
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Valeria Gasperi | + 1380 word(s) | 1380 | 2021-05-07 04:23:54 | | | |
| 2 | Lindsay Dong | Meta information modification | 1380 | 2021-05-07 09:35:09 | | |
Video Upload Options
Metabolic syndrome (MetS) is a complex pathophysiological state with incidence similar to that of a global epidemic and represents a risk factor for the onset of chronic non-communicable degenerative diseases (NCDDs), including cardiovascular disease (CVD), type 2 diabetes mellitus, chronic kidney disease, and some types of cancer. A plethora of literature data suggest the potential role of gut microbiota in interfering with the host metabolism, thus influencing several MetS risk factors.
1. Introduction
Metabolic syndrome (MetS) is defined by WHO as a pathological condition characterized by obesity, insulin resistance, hypertension, hyperlipidemia and waist-to-hip ratio; MetS is present if three or more of the above-mentioned criteria are present [1].
The complex ecosystem of microorganisms (including bacteria, viruses, protozoa, and fungi) living in different districts of the human body (gastrointestinal tract, skin, mouth, respiratory and urogenital systems) is defined as microbiota. Most microbiotas reside in the gastrointestinal tube [2]. The microbiota contains over 100 times more unique genes than those codified in the human genome [3]: it encompasses over 100 trillion microbes and 5000 different species, accounting for 5 million genes.
The habitual diet plays an important role in defining the composition of the intestinal microbiota and determining the microbial metabolites that can affect the host metabolism. In the literature, several studies have shown positive effects of some dietary models in MetS management. For example, adherence to the Mediterranean diet (MD) leads to significantly higher levels of total short-chain fatty acids (SCFAs), important gut microbiota metabolites that modulate immune–endocrine processes [4].
2. SCFA Beneficial Metabolic Effects
Intestinal bacteria play an important role in regulation of the host metabolism (influencing energy homeostasis, appetite and food eating behavior) and modulation of the immune system, through the production of SCFAs, vitamins, metabolites, and neuropeptides (Figure 1) [5][6].
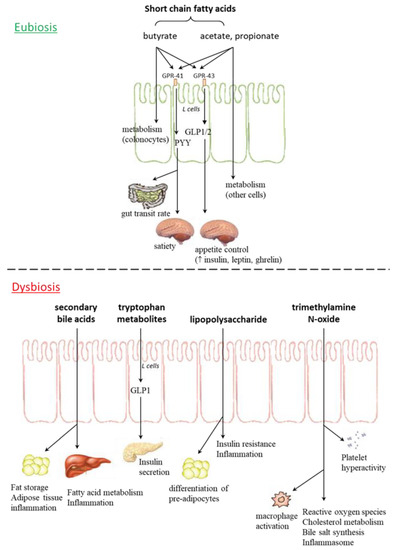
Figure 1. Gut microbiota metabolites in eubiosis and dysbiosis. GPR: G protein coupled receptor; GLP: glucagon-like peptide; PYY: peptide YY.
3. LPS and Endotoxemia
In healthy conditions, the integrity of the intestinal epithelial barrier, guaranteed by a tight junction network, blocks passage of antigens or microbe-derived endotoxins. Some pathological conditions give rise to gut microbiota perturbations (referred to as dysbiosis) and a subsequent impairment of intestinal barrier function (due to disorganized tight junction proteins, zonulin and occludin, in colonocytes); in these circumstances, microbial metabolites can cross the intestinal barrier and move to the bloodstream, triggering systemic pro-inflammatory signaling that, in turn, causes metabolic alterations (peripheral insulin resistance, hyperglycemia and non-alcoholic fatty liver disease (NAFLD)) in distant tissues [7][8].
Obesity, insulin resistance, and NAFLD, linked to MetS, are usually associated with low diversity in the gut microbiome and chronically higher levels of pro-inflammatory and microbiota derived lipopolysaccharide (LPS) in circulation [9]. A growing body of evidence suggests the potential role of LPS in obesity, insulin resistance, hepatic steatosis, and systemic and local inflammatory processes [7]. The ability of LPS to induce proliferation and adipogenesis is supported by in vitro and in vivo (mice models and human subjects) studies.
