose Derivatives
3.2.1. Hydroxyethyl Cellulose (HEC)
Hyroxyethyl cellulose is a non-ionic partially substituted poly(hydroxyethyl) ether of cellulose. It can be prepared by the reaction of cellulose with ethylene oxide under controlled and basic conditions with sodium hydroxide. The average number of ethylene oxide groups, attached to each glucose residue, is represented by the total molar substitution (MS), while the number of hydroxyl groups for every reacted glucose residue is represented by the degree of substitution (DS). Hydroxyethyl cellulose with DS = 1.5 and MS = 2.5 can be available with different molecular weight grades, corresponding to a different viscosity in aqueous media. L, M, H, and HH refers to low, medium, high, and very high viscosity, respectively. HEC can be dissolved in cold and hot water and it is not soluble in organic solvents. Hydroxyethyl cellulose of type L and M are very soluble in glycerin and present a good solubility in alcoholic solutions up to 60% ethanol [78]. Hydroxyethyl cellulose is not recommended to obtain gel formulations containing > 65% alcohol, because of the low solubility of this cellulose derivative and the turbid aspect (Table 1 and Table 2).
3.2.2. Sodium Carboxymethyl Cellulose (CMC)
CMC is an anionic rheological modifier, soluble in water at any temperature and giving clear colloidal systems at 1 to 6% [79]. It is available in different useful types according to DS and MS and it is classified by the letters “F” for food, “CS” for cosmetic, and “PH” for pharmaceutical use, according to American (USP), European (Ph. Eur), and Japanese (JP) pharmacopoeia [80][81][82]. Even though it is not soluble in a large number of organic solvents such as ethanol (95%), CMC is able to provide transparent systems in alcoholic solutions up to 40% ethanol. In higher amounts (up to 60% ethanol), it is possible to disperse CMC but obtain turbid systems. According to the literature, CMC is not useful for the preparation of hand sanitizers, being useful only for obtaining gels with ethanol up to 50% (Table 1).
3.2.3. Hydroxypropyl Methylcellulose (HPMC)
HPMC is a cellulose ether derivative widely applied in pharmaceutical formulations, and cosmetic and food products. The numeric code in the nomenclature indicates different types of HPMC, related to different percentages of methyl and hydroxypropyl groups and molecular weights [83][84]. HPMC is a widespread thickener for aqueous solutions and for a great number of binary solvent systems. Moreover, 2% HPMC (especially HPMC 2910) has a good solubility at high percentages of ethanol and isopropanol in water, allowing to obtain transparent gels with an appropriate viscosity [85] (Table 1 and Table 2).
4. Other Excipients in Hand Sanitizers
An important side effect in the use of hand rubs is skin dryness, due to over frequent application. Hydrating, refatting, and emollient agents can protect from the excessive drying effect of alcohol and detergents [86][87][88]. Glycerin is the most widespread humectant in sanitizing hand rubs [89]; in order to maintain the antimicrobial activity, the recommended concentration is 0.50 to 0.73%, because it still offers the necessary skin protection [90][91]. Glycerin is able to reduce the antimicrobial activity of several ABRs [92] if used at a concentration of 1.45% (v/v), and an excessively high concentration can extend the drying time of the hand rub, increasing the sticky sensation on the hands. Other emollients can be used to improve skin tolerance and consumer acceptance. Propylene glycol can be used at concentrations of 2 to 5%; ethylhexyl glycerin, dexpanthenol, and fatty alcohols can be added without decreasing antimicrobial efficacy [93]. Among several hydrating ingredients, Aloe vera gel has also been used in several cosmetic handrubs, increasing consumer interest as it is considered natural. It can be used in combination with glycerin or propylene glycol and can contribute to the firmness of gel formulations if used at very high concentrations.
References
- Carter, S.J. Aseptic Technique. Cooperand Gunn’s Dispensing for Pharmaceutical Students, 12th ed.; CBS Publishers and Distributors Pvt Ltd.: Delhi, India, 2000; pp. 494–540.
- Pickering, A.J.; Davis, J.; Boehm, A.B. Efficacy of Alcohol-Based Hand Sanitizer on Hands Soiled with Dirt and Cooking Oil. J Water Health 2011, 9, 429–433.
