Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Sara Bombace | + 3019 word(s) | 3019 | 2021-04-25 06:06:33 | | | |
| 2 | Lily Guo | Meta information modification | 3019 | 2021-05-07 03:17:31 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Bombace, S. Hypertrophic Cardiomyopathy. Encyclopedia. Available online: https://encyclopedia.pub/entry/9354 (accessed on 07 February 2026).
Bombace S. Hypertrophic Cardiomyopathy. Encyclopedia. Available at: https://encyclopedia.pub/entry/9354. Accessed February 07, 2026.
Bombace, Sara. "Hypertrophic Cardiomyopathy" Encyclopedia, https://encyclopedia.pub/entry/9354 (accessed February 07, 2026).
Bombace, S. (2021, May 06). Hypertrophic Cardiomyopathy. In Encyclopedia. https://encyclopedia.pub/entry/9354
Bombace, Sara. "Hypertrophic Cardiomyopathy." Encyclopedia. Web. 06 May, 2021.
Copy Citation
Hypertrophic cardiomyopathy (HCM) is a genetic cardiac disease that presents with cardiac hypertrophy. HCM phenocopies are clinical conditions that are phenotypically undistinguishable from HCM, but with a different underlying etiology. Cardiac tumors are rare entities that can sometimes mimic HCM in their echocardiographic appearance, thus representing an example of HCM phenocopy. At present, only case reports of tumoral HCM phenocopies can be found in literature.
Hypertrophic Cardiomyopathy
1. Introduction
Hypertrophic cardiomyopathy (HCM) is a disease that presents with thickening of the left ventricle (LV); it was first described in 1958 as a “benign cardiac tumor resulting in left ventricular outflow tract obstruction (LVOTO)” [1]. Since then, HCM has been thoroughly investigated and was eventually classified as a genetic heart disease [2], mostly due to mutations in genes encoding for sarcomeric proteins [3][4]. HCM has a high prevalence, reported to range from 1:200 to 1:500 [5]. The condition is characterized by inappropriate myocardial hypertrophy that cannot be explained by hemodynamic loading conditions alone [6]. Therefore, the diagnosis of HCM requires the exclusion of alternative etiologies of cardiac hypertrophy, including the so-called phenocopies.
HCM phenocopies are clinical conditions that present with the same phenotypic expression of HCM, but with a different etiology. They include the physiologic hypertrophic remodeling of the athlete’s heart, metabolic and storage diseases, infiltrative diseases such as amyloid cardiomyopathy, and primary and secondary cardiac tumors [7]. In particular, cardiac tumors are under recognized as HCM phenocopies, but their correct identification may significantly change the management and prognosis of the patient.
At present, only case reports can be found in literature and there are, to our knowledge, no comprehensive reviews on the oncological phenocopies of HCM. Therefore, we have collected and analyzed the case reports in which a cardiac tumor mimicked HCM to the point of misleading the diagnosis, in order to highlight any common patterns that can guide the correct diagnosis.
2. Methods
We performed a systematic review of the literature using PubMed for the following keywords: ‘hypertrophic cardiomyopathy’, ‘tumoral phenocopy’, ‘mimicking hypertrophic cardiomyopathy’, ‘asymmetric septal hypertrophy’, ‘cardiac tumor’, ‘cardiac metastasis’, ‘cardiac lymphoma’. We did not put any restriction criteria regarding date and original language of publication. Subsequently, we considered only publications about cardiac tumors overlapping with the diagnosis of HCM, i.e., tumoral phenocopies. All the resulting findings that matched our selection criteria were case reports.
3. Tumoral Phenocopies of Hypertrophic Cardiomyopathy (HCM) in Literature
We have collected and reviewed the case reports in literature, in which a cardiac tumor closely mimicked HCM to the point of misleading the diagnosis. These amounted to 25 case reports, which have been illustrated in Table 1. The cases in which cardiac magnetic resonance (CMR) was eventually performed are detailed in Table 2.