4. Nonalcoholic Fatty Liver Disease (NAFLD) and Microbiome
NAFLD is the hepatic manifestation of cardiometabolic syndrome. Systemic LPS concentration is significantly elevated in NAFLD compared to control groups, in both human and animal studies [10][11][12][13]. The gut microbiota contributes to liver fat deposition through modulation of the nuclear farnesoid X receptor (FXR), responsible for regulation of bile acid synthesis, and hepatic triglyceride accumulation [14][7][15]. After a meal, primary bile acids (chenodeoxycholic and cholic acids), stored in the gall bladder, are secreted in the duodenum, where they can be deconjugated by gut microbes, thus being metabolized into secondary bile acids in the colon [16]. Bacteria with the capability of producing secondary bile acids belong to Clostridium (clusters XIVa and XI) and Eubacterium [17][18].
Gut microbiota involvement in NAFLD genesis has been demonstrated by microbiota transplantation in recipient germ-free mice, as it generates fasting hyperglycemia, insulinemia and NAFLD; in particular, Lachnospiraceae bacterium 609 and Barnesiella intestinihominis species were specifically related to NAFLD [19].
5. Role of Bacterial TMAO and Development of Atherosclerosis
Trimethylamine N-oxide (TMAO) is a biomarker of risk for major adverse cardiovascular and cerebrovascular events, such as myocardial infarction and stroke (Figure 2): increased plasma TMAO concentrations have indeed been correlated with the accumulation of fatty depots in blood vessels, fatty liver, visceral obesity, and atherosclerosis [20][21][22][23][24][25][26][27][28].
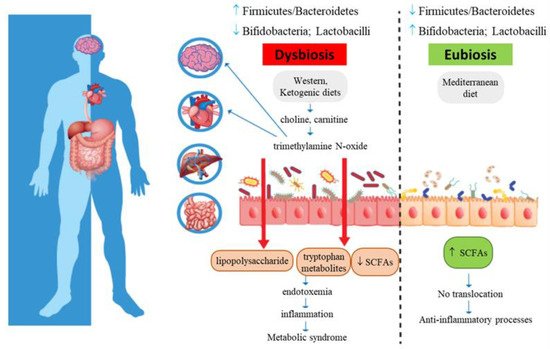
Figure 2. Metabolic syndrome and gut microbiota intestinal dysbiosis. Increased intestinal permeability causes translocation of lipopolysaccharide and tryptophan-derived metabolites, with subsequent metabolic endotoxemia and chronic low-grade systemic inflammation.
High TMAO serum levels are related to MetS and cardiovascular risk. TMAO may promote dyslipidemia by regulating hepatic lipogenesis and gluconeogenesis [3], macrophage scavenger receptors [29], while downregulating cholesterol and bile acid metabolism [30], as well as impairing macrophage reverse cholesterol transport [31], promoting movement of activated leukocytes to endothelial cells [32], activating NF-κB signaling [32], and enhancing platelet activation, thus promoting a pro-thrombotic phenotype [33] and inducing endothelial dysfunction through activation of the NLRP3 inflammasome [34]. In addition, TMAO also affects brain functions, as it induces neuronal senescence, increases oxidative stress, impairs mitochondrial function, inhibits mTOR signaling and upregulates expression of macrophage scavenger receptors and CD68, all phenomena that contribute to brain aging and cognitive impairment [32][28][35].
6. Gut Microbiota and Tryptophan Metabolism
Bacteria-derived indoles, produced from Trp metabolism, can modulate host physiological and pathological pathways, thus contributing to cardiovascular, metabolic, and brain disorders [36]. Trp metabolites are also involved in MetS. Indeed, in human patients with MetS, overactivation of indoleamine 2,3-dioxygenase and increased serum levels of kynurenine have been reported [37]. Indoxyl sulfate and p-cresyl sulfate are two other Trp metabolites that stimulate GLP-1 in L cells and subsequent insulin secretion from pancreatic β cells [38][39]. These two metabolites seem to be also related to chronic kidney disease, a MetS complication, and related risk factors (cardiovascular disease (CVD); hypertension, diabetes and hyperhomocysteinemia) [40][41].