- Hübner, N.-O.; Hübner, C.; Wodny, M.; Kampf, G.; Kramer, A. Effectiveness of alcohol-based hand disinfectants in a public administration: Impact on health and work performance related to acute respiratory symptoms and diarrhoea. BMC Infect. Dis. 2010, 10, 250.
- Golin, A.P.; Choi, D.; Ghahary, A. Hand sanitizers: A review of ingredients, mechanisms of action, modes of delivery, and efficacy against coronaviruses. Am. J. Infect. Control 2020, 48, 1062–1067.
- Madan, K.; Prashar, N.; Thakral, S. Comparative evaluation of efficacy of alcoholic vs. nonalcoholic hand san-itizers. Int. J. Life Sci. Biotechnol. Pharm. Res. 2012, 1, 173–177.
- Gold, N.A.; Avva, U. Alcohol Sanitizer; StatPearls Publishing: St. Petersburg, FL, USA, 2018. Available online: (accessed on 15 October 2020).
- Centers for Disease Control and Prevention (CDC): Hand Hygiene Recommendations. Guidance for Healthcare Providers about Hand Hygiene and COVID-19. Available online: (accessed on 3 November 2020).
- Oughton, M.T.; Loo, V.G.; Dendukuri, N.; Fenn, S.; Libman, M.D. Hand Hygiene with Soap and Water Is Superior to Alcohol Rub and Antiseptic Wipes for Removal ofClostridium difficile. Infect. Control Hosp. Epidemiol. 2009, 30, 939–944.
- Policy for Temporary Compounding of Certain Alcohol-Based Hand Sanitizer Products During the Public Health Emergency. Immediately in Effect Guidance for Industry. FDA, March 2020, updated February 10, 2021. Available online: (accessed on 1 March 2021).
- Simonne, A. Hand Hygiene and Hand Sanitizers. EDIS 2019, 2005, 1–4. Available online: (accessed on 25 October 2020).
- Todd, E.C.D.; Michaels, B.S.; Holahm, J.; Smith, D.; Greig, J.D.; Bartleson, C.A. Outbreaks Where Food Workers Have Been Implicated in the Spread of Foodborne Disease. Part 10. Alcohol-Based Antiseptics for Hand Disinfection and a Comparison of Their Effec-tiveness with Soaps. J. Food Prot. 2010, 73, 2128–2140.
- U.S. Centers for Disease Control and Prevention. Vessel Sanitation Program, OPRP-General Information on Hand Hygiene, Information Sheet. CDC (July 2019). Available online: (accessed on 14 October 2020).
- Dyer, D.L.; Gerenratch, K.B.; Wadhams, P.S. Testing a New Alcohol-Free Hand Sanitizer to Combat Infection. AORN J. 1998, 68, 239–251.
- Edmonds, S.L.; Macinga, D.R.; Mays-Suko, P.; Duley, C.; Rutter, J.; Jarvis, W.R.; Arbogast, J.W. Comparative efficacy of commercially available alcohol-based hand rubs and World Health Organization-recommended hand rubs: Formulation matters. Am. J. Infect. Control 2012, 40, 521–525.
- Sandora, T.J.; Taveras, E.M.; Shih, M.-C.; Resnick, E.A.; Lee, G.M.; Ross-Degnan, D.; Goldmann, N.A. A Randomized, Controlled Trial of a Multifaceted Intervention Including Alcohol-Based Hand Sanitizer and Hand-Hygiene Education to Reduce Illness Transmission in the Home. Pediatrics 2005, 116, 587–594.
- Boyce, J.; Chartier, Y.; Chraiti, M.; Cookson, B.; Damani, N.; Dharan, S. WHO Guidelines on Hand Hygiene in Health Care. First Global Patient Safety Challenge Clean Care Is Safer Care; World Health Organization: Geneva, Switzerland, 2009; Available online: (accessed on 25 October 2020).
- Boyce, J.M.; Pittet, D.; Healthcare Infection Control Practices Advisory Committee; HICPAC/SHEA/APIC/IDSA Hand Hygiene Task Force. Guideline for Hand Hygiene in Health-Care Settings. Recommendations of the Healthcare Infection Control Practices Advisory Committee and the HICPAC/SHEA/APIC/IDSA Hand Hygiene Task Force. Am. J. Infect. Control 2002, 20, S1–S46.