Table 1. Case reports of tumoral phenocopies and main features of cardiac tumors.
| Tumor | Typical Location | Histologic Features | CMR Features | Notes | Case Reports in Literature |
|---|---|---|---|---|---|
| Benign congenital | |||||
| Rhabdomyoma | Ventricle | Large and vacuolated myocytes (spider cell) | T1 isointense, T2 iso/hyperintense, no/minimal contrast enhancement | Strongly (80%) associated with tuberous sclerosis | [8] |
| Hamartoma of mature cardiac myocytes | Ventricle | Enlarged, highly disorganized cardiomyocytes | T1 Isointense T2 inhomogeneously hyperintense | Very similar to HCM | [9][10] |
| Cardiac fibroma | Ventricle and ventricular septum | Tumor cells resemble fibroblasts, calcification is common | T1 and T2 hypointense, heterogeneous late contrast enhancement with hypoenhancing core | Found in 3% of patients with Gorlin syndrome | [11][12][13][14] |
| Benign acquired | |||||
| Myxoma | Left atrium, right atrium, interatrial septum | Splinde-shaped cells (myxoma cells), calcification can be present | T1 iso/hypointense, T2 hyperintense, heterogeneous late contrast enhancement of >50% of the mass area | 5% are a manifestation of the Carney complex. In case of haemorrhage and haemosiderin deposition, hypointense in all sequences | [15] |
| Hemangioma | Left atrium, right atrium | Variably sized blood vessels | T1 iso/hyperintense, T2 hyperintense, peripheral nodular contrast enhancement and progressive centripetal fill | Sometimes with heterogeneous enhancement due to calcifications and septations of the mass | [16] |
| Lipomatous Hypertrophy | Interatrial septum | Brown fat and cardiac myocytes | T1 and T2 hyperintese, no contrast enhancement | Intensity reduced with fat suppression technique | [17][18][19] |
| Malignant | |||||
| Lymphoma | Right atrium | Malignant lymphocytes | T1 and T2 isointense, heterogeneous enhancement | Highly abnormal T2 relaxation times (up to 140 ms) with T2 -mapping | [20][21][22][23][24][25][26] |
| Metastases | Variable | Infiltrating malignant cells | Heterogeneous enhancement | [27][28][29][30] | |
| Metastases of melanoma | Variable | Infiltrating malignant cells | T1 hyperintense, T2 hypointense | Due to the T1 relaxation time-shortening properties of melanin | [31][32] |
Table 2. Case reports describing a tumoral phenocopy of hypertrophic cardiomyopathy (HCM) in which cardiac magnetic resonance (CMR) was performed.
| Tumoral Phenocopy of HCM | Patient | Symptoms | ECG Suggestive | Echocardiography | CMR Depiction | Notes | Reference | ||
|---|---|---|---|---|---|---|---|---|---|
| Age | Gender | Mass | Mass | Diagnostic | |||||
| Rhabdomyoma | 50 | Male | Yes | No | No | Yes | Yes | [8] | |
| Hamartoma of mature cardiac myocytes | 33 | Male | Yes | NR | Not at first evaluation | Yes | No | Definitive diagnosis obtained with histology of the tumor excised | [9] |
| Hamartoma of mature cardiac myocytes | 41 | Female | Yes | Yes | Not at first evaluation | Yes | No | Definitive diagnosis obtained with histology of biopsy | [10] |
| Cardiac fibroma | 55 | Male | Yes | Yes | Not at first evaluation | Yes | Yes | Precedent misdiagnosis of HCM | [12] |
| Cardiac fibroma | 40 | Male | No | Yes | Inadequate window | Yes | Yes | [11] | |
| Cardiac fibroma | 59 | Female | Yes | Yes | No | Yes | Yes | [14] | |
| Myxoma | 5 | Male | No | Yes | Not at first evaluation | Yes | Yes | [15] | |
| Cardiac angioma | 20 | Female | Yes | Yes | No | No | No | Precedent misdiagnosis of HCM; definitive diagnosis obtained with histology of septal myectomy sample | [16] |
| Lipomatous hypertrophy of interventricular septum | 46 | Male | No | No | No | Yes | Yes | [17] | |
| Lipomatous hypertrophy of interventricular septum | 17 | Male | No | No | No | Yes | Yes | [18] | |
| Non-Hodgkin Lymphoma | 38 | Male | No | NR | No | No | Yes | At CMR myocardial involvement compatible with lymphoproliferative disease | [20] |
| Burkitt’s Lymphoma | 34 | Female | No | Yes | No | No | No | Concomitant oncological history; definitive diagnosis with 18-FDG PET and regression after chemotheraphy | [21] |
| Metastases Melanoma | 45 | Female | No | NR | NR | Yes | Yes | Known history of malignant melanoma | [31] |
| Metastases Hepatocellular Carcinoma | 61 | Male | Yes | Yes | Not at first evaluation | Yes | No | Previous diagnosis of HCC and misdiagnosis of HCM; myocardial biopsy needed for conclusive diagnosis | [27] |
Generally, the patient would present with cardiologic symptoms and/or electrocardiographic features that could be attributable to cardiac hypertrophy. Concurrently, at echocardiography, the walls of the heart were invariably thickened, but did not plainly suggest the presence of a tumoral mass or lesion. In fact, the location and appearance of the tumoral phenocopies that most closely mimic HCM tend to overlap with the classic patterns of LV hypertrophy (LVH) due to HCM itself, i.e., at the interventricular septum and LV apex.