7. Dietary Strategies for MetS Management and Gut Microbiota Modulation
In MetS patients, nutritional intervention should firstly aim at reducing CVD and type 2 diabetes risk and usually includes reduction of body weight by 7–10%, followed by weight maintenance and lifestyle changes (such as increases in physical activity and stopping cigarette smoking). Losing as little as 5% of initial weight results in insulin sensitivity improvement, serum triglycerides and LDL-cholesterol reduction and decrease in systolic and diastolic blood pressure [42][43].
Obesity is one of the five fundamental features of MetS; the relationship between obesity and microbes is clearly established. Colonization of germ-free mice with gut microbiotas derived from obese subjects led to increases in total weight, compared with transplantation of lean gut microbiotas [44]. Similar results have been observed in humans, where obesity developed after fecal microbiota transplantation from overweight donors [45], whereas transplantation of lean microbiotas into individuals with MetS improved insulin sensitivity [46]. In obese subjects, main microbiota changes concern the reduction of Bacteroidetes and a proportional increase in Firmicutes and Actinobacteria [47][48][49][50]; increase in Bacteroidetes in overweight subjects has also been reported [51]. However, conflicting data still exist [48][52][53], especially considering differences in age, sex, physiological state, and ethnicity; therefore, we are far away from understanding the real Firmicutes/Bacteroidetes ratio in obesity, and further studies are recommended.
Several in vivo studies have underlined the relevance of dietary fat quality on metabolic health and gut microbiota composition. Patterson and co-workers [54] demonstrated that mice fed with high-fat diets of different compositions displayed peculiar microbial ecosystems: dietary saturated fatty acids (palm oil) were associated with a low abundance of Bacteroidetes, obesity and MetS; mono-unsaturated fatty acids (MUFAs; olive oil) led to an increase in the Bacteroidaceae family; ω-3 PUFAs (flaxseed/fish oil) increased EPA and DHA concentrations, as well as the intestinal abundance of Bifidobacterium genus.
MD adherence reduces chronic inflammation, improves lipid profile, insulin sensitivity and endothelial function, while decreasing CVD incidence and all causes of mortality (myocardial infarction, stroke, cancer) [128,129]. High adherence to MD positively impacts the gut microbiota composition and microbial metabolomes.
Some findings indicated that nutritional ketosis, induced by very low carbohydrate ketogenic diets (VLCKD; <10% carbohydrates per day), allowed weight management and improved metabolic and inflammatory markers, including lipids, glycated hemoglobin, high-sensitivity C-reactive protein, fasting insulin and glucose levels [55][56].
A diet rich in proteins and low in carbohydrates promotes dysbiosis, causing an increase in pro-inflammatory bacteria and a reduction in SCFA-producing bacteria, with subsequent changes in bacterial metabolites; indeed, increased branched-chain fatty acids, indoxyl sulfate, p-cresol sulfate and TMAO have been observed with this dietary regimen [57][58][59].
Epidemiological evidence identifies the benefits of vegetarian dietary patterns (rich in fiber, but low in EPA and DHA) in both the prevention and treatment of MetS, CVD mortality and risk of coronary heart disease [60][61].
References
- Saklayen, M.G. The Global Epidemic of the Metabolic Syndrome. Curr. Hypertens. Rep. 2018, 20, 12.
- Pascale, A.; Marchesi, N.; Marelli, C.; Coppola, A.; Luzi, L.; Govoni, S.; Giustina, A.; Gazzaruso, C. Microbiota and metabolic diseases. Endocrine 2018, 61, 357–371.
- Canyelles, M.; Tondo, M.; Cedó, L.; Farràs, M.; Escolà-Gil, J.; Blanco-Vaca, F. Trimethylamine N-Oxide: A Link among Diet, Gut Microbiota, Gene Regulation of Liver and Intestine Cholesterol Homeostasis and HDL Function. Int. J. Mol. Sci. 2018, 19, 3228.