- Seto, W.; Tsang, D.; Yung, R.; Ching, T.; Ng, T.; Ho, M.; Ho, L.; Peiris, J. Effectiveness of precautions against droplets and contact in prevention of nosocomial transmission of severe acute respiratory syndrome (SARS). Lancet 2003, 361, 1519–1520.
- Manocha, S.; Walley, K.R.; Russell, J.A. Severe acute respiratory distress syndrome (SARS): A critical care perspective. Crit. Care Med. 2003, 31, 2684–2692.
- Fendler, E.; Groziak, P. Efficacy of Alcohol-Based Hand Sanitizers against Fungi and Viruses. Infect. Control Hosp. Epidemiol. 2002, 23, 61–62.
- Gerberding, J.L.; Fleming, M.W.; Snider, D.E., Jr.; Thacker, S.B.; Ward, J.W.; Hewitt, S.M.; Wilson, R.J.; Heilman, M.A.; Doan, Q.M. Morbidity and Mortality Weekly Report. Guideline for Hand Hygiene in Health-Care Settings; Recommendations of the Healthcare Infection Control Practices Advisory Committee and the HICPAC/SHEA/APIC/IDSA Hand Hygiene Task Force; Centers for Disease Control: Atlanta, GA, USA, 2002; Volume 51.
- Ionidis, G.; Hübscher, J.; Jack, T.; Becker, B.; Bischoff, B.; Todt, D.; Hodasa, V.; Brill, F.H.H.; Steinmann, E.; Steinmann, J. Development and virucidal activity of a novel alcohol-based hand disinfectant supplemented with urea and citric acid. BMC Infect. Dis. 2016, 16, 77.
- Ansari, S.A.; Springthorpe, V.S.; Sattar, S.A.; Rivard, S.; Rahman, M. Potential role of hands in the spread of respiratory viral infections: Studies with human parainfluenza virus 3 and rhinovirus 14. J. Clin. Microbiol. 1991, 29, 2115–2119.
- Sattar, S. Microbicides and the environmental control of nosocomial viral infections. J. Hosp. Infect. 2004, 56, 64–69.
- Kramer, A.; Rudolph, P.; Kampf, G.; Pittet, D. Limited efficacy of alcohol-based hand gels. Lancet 2002, 359, 1489–1490.
- Dharan, S.; Hugonnet, S.; Sax, H.; Pittet, D. Comparison of Waterless Hand Antisepsis Agents at Short Application Times: Raising the Flag of Concern. Infect. Control Hosp. Epidemiol. 2003, 24, 160–164.
- Kampf, G. How effective are hand antiseptics for the postcontamination treatment of hands when used as recommended? Am. J. Infect. Control 2008, 36, 356–360.
- Erasmus, V.; Daha, T.J.; Brug, H.; Richardus, J.H.; Behrendt, M.D.; Vos, M.C.; Van Beeck, E.F. Systematic Review of Studies on Compliance with Hand Hygiene Guidelines in Hospital Care. Infect. Control Hosp. Epidemiol. 2010, 31, 283–294.
- Guide to Local Production: WHO-Recommended Handrub Formulations; World Health Organization: Geneva, Switzerland, 2010; Available online: (accessed on 3 November 2020).
- Matthew, Z.U.S. FDA Hand Sanitizer Solutions Explained. Cosmetic and Toiletries May 2020. Available online: (accessed on 3 November 2020).
- McDonnell, G.; Russell, A.D. Antiseptics and Disinfectants: Activity, Action, and Resistance. Clin. Microbiol. Rev. 1999, 12, 147–179.
- Van Assel, A.J.; Te Giel, M.C. Pathogen resistance and adaptation to disinfectants and sanitisers. In Understanding Pathogen Behaviour; Elsevier Ltd.: Amsterdam, The Netherlands, 2005; pp. 484–506.
- Bloomfield, S.F.; Arthur, M. Mechanisms of inactivation and resistance of spores to chemical biocides. J. Appl. Bacteriol. 1994, 76, 91–104.
- Goldsmith, C.S.; Tatti, K.M.; Ksiazek, T.G.; Rollin, P.E.; Comer, J.A.; Lee, W.W.; Rota, P.A.; Bankamp, B.; Bellini, W.J.; Zaki, S.R. Ultrastructural Characterization of SARS Coronavirus. Emerg. Infect. Dis. 2004, 10, 320–326.