The tumoral phenocopy was eventually recognized only with II level imaging—most frequently with CMR—when the patient’s condition invariably worsened prompting further investigation, or incidentally during other examinations and imaging tests.
3.1. Inadequate Acoustic Window
In some cases, cardiac tumors mimic HCM in their symptoms and electrocardiogram (ECG), and remain hidden from echocardiographic recognition by lingering in acoustic shadows. Torres et al. described the case of a 40-year-old male with an incidental detection of apical hypertrophy at routine ECG analysis [11]. The consequent echocardiography was inconclusive due to an inadequate ultrasound window. Eventually, CMR was needed to detect the mass at the apex of the LV, and it also allowed the diagnosis of cardiac fibroma: the mass was isointense compared to the muscle on T1 weighted images, and hypointense on the first pass study, whilst being strongly enhanced on delayed imaging, on contrast-enhanced T2 weighted images.
3.2. Adequate Acoustic Window
Echocardiography may miss the correct diagnosis even in patients with an adequate ultrasound window. Hovasse et al. presented a case of a 50-year-old male with exertional dyspnea and a systolic murmur [8]. Transthoracic echocardiography initially pointed to a diagnosis of obstructive HCM (OHCM) with systolic anterior motion (SAM) of the anterior mitral leaflet, without further suspicion of any alternative diagnosis. Nonetheless, the patient underwent CMR for risk stratification and assessment of fibrosis. At CMR the presence of a lobular mass was detected, and this prompted a correction of the diagnosis to that of benign myocardial tumor. Furthermore, through tissue characteristics analysis, the type of tumor could be determined as a rhabdomyoma, displaying the same T1 weighted and T2 weighted signals as the normal myocardium.
Similarly, in a case presented by Papadopoulos et al., a 46-year-old male underwent echocardiographic imaging which appeared suggestive of asymmetrical LVH [17]. However, further investigation with CMR, originally aimed at the evaluation of myocardial fibrosis, showed extensive adipose infiltration of the interventricular septum in a heterogeneous pattern and not well-demarcated, highly compatible with the diagnosis of lipomatous hypertrophy of the interventricular septum instead.
3.3. Apical HCM
In case of apical left ventricular masses, the differential diagnosis with apical HCM (AHCM) is particularly challenging with echocardiography, because of the variable presentation of AHCM, and because both instances are characterized by a diffuse thickening of the LV apex. Veinot et al. reported a case of a 55-year-old male with a cardiac fibroma, who carried the diagnosis of AHCM for more than 10 years [12]. He never underwent level II imaging, i.e., CMR, for example. At echocardiographic reassessment, performed due to worsening of the symptoms, a large mass involving the lateral and apical left ventricular walls with areas of calcification was revealed that had never been noticed in previous exams. A subsequent CMR confirmed the presence of the calcified mass, with no contrast enhancement, and strongly suggested the diagnosis of a cardiac fibroma. Surgical excision of the tumor was performed, and the diagnosis was confirmed.
3.4. Malignant Phenocopies Red Flags
3.4.1. Echocardiographic Red Flags
In case of hypertrophy of the interventricular septum at echocardiography, some features may be considered as red flags: among these are a concomitant thickening of the right ventricle, of the atrial walls or of the interatrial septum, and pericardial and/or pleural effusion, because they imply a higher probability of malignancy, and make the diagnosis of a common HCM less likely (Figure 1). Representative of this is the case reported by Kuchynka et al. of a 38-year-old male, with a month-long history of abdominal lymphadenopathy, whose echocardiographic imaging at workup gave grounds for suspicion of HCM [20]. Upon renewed ultrasound investigation, the thickening of the interventricular septum was confirmed, but also a thickening of the right ventricle’s free wall and of the interatrial septum, with a large pericardial effusion, became evident. The ensuing CMR showed diffuse heterogenous late gadolinium enhancement (LGE) in the thickened segments of the myocardium, highly suggestive of a lymphoproliferative disease. Eventually, histological analysis of an enlarged lymph node confirmed the diagnosis of non-Hodgkin lymphoma. After chemotherapy, complete remission was achieved, with normalization of ventricular wall thickness and resorption of the pericardial effusion.