- Garcia-Mantrana, I.; Selma-Royo, M.; Alcantara, C.; Collado, M.C. Shifts on Gut Microbiota Associated to Mediterranean Diet Adherence and Specific Dietary Intakes on General Adult Population. Front. Microbiol. 2018, 9, 890.
- Hartstra, A.V.; Bouter, K.E.C.; Bäckhed, F.; Nieuwdorp, M. Insights Into the Role of the Microbiome in Obesity and Type 2 Diabetes. Diabetes Care 2015, 38, 159–165.
- Marchesi, J.R.; Adams, D.H.; Fava, F.; Hermes, G.D.A.; Hirschfield, G.M.; Hold, G.; Quraishi, M.N.; Kinross, J.; Smidt, H.; Tuohy, K.M.; et al. The gut microbiota and host health: A new clinical frontier. Gut 2016, 65, 330–339.
- Cani, P.D.; Delzenne, N.M. Involvement of the gut microbiota in the development of low grade inflammation associated with obesity: Focus on this neglected partner. Acta Gastroenterol. Belg. 2010, 73, 267–269.
- Fändriks, L. Roles of the gut in the metabolic syndrome: An overview. J. Intern. Med. 2017, 281, 319–336.
- Le Chatelier, E.; Nielsen, T.; Qin, J.; Prifti, E.; Hildebrand, F.; Falony, G.; Almeida, M.; Arumugam, M.; Batto, J.-M.; Kennedy, S.; et al. Richness of human gut microbiome correlates with metabolic markers. Nature 2013, 500, 541–546.
- Yang, S.Q.; Lin, H.Z.; Lane, M.D.; Clemens, M.; Diehl, A.M. Obesity increases sensitivity to endotoxin liver injury: Implications for the pathogenesis of steatohepatitis. Proc. Natl. Acad. Sci. USA 1997, 94, 2557–2562.
- Thuy, S.; Ladurner, R.; Volynets, V.; Wagner, S.; Strahl, S.; Königsrainer, A.; Maier, K.-P.; Bischoff, S.C.; Bergheim, I. Nonalcoholic Fatty Liver Disease in Humans Is Associated with Increased Plasma Endotoxin and Plasminogen Activator Inhibitor 1 Concentrations and with Fructose Intake. J. Nutr. 2008, 138, 1452–1455.
- Harte, A.L.; da Silva, N.F.; Creely, S.J.; McGee, K.C.; Billyard, T.; Youssef-Elabd, E.M.; Tripathi, G.; Ashour, E.; Abdalla, M.S.; Sharada, H.M.; et al. Elevated endotoxin levels in non-alcoholic fatty liver disease. J. Inflamm. 2010, 7, 15.
- Sharifnia, T.; Antoun, J.; Verriere, T.G.C.; Suarez, G.; Wattacheril, J.; Wilson, K.T.; Peek, R.M.; Abumrad, N.N.; Flynn, C.R. Hepatic TLR4 signaling in obese NAFLD. Am. J. Physiol. Liver Physiol. 2015, 309, G270–G278.
- Cani, P.D.; Delzenne, N.M. Interplay between obesity and associated metabolic disorders: New insights into the gut microbiota. Curr. Opin. Pharmacol. 2009, 9, 737–743.
- Cani, P.D.; Joly, E.; Horsmans, Y.; Delzenne, N.M. Oligofructose promotes satiety in healthy human: A pilot study. Eur. J. Clin. Nutr. 2006, 60, 567–572.
- de Aguiar Vallim, T.Q.; Tarling, E.J.; Edwards, P.A. Pleiotropic Roles of Bile Acids in Metabolism. Cell Metab. 2013, 17, 657–669.
- Kitahara, M.; Takamine, F.; Imamura, T.; Benno, Y. Clostridium hiranonis sp. nov., a human intestinal bacterium with bile acid 7alpha-dehydroxylating activity. Int. J. Syst. Evol. Microbiol. 2001, 51, 39–44.
- Hylemon, P.B.; Harris, S.C.; Ridlon, J.M. Metabolism of hydrogen gases and bile acids in the gut microbiome. FEBS Lett. 2018, 592, 2070–2082.
