
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Jackson Scharf | + 4907 word(s) | 4907 | 2021-02-19 04:05:05 | | | |
| 2 | Jackson Scharf | Meta information modification | 4907 | 2021-05-05 20:52:43 | | | | |
| 3 | Bruce Ren | -2216 word(s) | 2691 | 2021-05-06 04:15:45 | | | | |
| 4 | Jackson Scharf | + 13 word(s) | 2704 | 2021-05-10 22:18:38 | | | | |
| 5 | Conner Chen | Meta information modification | 2704 | 2021-09-22 02:31:25 | | |
Video Upload Options
The advent of optical coherence tomography angiography (OCTA) has facilitated remarkable advancements in our ability to image the blood vessels of the retina and choroid. This is particularly true of the choriocapillaris (CC), the blood vessel bed that feeds the outer retina. OCTA has more clearly defined the integral role of the CC in age-related macular degeneration (AMD), the leading cause of vision loss people over 50 years old. OCTA imaging shows that the choriocapillaris is impaired in intermediate and advanced non-neovascular AMD, and the severity of impairment may predict the advancement of disease. In advanced non-neovascular AMD, the choriocapillaris is severely impaired underneath the area of geographic atrophy, and the level of impairment surrounding geographic atrophy can predict the rate of atrophy enlargement. Macular neovascularization, harmful new blood vessels that grow in neovascular AMD, can be readily identified and classified using OCTA. It is still unclear however if neovascularization features with OCTA can predict the lesion’s level of activity. However, the choriocapillaris surrounding macular neovascularization is impaired while the more peripheral choriocapillaris is spared, implying that choriocapillaris disease may drive the growth of these new blood vessels. With continued innovation in OCTA image acquisition and analysis methods, new discoveries in AMD are set to follow.
Jackson Scharf, Giulia Corradetti, MD, Federico Corvi, MD, SriniVas Sadda, MD, David Sarraf, MD
Age-related macular degeneration (AMD), the leading cause of vision loss in people 50 years or older, profoundly affects the quality of life of almost 200 million people worldwide[1]. The disease causes progressive loss of the patient’s central vision and can lead to central blindness. In the late stages, AMD severely impacts the patient’s reading ability, mobility, and emotional quality of life [2]. Currently no treatment exists for non-neovascular AMD, the most common AMD subtype, highlighting the importance of research to better understand the disease.
While significant advancements have been made in the understanding of AMD, a complete picture of the disease has not yet been elucidated. AMD affects the retina, the neural tissue at the back of the eye that receives light and converts it into signals that are sent to the brain. Specifically, it affects the macula, the area of the retina that mediates central vision. The retina is composed of many layers, of which only the outermost are affected by AMD. The outer retina contains the rods and cones, the photoreceptor cells that convert light into neurologic signals. These cells are supported underneath by two layers called the retinal pigment epithelium (RPE) and Bruch’s membrane. Each of these layers receive blood flow from a vascular layer below it called the choroid. The most superficial layer of the choroid, the choriocapillaris, provides a bed of capillaries which nourish the outer retina. AMD causes degeneration of the outer retina, which contains the photoreceptor cells, the RPE, Bruch’s membrane, and the choriocapillaris.
As the outer retina’s source of blood flow and connection to the systemic system, the role of the choriocapillaris in AMD is of great interest. However, as the choriocapillaris is relatively thin and located underneath the retina, imaging of the layer has proven challenging. With the advent of optical coherence tomography angiography (OCTA), however, blood flow in the choriocapillaris can be identified with far greater detail than ever before. OCTA is a relatively new imaging modality that allows for three-dimensional visualization of the retinal and choroidal circulation without the need for dye injection. This advancement has heralded critical new insights into the pathogenesis of AMD and is poised to become an important part of the clinical care of AMD patients.