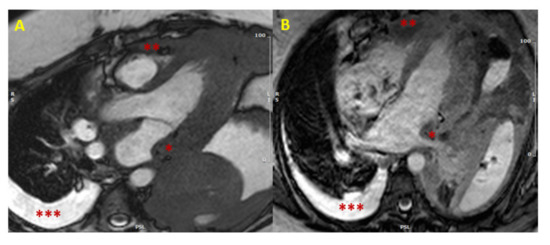
Figure 1. Cardiac lymphoma at CMR. SSFP images in long axis (3-chamber view in Panel (A), and 4-chamber view in Panel (B)) showing concomitant hypertrophy of left atrial wall (single asterisk *) and RV (two asterisks **). The presence of multiple areas of hypertrophy and pleural effusion (three asterisks ***) are “red flags” for malignancy. (CMR = cardiac magnetic resonance; SSFP = steady-state free precession; LV = left ventricle; RV = right ventricle).
3.4.2. Clinical Red Flags
A prior clinical history of malignancy should be considered as a red flag, too. In fact, secondary cardiac metastases can occur many years after the diagnosis of the primary tumor and, therefore, any recent diagnosis of HCM in a patient with a prior history of malignancy should be carefully investigated, in order to exclude a metastatic phenocopy of HCM: to this end, CMR serves as an excellent, unrivaled tool. This was evident in the case of a 61-year-old hepatocellular carcinoma (HCC) patient treated with radiofrequency ablation of the liver lesion, and a precedent diagnosis of HCM, presented by Greco et al. [27]. After a syncope-like episode, echocardiographic re-evaluation detected a large isoechogenic ventricular septum size (approximately 5.58 cm), extending to the cardiac apex. Concomitantly, at body computed tomography (CT) scan, a distant recurrence of the previously treated nodular lesion was found in the liver. A final CMR unmasked the lesion in the heart as causative of the abnormal thickness of the interventricular septum. The lesion displayed tenuous polylobate gadolinium enhancement and caused significant restriction of the outflow of left and right ventricles during systole. A myocardial biopsy eventually confirmed the infiltration of a well-differentiated HCC metastasis in the ventricular septum.
Since some cardiac tumors can be associated to genetic syndromes, a complete and thorough physical examination is necessary in every patient with a new diagnosis of HCM, in order to exclude syndromic traits. The presence of any signs and symptoms attributable to genetic syndromes such as tuberous sclerosis, Gorlin syndrome and Carney complex, imposes the need of a II level evaluation, in order to exclude an oncologic phenocopy of HCM. Pawloska et al. reported a case of a 41-year-old male patient with a longstanding history of HCM, that was treated with the positioning of an implanted cardioverter-defibrillator (ICD), seven years before [13]. None of the multiple precedent transthoracic echocardiographic examinations ever described the presence of a cardiac mass, or suggested an alternative diagnosis. Only a transesophageal echocardiography, eventually performed for the evaluation of atrial and ventricular lead failure of the ICD, and a subsequent chest CT, revealed the presence of a cardiac fibroma; of note, the ICD precluded the execution of CMR in this patient. The chest CT also displayed a bifid third rib, and therefore Gorlin syndrome was suspected. A more accurate history taking, and a meticulous physical examination ascertained other findings, such as a history of multiple basal cell carcinomas, macrocephaly, and plantar pitting. In conclusion, the final diagnosis of Gorlin syndrome was warranted.
4. Imaging of Tumoral Phenocopies
4.1. Echocardiography
Some cardiac tumors may closely mimic HCM in their echocardiographic appearance. At ultrasound imaging, a diagnosis of HCM can be made when the maximal end-diastolic wall thickness is >15 mm anywhere in the LV, in the absence of other causes of LV hypertrophy (LVH) [6][33].
In HCM nearly any part of the ventricular wall may become hypertrophic [33][34]. The classical phenotype of HCM, however, presents with asymmetric LVH of the basal anterior septum, displaying hypertrophy of the basal interventricular anterior septum in continuity with the anterior free wall. Albeit less commonly, HCM may as well cause LVH with non-septal asymmetric patterns, as well as with symmetric and concentric patterns [34]. Of note, apical HCM (AHCM), classically known as Yamaguchi syndrome, is a specific and rare variant of HCM with the isolated thickening of the LV apex [35].