- Le Roy, T.; Llopis, M.; Lepage, P.; Bruneau, A.; Rabot, S.; Bevilacqua, C.; Martin, P.; Philippe, C.; Walker, F.; Bado, A.; et al. Intestinal microbiota determines development of non-alcoholic fatty liver disease in mice. Gut 2013, 62, 1787–1794.
- Randrianarisoa, E.; Lehn-Stefan, A.; Wang, X.; Hoene, M.; Peter, A.; Heinzmann, S.S.; Zhao, X.; Königsrainer, I.; Königsrainer, A.; Balletshofer, B.; et al. Relationship of Serum Trimethylamine N-Oxide (TMAO) Levels with early Atherosclerosis in Humans. Sci. Rep. 2016, 6, 26745.
- Zhu, L.; Baker, S.S.; Gill, C.; Liu, W.; Alkhouri, R.; Baker, R.D.; Gill, S.R. Characterization of gut microbiomes in nonalcoholic steatohepatitis (NASH) patients: A connection between endogenous alcohol and NASH. Hepatology 2013, 57, 601–609.
- Stremmel, W.; Schmidt, K.V.; Schuhmann, V.; Kratzer, F.; Garbade, S.F.; Langhans, C.-D.; Fricker, G.; Okun, J.G. Blood Trimethylamine-N-Oxide Originates from Microbiota Mediated Breakdown of Phosphatidylcholine and Absorption from Small Intestine. PLoS ONE 2017, 12, e0170742.
- Velasquez, M.; Ramezani, A.; Manal, A.; Raj, D. Trimethylamine N-Oxide: The Good, the Bad and the Unknown. Toxins 2016, 8, 326.
- Vogt, N.M.; Romano, K.A.; Darst, B.F.; Engelman, C.D.; Johnson, S.C.; Carlsson, C.M.; Asthana, S.; Blennow, K.; Zetterberg, H.; Bendlin, B.B.; et al. The gut microbiota-derived metabolite trimethylamine N-oxide is elevated in Alzheimer’s disease. Alzheimers Res. Ther. 2018, 10, 124.
- Kanitsoraphan, C.; Rattanawong, P.; Charoensri, S.; Senthong, V. Trimethylamine N-Oxide and Risk of Cardiovascular Disease and Mortality. Curr. Nutr. Rep. 2018, 7, 207–213.
- Schugar, R.C.; Shih, D.M.; Warrier, M.; Helsley, R.N.; Burrows, A.; Ferguson, D.; Brown, A.L.; Gromovsky, A.D.; Heine, M.; Chatterjee, A.; et al. The TMAO-Producing Enzyme Flavin-Containing Monooxygenase 3 Regulates Obesity and the Beiging of White Adipose Tissue. Cell Rep. 2017, 19, 2451–2461.
- Tang, W.H.W.; Wang, Z.; Kennedy, D.J.; Wu, Y.; Buffa, J.A.; Agatisa-Boyle, B.; Li, X.S.; Levison, B.S.; Hazen, S.L. Gut Microbiota-Dependent Trimethylamine N -Oxide (TMAO) Pathway Contributes to Both Development of Renal Insufficiency and Mortality Risk in Chronic Kidney Disease. Circ. Res. 2015, 116, 448–455.
- Koeth, R.A.; Wang, Z.; Levison, B.S.; Buffa, J.A.; Org, E.; Sheehy, B.T.; Britt, E.B.; Fu, X.; Wu, Y.; Li, L.; et al. Intestinal microbiota metabolism of l-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 2013, 19, 576–585.
- Falony, G.; Vieira-Silva, S.; Raes, J. Microbiology Meets Big Data: The Case of Gut Microbiota–Derived Trimethylamine. Annu. Rev. Microbiol. 2015, 69, 305–321.
- Janeiro, M.; Ramírez, M.; Milagro, F.; Martínez, J.; Solas, M. Implication of Trimethylamine N-Oxide (TMAO) in Disease: Potential Biomarker or New Therapeutic Target. Nutrients 2018, 10, 1398.