- Kampf, G.; Kramer, A. Epidemiologic Background of Hand Hygiene and Evaluation of the Most Important Agents for Scrubs and Rubs. Clin. Microbiol. Rev. 2004, 17, 863–893.
- Howes, L. What Is Hand Sanitizer, and Does It Keep Your Hands Germ-Free? Chem. Eng. News 2020, 98, 12. Available online: (accessed on 15 October 2020).
- Visscher, M.; Davis, J.; Wickett, R. Effect of topical treatments on irritant hand dermatitis in health care workers. Am. J. Infect. Control 2009, 37, 842.e1–842.e11.
- Pittet, D. Compliance with hand disinfection and its impact on hospital-acquired infections. J. Hosp. Infect. 2001, 48, S40–S46.
- Winnefeld, M.; Richard, M.; Drancourt, M.; Grob, J.-J. Skin tolerance and effectiveness of two hand decontamination procedures in everyday hospital use. Br. J. Dermatol. 2000, 143, 546–550.
- Greenaway, R.; Ormandy, K.; Fellows, C.; Hollowood, T. Impact of hand sanitizer format (gel/foam/liquid) and dose amount on its sensory properties and acceptability for improving hand hygiene compliance. J. Hosp. Infect. 2018, 100, 195–201.
- Ale, I.S.; Maibach, H.I. Irritant contact dermatitis. Rev. Environ. Health 2014, 29, 195–206.
- Angelova-Fischer, I.; Dapic, I.; Hoek, A.-K.; Jakasa, I.; Fischer, T.W.; Zillikens, D.; Kezic, S. Skin Barrier Integrity and Natural Moisturising Factor Levels After Cumulative Dermal Exposure to Alkaline Agents in Atopic Dermatitis. Acta Derm. Venereol. 2014, 94, 640–644.
- Show Me the Science—When & How to Use Hand Sanitizer in Community Settings. CDC 2019. Available online: (accessed on 15 October 2020).
- Hadaway, A. Handwashing: Clean Hands Save Lives. J. Consum. Health Internet 2020, 24, 43–49.
- Brannonpeppas, L. Dynamic and equilibrium swelling behaviour of pH-sensitive hydrogels containing 2-hydroxyethyl methacrylate. Biomaterials 1990, 11, 635–644.
- Jones, D.S.; Andrews, G.P.; Gorman, S.P. Characterization of crosslinking effects on the physicochemical and drug diffusional properties of cationic hydrogels designed as bioactive urological biomaterials. J. Pharm. Pharmacol. 2005, 57, 1251–1259.
- Sastry, S.K.; Lakonishok, M.; Wu, S.; Truong, T.Q.; Huttenlocher, A.; Turner, C.E.; Horwitz, A.F. Quantitative Changes in Integrin and Focal Adhesion Signaling Regulate Myoblast Cell Cycle Withdrawal. J. Cell Biol. 1999, 144, 1295–1309.
- Hoffman, A.S. Hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2002, 54, 3–12.
- Ganji, F.; Vasheghani-Farahani, S.; Vasheghani-Farahani, E. Theoretical description of hydrogel swelling: A review. Iran Polym. J. 2010, 19, 375–398.
- Chirani, N.; Gritsch, L.; Motta, F.L.; Fare, S. History and Applications of Hydrogels. J. Biomed. Sci. 2015, 4.
- Wichterle, O.; Lím, D. Hydrophilic Gels for Biological Use. Nat. Cell Biol. 1960, 185, 117–118.
- Seeliger, M.A.; Breward, S.E.; Friedler, A.; Schon, O.; Itzhaki, L.S. Cooperative organization in a macromolecular complex. Nat. Struct. Mol. Biol. 2003, 10, 718–724.
- Silva, S.S.; Mano, J.F.; Reis, R.L. Potential applications of natural origin polymer-based systems in soft tissue regeneration. Crit. Rev. Biotechnol. 2010, 30, 200–221.
- Li, F.; Li, S.; El Ghzaoui, A.; Nouailhas, A.H.; Zhuo, R. Synthesis and Gelation Properties of PEG−PLA−PEG Triblock Copolymers Obtained by Coupling Monohydroxylated PEG−PLA with Adipoyl Chloride. Langmuir 2007, 23, 2778–2783.
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121.
- Maitra, J.; Shukla, V.K. Cross-linking in Hydrogels—A Review. Am. J. Polym. Sci. 2014, 4, 25–31.