Some secondary ancillary findings are typically associated with HCM at echocardiography, although they are not required for the diagnosis: these include hypertrophic and apically displaced papillary muscles, an anomalous insertion of the papillary muscle directly at the anterior leaflet of the mitral valve in the absence of chordae tendinae, elongated mitral valve leaflets, and myocardial clefts [33].
Benign cardiac tumors can range from pedunculated to sessile masses, protruding or not at various degrees into the cardiac chambers. These masses can be clearly visible at ultrasound imaging, but an incorrect diagnosis of HCM can be made when the tumor is not morphologically well-demarcated, when the location and appearance overlaps with the classic HCM patterns of LVH, or when it hides in areas of acoustic shadow.
Lymphomas may mimic HCM very well, too, because they appear as diffuse thickening of the cardiac walls without any apparent intracardiac mass, and thus evade echocardiographic recognition with ease. However, the presence of pericardial effusion, which often accompanies the myocardial thickening in these patients, suggests the possibility of a malignancy, rather than HCM [20][21][22][23][24][25][26].
On the contrary, there seem to be no cases in literature, in which cardiac sarcomas are misdiagnosed as HCM at echocardiography. Cardiac sarcomas usually arise from either of the atria, or from the pericardium [36] and present as an intracavitary mass [37] or as recurrent pericardial effusion [38]; their distinct manifestation usually allows echocardiography to be effective for the initial identification of the lesion.
Cardiac metastases involving the myocardium can often present with the same phenotype of HCM, displaying thickening of myocardial walls or mass formation, which can be misinterpreted as HCM [39].
4.2. Second-Level Imaging
With the use of CMR, the distinction between HCM and its tumoral phenocopies at workup is significantly improved, due to the ability of CMR in soft tissue characterization [40].
CMR is well recognized as the imaging modality of choice for the detection and evaluation of cardiac tumors [36][41][42][43]. A comprehensive study for the characterization of areas of myocardial hypertrophy should include T1 and T2 short-tau inversion recovery (STIR) fat-suppressed images, parametric sequences for native T1 and extracellular volume (ECV) calculation, first pass perfusion imaging and LGE sequences. CMR gives detailed information about tumor location, dimension, morphology and hemodynamic effect, about tissue composition (fat–cellularity–fibrosis) and perfusion, and about infiltration of the surrounding tissues. Of note, microcalcifications may be missed at CMR. Parametric sequences are of paramount interest and are still underutilized in the setting of oncologic phenocopies of HCM: typically, HCM shows increased native T1 and ECV values. In the case of cardiac tumors, ECV calculation often show normal/reduced values (of 25% or less) since the hypertrophic myocardium is characterized by tumor hypercellularity rather than extracellular volume expansion due to fiber disarray [44].
Moreover, some cardiac tumors display a specific CMR signal, which make a confident diagnosis possible, even without the use of contrast media. For example, cardiac metastases of melanomas are typically hyperintense in T1-weighted and hypointense in T2-weighted pre-contrast images.
However, in some cardiac tumors, such as Hamartoma of mature cardiac myocytes, CMR fails to detect the presence of a mass [9][10].
In these cases, CMR can be complemented by other imaging techniques: these include PET-CT and 18-FDG PET [21]. The latter can be particularly advantageous in this clinical setting, documenting the presence or absence of metabolically active tissue [45]. These techniques require a consistent suspicion of a tumor to be executed, that can be strengthened by the CMR evidence of a normal conventional and T1–T2 mapping features in non hypertrophic segments.

Figure 2. Cardiac fibroma at CMR. Focal myocardial thickening of mid-basal infero-lateral wall, with isointense signal in basal SSFP sequences, mimicking HCM (Panel (A,B)). Heterogeneous LGE with a capsulated appearance of the focal hypertrophy suggesting cardiac fibroma (Panel (C)). (CMR = cardiac magnetic resonance; SSFP = steady-state free precession; HCM = hypertrophic cardiomyopathy; LGE = late gadolinium enhancement).
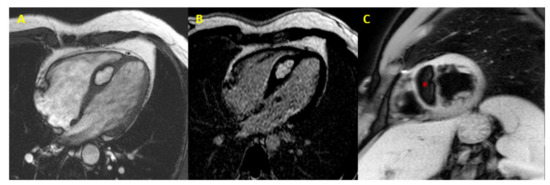
Figure 3. Cardiac lipoma at CMR. CMR image (4-chamber view) showing septal hypertrophy characterized by hyperintense signal in SSFP and LGE sequences (Panel (A,B)), and signal suppression (* asterisk in Panel (C)) in fat-saturated images (CMR = cardiac magnetic resonance; SSFP = steady-state free precession; LGE = late gadolinium enhancement).