- Kzhyshkowska, J.; Neyen, C.; Gordon, S. Role of macrophage scavenger receptors in atherosclerosis. Immunobiology 2012, 217, 492–502.
- Seldin, M.M.; Meng, Y.; Qi, H.; Zhu, W.; Wang, Z.; Hazen, S.L.; Lusis, A.J.; Shih, D.M. Trimethylamine N-Oxide Promotes Vascular Inflammation Through Signaling of Mitogen-Activated Protein Kinase and Nuclear Factor-κB. J. Am. Heart Assoc. 2016, 5, e002767.
- Zhu, W.; Gregory, J.C.; Org, E.; Buffa, J.A.; Gupta, N.; Wang, Z.; Li, L.; Fu, X.; Wu, Y.; Mehrabian, M.; et al. Gut Microbial Metabolite TMAO Enhances Platelet Hyperreactivity and Thrombosis Risk. Cell 2016, 165, 111–124.
- Chen, M.; Zhu, X.; Ran, L.; Lang, H.; Yi, L.; Mi, M. Trimethylamine-N-Oxide Induces Vascular Inflammation by Activating the NLRP3 Inflammasome Through the SIRT3-SOD2-mtROS Signaling Pathway. J. Am. Heart Assoc. 2017, 6.
- Minett, T.; Classey, J.; Matthews, F.E.; Fahrenhold, M.; Taga, M.; Brayne, C.; Ince, P.G.; Nicoll, J.A.R.; Boche, D. Microglial immunophenotype in dementia with Alzheimer’s pathology. J. Neuroinflamm. 2016, 13, 135.
- Agus, A.; Planchais, J.; Sokol, H. Gut Microbiota Regulation of Tryptophan Metabolism in Health and Disease. Cell Host Microbe 2018, 23, 716–724.
- Mallmann, N.H.; Lima, E.S.; Lalwani, P. Dysregulation of Tryptophan Catabolism in Metabolic Syndrome. Metab. Syndr. Relat. Disord. 2018, 16, 135–142.
- Gao, H.; Liu, S. Role of uremic toxin indoxyl sulfate in the progression of cardiovascular disease. Life Sci. 2017, 185, 23–29.
- Chimerel, C.; Emery, E.; Summers, D.K.; Keyser, U.; Gribble, F.M.; Reimann, F. Bacterial Metabolite Indole Modulates Incretin Secretion from Intestinal Enteroendocrine L Cells. Cell Rep. 2014, 9, 1202–1208.
- Weiner, D.E. Chronic Kidney Disease as a Risk Factor for Cardiovascular Disease and All-Cause Mortality: A Pooled Analysis of Community-Based Studies. J. Am. Soc. Nephrol. 2004, 15, 1307–1315.
- Longenecker, J.C. Traditional Cardiovascular Disease Risk Factors in Dialysis Patients Compared with the General Population: The Choice Study. J. Am. Soc. Nephrol. 2002, 13, 1918–1927.
- Klein, S.; Burke, L.E.; Bray, G.A.; Blair, S.; Allison, D.B.; Pi-Sunyer, X.; Hong, Y.; Eckel, R.H. Clinical Implications of Obesity With Specific Focus on Cardiovascular Disease. Circulation 2004, 110, 2952–2967.
- Grundy, S.M.; Cleeman, J.I.; Daniels, S.R.; Donato, K.A.; Eckel, R.H.; Franklin, B.A.; Gordon, D.J.; Krauss, R.M.; Savage, P.J.; Smith, S.C.; et al. Diagnosis and Management of the Metabolic Syndrome. Circulation 2005, 112, 2735–2752.
- Ridaura, V.K.; Faith, J.J.; Rey, F.E.; Cheng, J.; Duncan, A.E.; Kau, A.L.; Griffin, N.W.; Lombard, V.; Henrissat, B.; Bain, J.R.; et al. Gut Microbiota from Twins Discordant for Obesity Modulate Metabolism in Mice. Science 2013, 341, 1241214.
- Alang, N.; Kelly, C.R. Weight Gain after Fecal Microbiota Transplantation. Open Forum Infect. Dis. 2015, 2, ofv004.