- Singhal, R.; Gupta, K. A Review: Tailor-made Hydrogel Structures (Classifications and Synthesis Parameters). Polym. Technol. Eng. 2015, 55, 54–70.
- Ogata, T.; Nagayoshi, K.; Nagasako, T.; Kurihara, S.; Nonaka, T. Synthesis of hydrogel beads having phosphinic acid groups and its adsorption ability for lanthanide ions. React. Funct. Polym. 2006, 66, 625–633.
- Dragan, E.S.; Dinu, M.V. Interpenetrating Polymer Network Composite Cryogels with Tailored Porous Morphology and Sorption Properties. Adv. Struct. Saf. Stud. 2015, 1286, 239–252.
- Peppas, N.A. Biomedical Applications of Hydrogels Handbook. In Biomedical Applications of Hydrogels Handbook; Metzler, J.B., Ed.; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2010; pp. 203–225.
- Kabir, S.F.; Sikdar, P.P.; Haque, B.; Bhuiyan, M.R.; Ali, A.; Islam, M.N. Cellulose-based hydrogel materials: Chemistry, properties and their prospective ap-plications. Prog. Biomater. 2018, 7, 153–174.
- Sennakesavan, G.; Mostakhdemin, M.; Dkhar, L.; Seyfoddin, A.; Fatihhi, S. Acrylic acid/acrylamide based hydrogels and its properties—A review. Polym. Degrad. Stab. 2020, 180, 109308.
- Koetting, M.C.; Peters, J.T.; Steichen, S.D.; Peppas, N.A. Stimulus-responsive hydrogels: Theory, modern advances, and applications. Mater. Sci. Eng. R Rep. 2015, 93, 1–49.
- Ghorbani, S.; Eyni, H.; Bazaz, S.R.; Nazari, H.; Asl, L.S.; Zaferani, H.; Kiani, V.; Mehrizi, A.A.; Soleimani, M. Hydrogels Based on Cellulose and its Derivatives: Applications, Synthesis, and Characteristics. Polym. Sci. Ser. A 2018, 60, 707–722.
- Vedadghavami, A.; Minooei, F.; Mohammadi, M.H.; Khetani, S.; Kolahchi, A.R.; Mashayekhan, S.; Sanati-Nezhad, A. Manufacturing of hydrogel biomaterials with controlled mechanical properties for tissue engineering applications. Acta Biomater. 2017, 62, 42–63.
- Peppas, N.A.; Sahlin, J.J. Hydrogels as mucoadhesive and bioadhesive materials: A review. Biomaterials 1996, 17, 1553–1561.
- Oyen, M.L. Mechanical characterisation of hydrogel materials. Int. Mater. Rev. 2013, 59, 44–59.
- Pape, A.C.H.; Bastings, M.M.C.; Kieltyka, R.E.; Wyss, H.M.; Voets, I.K.; Meijer, E.W.; Dankers, P.Y.W. Mesoscale characterization of su-pramolecular transient networks using SAXS and rheology. Int. J. Mol. Sci. 2014, 15, 1096–1111.
- Larson, R.G. The Structure and Rheology of Complex Fluids; Oxford University Press: New York, NY, USA, 1999.
- Weitz, D.; Wyss, H.; Larsen, R. Oscillatory rheology: Measuring the viscoelastic behaviour of soft materials, GIT Laboratory. J. Eur. 2007, 11, 68–70. Available online: (accessed on 20 October 2020).
- Surini, S.; Amirtha, N.I.; Lestari, D.C. Formulation and effectiveness of a hand sanitizer gel produced using salam bark extract. Int. J. Appl. Pharm. 2018, 10, 216–220.
- Zatz, J.L.; Kushla, G.P. Pharmacheutical Dosage Form: Disperse System, 2nd ed.; Lieberman, H.A., Rieger, M.M., Banker, G.S., Eds.; Marcel Dekker: New York, NY, USA, 1996; pp. 399–421.
- Berardi, A.; Perinelli, D.R.; Merchant, H.A.; Bisharat, L.; Basheti, I.A.; Bonacucina, G.; Cespi, M.; Palmieri, G.F. Hand sanitisers amid CoViD-19: A critical review of alcohol-based products on the market and formulation approaches to respond to increasing demand. Int. J. Pharm. 2020, 584, 119431.