References
- Teare, D. Asymmetrical hypertrophy of the heart in young adults. Br. Heart J. 1958, 20, 1.
- Richard, P.; Charron, P.; Carrier, L.; Ledeuil, C.; Cheav, T.; Pichereau, C.; Benaiche, A.; Isnard, R.; Dubourg, O.; Burban, M.; et al. Hypertrophic cardiomyopathy. Circulation 2003, 107, 2227–2232.
- Alfares, A.A.; Kelly, M.A.; McDermott, G.; Funke, B.H.; Lebo, M.S.; Baxter, S.B.; Shen, J.; McLaughlin, H.M.; Clark, E.H.; Babb, L.J.; et al. Results of clinical genetic testing of 2,912 probands with hypertrophic cardiomyopathy: Expanded panels offer limited additional sensitivity. Genet. Med. 2015, 17, 880–888.
- Ingles, J.; Sarina, T.; Yeates, L.; Hunt, L.; Macciocca, I.; McCormack, L.; Winship, I.; McGaughran, J.; Atherton, J.; Semsarian, C. Clinical predictors of genetic testing outcomes in hypertrophic cardiomyopathy. Genet. Med. 2013, 15, 972–977.
- Semsarian, C.; Ingles, J.; Maron, M.S.; Maron, B.J. New perspectives on the prevalence of hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 2015, 65, 1249–1254.
- Authors/Task Force Members; Elliott, P.M.; Anastasakis, A.; Borger, M.A.; Borggrefe, M.; Cecchi, F.; Charron, P.; Hagege, A.A.; Lafont, A.; Limongelli, G.; et al. 2014 ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy: The task force for the diagnosis and management of hypertrophic cardiomyopathy of the European Society of Cardiology (ESC). Eur. Heart J. 2014, 35, 2733–2779.
- Bogaert, J.; Olivotto, I. MR Imaging in hypertrophic cardiomyopathy: From magnet to bedside. Radiology 2014, 273, 329–348.
- Hovasse, T.; Unterseeh, T.; Garot, J. Unusual form of ‘obstructive hypertrophic cardiomyopathy’: Unique contribution of comprehensive cardiovascular magnetic resonance. Eur. Heart J. 2008, 30, 384.
- Dinh, M.H.; Galvin, J.M.; Aretz, T.H.; Torchiana, D.F. Left ventricular hamartoma associated with ventricular tachycardia. Ann. Thorac. Surg. 2001, 71, 1673–1675.
- Gilman, G.; Wright, R.S.; Glockner, J.F.; Starrett, R.S.; Hansen, W.H.; Sinak, L.J.; Tazelaar, H.D.; Tajik, A.J.; Dearani, J.A. Ventricular septal hamartoma mimicking hypertrophic cardiomyopathy in a 41-year-old woman presenting with paroxysmal supraventricular tachycardia. J. Am. Soc. Echocardiogr. 2005, 18, 272–274.
- Torres, I.R.; Bonmatí, L.M.; González, A.P. Tumor cardíaco que simula miocardiopatía hipertrófica. Rev. Esp. Cardiol. 2003, 56, 734.
- Veinot, J.; Omurchu, B.; Tazelaar, H.; Orszulak, T.; Seward, J. Cardiac fibroma mimicking apical hypertrophic cardiomyopathy: A case report and differential diagnosis. J. Am. Soc. Echocardiogr. 1996, 9, 94–99.
- Ateńska-Pawłowska, J.I.; Sterliński, M.; Oręziak, A.; Sobiech, M.; Lewandowski, M. Left ventricular tumour masquerading as hypertrophic cardiomyopathy: How one can be misled by transthoracic echocardiography. Kardiol. Polska 2017, 75, 280.
- Nucifora, G.; Pasotti, E.; Pedrazzini, G.; Moccetti, T.; Faletra, F.F.; Gallino, A. Cardiac fibroma mimicking hypertrophic cardiomyopathy: Role of magnetic resonance imaging in the differential diagnosis. Int. J. Cardiol. 2012, 154, e11–e13.
- Mani, A.; Gopalakrishnan, A.; Ayyappan, A.; Valaparambil, A. Image of the month: Ventricular myxoma mimicking hypertrophic cardiomyopathy. Clin. Med. 2019, 19, 131–132.
- Brown, M.R.; Dettrick, A.; Javorsky, G.; McKenzie, S.C.; Platts, D.G. Indolent cardiac angioma mimicking hypertrophic obstructive cardiomyopathy and causing right ventricular outflow tract obstruction. Eur. Heart J. Cardiovasc. Imaging 2013, 14, 718.