- Vrieze, A.; Van Nood, E.; Holleman, F.; Salojärvi, J.; Kootte, R.S.; Bartelsman, J.F.W.M.; Dallinga–Thie, G.M.; Ackermans, M.T.; Serlie, M.J.; Oozeer, R.; et al. Transfer of Intestinal Microbiota from Lean Donors Increases Insulin Sensitivity in Individuals With Metabolic Syndrome. Gastroenterology 2012, 143, 913–916.e7.
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006, 444, 1027–1031.
- Turnbaugh, P.J.; Hamady, M.; Yatsunenko, T.; Cantarel, B.L.; Duncan, A.; Ley, R.E.; Sogin, M.L.; Jones, W.J.; Roe, B.A.; Affourtit, J.P.; et al. A core gut microbiome in obese and lean twins. Nature 2009, 457, 480–484.
- Santacruz, A.; Collado, M.C.; García-Valdés, L.; Segura, M.T.; Martín-Lagos, J.A.; Anjos, T.; Martí-Romero, M.; Lopez, R.M.; Florido, J.; Campoy, C.; et al. Gut microbiota composition is associated with body weight, weight gain and biochemical parameters in pregnant women. Br. J. Nutr. 2010, 104, 83–92.
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Human gut microbes associated with obesity. Nature 2006, 444, 1022–1023.
- Schwiertz, A.; Taras, D.; Schäfer, K.; Beijer, S.; Bos, N.A.; Donus, C.; Hardt, P.D. Microbiota and SCFA in Lean and Overweight Healthy Subjects. Obesity 2010, 18, 190–195.
- Collado, M.C.; Isolauri, E.; Laitinen, K.; Salminen, S. Distinct composition of gut microbiota during pregnancy in overweight and normal-weight women. Am. J. Clin. Nutr. 2008, 88, 894–899.
- Mai, V.; McCrary, Q.M.; Sinha, R.; Glei, M. Associations between dietary habits and body mass index with gut microbiota composition and fecal water genotoxicity: An observational study in African American and Caucasian American volunteers. Nutr. J. 2009, 8, 49.
- Patterson, E.; O’Doherty, R.M.; Murphy, E.F.; Wall, R.; O’Sullivan, O.; Nilaweera, K.; Fitzgerald, G.F.; Cotter, P.D.; Ross, R.P.; Stanton, C. Impact of dietary fatty acids on metabolic activity and host intestinal microbiota composition in C57BL/6J mice. Br. J. Nutr. 2014, 111, 1905–1917.
- Gershuni, V.M.; Yan, S.L.; Medici, V. Nutritional Ketosis for Weight Management and Reversal of Metabolic Syndrome. Curr. Nutr. Rep. 2018, 7, 97–106.
- Kosinski, C.; Jornayvaz, F. Effects of Ketogenic Diets on Cardiovascular Risk Factors: Evidence from Animal and Human Studies. Nutrients 2017, 9, 517.
- De Angelis, M.; Garruti, G.; Minervini, F.; Bonfrate, L.; Portincasa, P.; Gobbetti, M. The Food-gut Human Axis: The Effects of Diet on Gut Microbiota and Metabolome. Curr. Med. Chem. 2019, 26, 3567–3583.
- Gentile, C.L.; Weir, T.L. The gut microbiota at the intersection of diet and human health. Science 2018, 362, 776–780.
- Altuntas, Y. Microbiota and Metabolic Syndrome. Turk Kardiyol. Dern. Ars. 2017, 45, 286–296.
- Rizzo, N.S.; Sabate, J.; Jaceldo-Siegl, K.; Fraser, G.E. Vegetarian Dietary Patterns Are Associated With a Lower Risk of Metabolic Syndrome: The Adventist Health Study 2. Diabetes Care 2011, 34, 1225–1227.
- Kahleova, H.; Levin, S.; Barnard, N.D. Vegetarian Dietary Patterns and Cardiovascular Disease. Prog. Cardiovasc. Dis. 2018, 61, 54–61.