- TDS-237: Neutralizing Carbopol® and PemulenTM Polymers in Aqueous and Hydroalcoholic Systems. Lubrizol 2009. Available online: (accessed on 5 November 2020).
- SEPIMAX ZEN™—Multifunctional Powder Polymer for ZEN Attitude, SEPPIC 2019. Available online: (accessed on 11 November 2020).
- CARBOPOL® ULTREZ 20 POLYMER, Lubrizol 2020. Available online: (accessed on 1 November 2020).
- Fresno, M.; Ramírez, A.; Jimenez, M. Systematic study of the flow behaviour and mechanical properties of Carbopol® Ultrez™ 10 hydroalcoholic gels. Eur. J. Pharm. Biopharm. 2002, 54, 329–335.
- Ashland. Who Helps Formulate for Healthy Hands? We Do. Rheology Modifiers for Hand Sanitizer Formulations. 2018. Available online: (accessed on 21 October 2020).
- Ashland. BlanoseTM Refined Sodium Carboxymethyl Cellulose. 2012. Available online: (accessed on 13 November 2020).
- USP—United States Pharmacopeia and National Formulary (43–NF 38). In United States Pharmacopeial Convention; The U.S. Pharmacopeia (USP): Rockville, MD, USA, 2020.
- Ph. Eur.—The European Pharmacopoeia, 10th ed.; Council of Europe: Strasbourg, France, 2020.
- The Japanese Pharmacopoeia, 17th ed.; PMDR, Yakuji Nippo, Ltd.: Chiyoda-ku, Tokyo, Japan, 2017.
- Dow Chemical. Methocel Cellulose Ethers Technical Handbook; The Dow Chemical Company: Midland, MI, USA, 2002.
- Li, C.L.; Martini, L.G.; Ford, J.L.; Roberts, M. The use of hypromellose in oral drug delivery. J. Pharm. Pharmacol. 2005, 57, 533–546.
- Hand Sanitizer Gel Using Tylopur® DG-4T. ShinEtsu 2020. Available online: (accessed on 10 October 2020).
- Ahmed-Lecheheb, D.; Cunat, L.; Hartemann, P.; Hautemanière, A. Prospective observational study to assess hand skin condition after application of alcohol-based hand rub solutions. Am. J. Infect. Control 2012, 40, 160–164.
- Harbarth, S.; Pittet, D.; Grady, L.; Zawacki, A.; Potter-Bynoe, G.; Samore, M.H.; Goldmann, D.A. Interventional study to evaluate the impact of an alcohol-based hand gel in improving hand hygiene compliance. Pediatr. Infect. Dis. J. 2002, 21, 489–495.
- Kramer, A.; Bernig, T.; Kampf, G. Clinical double-blind trial on the dermal tolerance and user acceptability of six alcohol-based hand disinfectants for hygienic hand disinfection. J. Hosp. Infect. 2002, 51, 114–120.
- Houben, E.; De Paepe, K.; Rogiers, V. Skin condition associated with intensive use of alcoholic gels for hand disinfection: A combination of biophysical and sensorial data. Contact Dermat. 2006, 54, 261–267.
- Menegueti, M.G.; Laus, A.M.; Ciol, M.A.; Auxiliadora-Martins, M.; Basile-Filho, A.; Gir, E.; Pires, D.; Pittet, D.; Bellissimo-Rodrigues, F. Glycerol content within the WHO ethanol-based handrub formulation: Balancing tolerability with antimicrobial efficacy. Antimicrob. Resist. Infect. Control 2019, 8, 1–8.
- Suchomel, M.; Weinlich, M.; Kundi, M. Influence of glycerol and an alternative humectant on the immediate and 3-h bactericidal efficacies of two isopropanol-based antiseptics in laboratory experiments in vivo according to EN 12791. Antimicrob. Resist. Infect. Control 2017, 6, 72.
- Suchomel, M.; Rotter, M.; Weinlich, M.; Kundi, M. Glycerol significantly decreases the three hour efficacy of alcohol-based surgical hand rubs. J. Hosp. Infect. 2013, 83, 284–287.
- Handbook of Cosmetic Science and Technology; Apple Academic Press: Palm Bay, FL, USA, 2014.
More
Information
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.7K
Revisions:
3 times
(View History)
Update Date:
08 May 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