- Papadopoulos, C.E.; Matsiras, S.; Vassilikos, V. A rare case of lipomatous hypertrophy of the interventricular septum. J. Cardiovasc. Ultrasound 2016, 24, 170–171.
- Stephant, E.; Barthelet, M.; Leroux, P.-Y.; Revel, D. Lipomatous hypertrophy of the interventricular septum. Circulation 2008, 118, e71–e72.
- Nikolić, A.; Jovovic, L.; Angelkov, L.; Oprić, M. Uncommon presentation of asymmetric hypertrophic cardiomyopathy or something else. Int. J. Cardiol. 2007, 121, e17–e18.
- Kuchynka, P.; Palecek, T.; Lambert, L.; Masek, M.; Knotkova, V. Cardiac involvement in lymphoma mimicking hypertrophic cardiomyopathy. Kardiol. Polska 2018, 76, 1278.
- Bergler-Klein, J.; Knoebl, P.; Kos, T.; Streubel, B.; Becherer, A.; Schwarzinger, I.; Maurer, G.; Binder, T. Myocardial involvement in a patient with Burkitt’s lymphoma mimicking hypertrophic cardiomyopathy. J. Am. Soc. Echocardiogr. 2003, 16, 1326–1330.
- Cabin, H.S.; Costello, R.M.; Vasudevan, G.; Maron, B.J.; Roberts, W.C. Cardiac lymphoma mimicking hypertrophic cardiomyopathy. Am. Heart J. 1981, 102, 466–468.
- Kim, D.-H.; Kim, Y.-H.; Song, W.-H.; Ahn, J.-C. Primary cardiac lymphoma presenting as an atypical type of hypertrophic cardiomyopathy. Echocardiography 2013, 31, 115–119.
- Bin Yap, L.; Faizura, I.; Khan, S.K.A.; Tamin, S.S.; Chew, D.S.P. Diff use cardiac B-cell lymphoma presenting with pericardial eff usion or mimicking hypertrophic cardiomyopathy. Acta Cardiol. 2015, 70, 367–368.
- Lee, P.; Woo, K.; Chow, L.T.; Ng, H.; Chan, W.W.; Yu, C.; Lo, A.W. Images in cardiovascular medicine. Diffuse infiltration of lymphoma of the myocardium mimicking clinical hypertrophic cardiomyopathy. Circulation 2006, 113, e662–e664.
- Santhosh, S.; Bahl, A.; Saikia, U.N.; Lad, D.; Mittal, B.R.; Malhotra, P.; Varma, S. FDG PET/CT in the staging and follow-up of primary cardiac ‘T’ cell lymphoma presenting as hypertrophic cardiomyopathy. J. Nucl. Cardiol. 2015, 23, 581–584.
- Greco, A.; De Masi, R.; Orlando, S.; Metrangolo, A.; Zecca, V.; Morciano, G.; Bagordo, F.; Piccinni, G.; De Donno, A. Metastases of hepatocellular carcinoma misdiagnosed as isolated hypertrophic cardiomyopathy. Ann. Hepatol. 2017, 16, 966–969.
- Karass, M.; Mondal, P.; Alkayem, M.; Ojo, A.; Aronow, W.S.; Puccio, C. A rare presentation of acute heart failure secondary to aggressive uterine leiomyosarcoma metastatic to the myocardium initially diagnosed as hypertrophic obstructive cardiomyopathy. Ann. Transl. Med. 2016, 4, 374.
- Ciesek, S.; Kirchhoff, T.; Niehaus, M.; Manns, M.P.; Greten, T.F. Verdacht auf neu aufgetretene hypertrophe obstruktive Kardiomyopathie bei hepatozellulärem Karzinom. DMW Dtsch. Med. Wochenschr. 2008, 133, 2209–2212.
- Kondo, T.; Kitazawa, R.; Kawata, E.; Mori, K.; Kitazawa, S. Diffuse cardiac lymphatic involvement by metastatic neuroendocrine carcinoma mimicking hypertrophic cardiomyopathy: A case report. Cases J. 2009, 2, 9127.
- Lopci, E.; Monti, L.; Balzarini, L.; Chiti, A. Cardiac and acoustic metastases in relapsing melanoma. Clin. Nucl. Med. 2013, 38, e85–e88.
- Friedel, I.; Burchert, W.; Horstkotte, D.; Faber, L. A Rare Case of Cardiac Melanoma Mimicking Hypertrophic Cardiomyopathy. Can. J. Cardiol. 2017, 33, 950.e1–950.e3.
- Ommen, S.R.; Mital, S.; Burke, M.A.; Day, S.M.; Deswal, A.; Elliott, P.; Evanovich, L.L.; Hung, J.; Joglar, J.A.; Kantor, P.; et al. 2020 AHA/ACC guideline for the diagnosis and treatment of patients with hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 2020, 76, e159–e240.
- Maron, M.S.; Maron, B.J.; Harrigan, C.; Buros, J.; Gibson, C.M.; Olivotto, I.; Biller, L.; Lesser, J.R.; Udelson, J.E.; Manning, W.J.; et al. Hypertrophic cardiomyopathy phenotype revisited after 50 years with cardiovascular magnetic resonance. J. Am. Coll. Cardiol. 2009, 54, 220–228.
- Eriksson, M.J.; Sonnenberg, B.; Woo, A.; Rakowski, P.; Parker, T.G.; Wigle, E.; Rakowski, H. Long-term outcome in patients with apical hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 2002, 39, 638–645.
- Siontis, B.L.; Zhao, L.; Leja, M.; McHugh, J.B.; Shango, M.M.; Baker, L.H.; Schuetze, S.M.; Chugh, R. Primary cardiac sarcoma: A rare, aggressive malignancy with a high propensity for brain metastases. Sarcoma 2019, 2019, 1960593.
- Suehiro, S.; Matsuda, M.; Hirata, T.; Taniyama, D.; Kuraoka, K.; Takasaki, T.-I.; Segawa, T.; Oka, T.; Tamura, R.; Sugino, H. Primary cardiac rhabdomyosarcoma developed after receiving radiotherapy for left breast cancer 18 years prior. J. Cardiol. Cases 2017, 15, 181–183.
- Riles, E.; Gupta, S.; Wang, D.D.; Tobin, K. Primary cardiac angiosarcoma: A diagnostic challenge in a young man with recurrent pericardial effusions. Exp. Clin. Cardiol. 2012, 17, 39–42.
- Maleszewski, J.J.; Anavekar, N.S.; Moynihan, T.J.; Klarich, K.W. Pathology, imaging, and treatment of cardiac tumours. Nat. Rev. Cardiol. 2017, 14, 536–549.
- Leiner, T.; Bogaert, J.; Friedrich, M.G.; Mohiaddin, R.; Muthurangu, V.; Myerson, S.; Powell, A.J.; Raman, S.V.; Pennell, D.J. SCMR Position Paper (2020) on clinical indications for cardiovascular magnetic resonance. J. Cardiovasc. Magn. Reson. 2020, 22, 1–37.
- Ferrari, V. The EACVI Textbook of Cardiovascular Magnetic Resonance; Lombardi, M., Plein, S., Petersen, S., Eds.; Oxford University Press: Oxford, UK, 2018.
- Fussen, S.; De Boeck, B.W.; Zellweger, M.J.; Bremerich, J.; Goetschalckx, K.; Zuber, M.; Buser, P.T. Cardiovascular magnetic resonance imaging for diagnosis and clinical management of suspected cardiac masses and tumours. Eur. Heart J. 2011, 32, 1551–1560.
- Pazos-López, P.; Pozo, E.; Siqueira, M.E.; García-Lunar, I.; Cham, M.; Jacobi, A.; Macaluso, F.; Fuster, V.; Narula, J.; Sanz, J. Value of CMR for the differential diagnosis of cardiac masses. JACC Cardiovasc. Imaging 2014, 7, 896–905.
- Moscatelli, S.; Nardi, B.; Indolfi, E.; Fazzari, F.; Montini, O.; Coppini, L.; Monti, L. P586An unusual phenocopy of hypertrophic cardiomyopathy: A case report. Eur. Heart J. Cardiovasc. Imaging 2019, 20, 586.
- Gallamini, A.; Zwarthoed, C.; Borra, A. Positron emission tomography (PET) in oncology. Cancers 2014, 6, 1821–1889.
- Basso, C.; Rizzo, S.; Valente, M.; Thiene, G. Cardiac masses and tumours. Heart 2016, 102, 1230–1245.
- Burke, A.; Tavora, F. The 2015 WHO classification of tumors of the heart and pericardium. J. Thorac. Oncol. 2016, 11, 441–452.
More
Information
Subjects:
Cardiac & Cardiovascular Systems
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
930
Revisions:
2 times
(View History)
Update Date:
07 May 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