OCTA of the Choriocapillaris in AMD
Choriocapillaris disease is associated with outer retinal, RPE and Bruch’s membrane disruption in all stages of AMD. Several histopathologic studies, studies that examine dead tissue under a microscope, have in fact shown that the density of blood vessels in the choriocapillaris decreases with increasing AMD severity [3][4]. Various OCTA studies, discussed below, also have shown that the choriocapillaris is distinctly altered in the eyes of patients with intermediate and late-stage AMD, and that the health of the choriocapillaris may even predict further progression of the disease.
AMD exists in non-neovascular and neovascular forms, and in early, intermediate and late stages. In the non-neovascular form, lipoprotein deposits called drusen form underneath the macula in the early and intermediate stages while the outer retina, RPE and choriocapillaris degenerate and decay in the late stage. This pattern in the late stage is termed geographic atrophy. In the neovascular form, new blood vessels proliferate, often from the choriocapillaris, which leak and increase the risk of vision loss. Alterations in the choriocapillaris can be captured with OCTA in the intermediate and late stages of both forms of AMD.
OCTA of the Choriocapillaris in Non-Neovascular AMD
Intermediate Non-Neovascular Intermediate AMD
In intermediate, non-neovascular AMD, early outer retinal abnormalities are associated with impairment of the choriocapillaris. Drusen, the hallmark feature of early and intermediate AMD, is associated with progressive disruption of the choriocapillaris [5]. OCTA analysis of intermediate AMD eyes shows impaired blood flow through the choriocapillaris [6], particularly beneath and surrounding drusen [7]. Even these early changes may functionally impact the patient’s vision. The level of impairment of blood flow through the choriocapillaris correlates with reduced sensitivity of the macula in eyes with early or intermediate AMD [8]. In these ways, the health of the choriocapillaris on OCTA is a meaningful indicator of the severity of disease in AMD.
The health of the choriocapillaris on OCTA may also have predictive power in determining the advancement of intermediate AMD. Areas of the choriocapillaris in which blood flow is not detected are termed “flow deficits.” In patients with macular drusen, choriocapillaris flow deficit predicts both the enlargement of the existing drusen and the development of new drusen [9]. Impairment of the choriocapillaris may also indicate progression to more advanced stages of the disease. Greater choriocapillaris flow deficit can be a predictor of progression to RPE and outer retinal atrophy [10]. Similarly, flow deficit is greater in intermediate AMD eyes that progress to the advanced, atrophic stage [11]. Guided by these findings, OCTA of the choriocapillaris may provide useful risk stratification or predictive benefits in the future.
Analysis of the choriocapillaris in AMD must be considered in the context of the normal aging of the retina. Age is highly correlated with decreasing density of the choriocapillaris, particularly in the macula, a finding that has been demonstrated in both histopathologic [12] and OCTA studies [6]. Increased choriocapillaris flow deficits, as discussed above, can also be attributed to normal aging. The OCTA findings associated with normal aging and the OCTA findings that occur during transition to early AMD are not yet well defined.
Advanced Non-Neovascular AMD
In non-neovascular advanced AMD, atrophy of the outer retina, RPE and choriocapillaris occurs, termed “geographic atrophy” (GA). Because of CC attenuation, it is not surprising that patients with advanced non-neovascular AMD show choriocapillaris alterations on OCTA. OCTA in fact allows for precise demarcation of the area of atrophy as accurately as fundus autofluorescence [13], the gold standard for GA demarcation. As the disease progresses to GA, the choriocapillaris exhibits significant impairment underneath the area of atrophy [14][15][14, 15], and{Alagorie, 2019 #348} then is lost completely. It is important to note that the choriocapillaris is also impaired in the peripheral macula, outside of the areas of geographic atrophy [15][16][17]. The choriocapillaris in the zone immediately surrounding geographic atrophy shows the greatest impairment on OCTA and predicts the rate of atrophy enlargement (Figure 1) [18][19]. These findings again indicate the critical and possibly predictive role of the choriocapillaris and its impairment in the development and progression of advanced AMD.
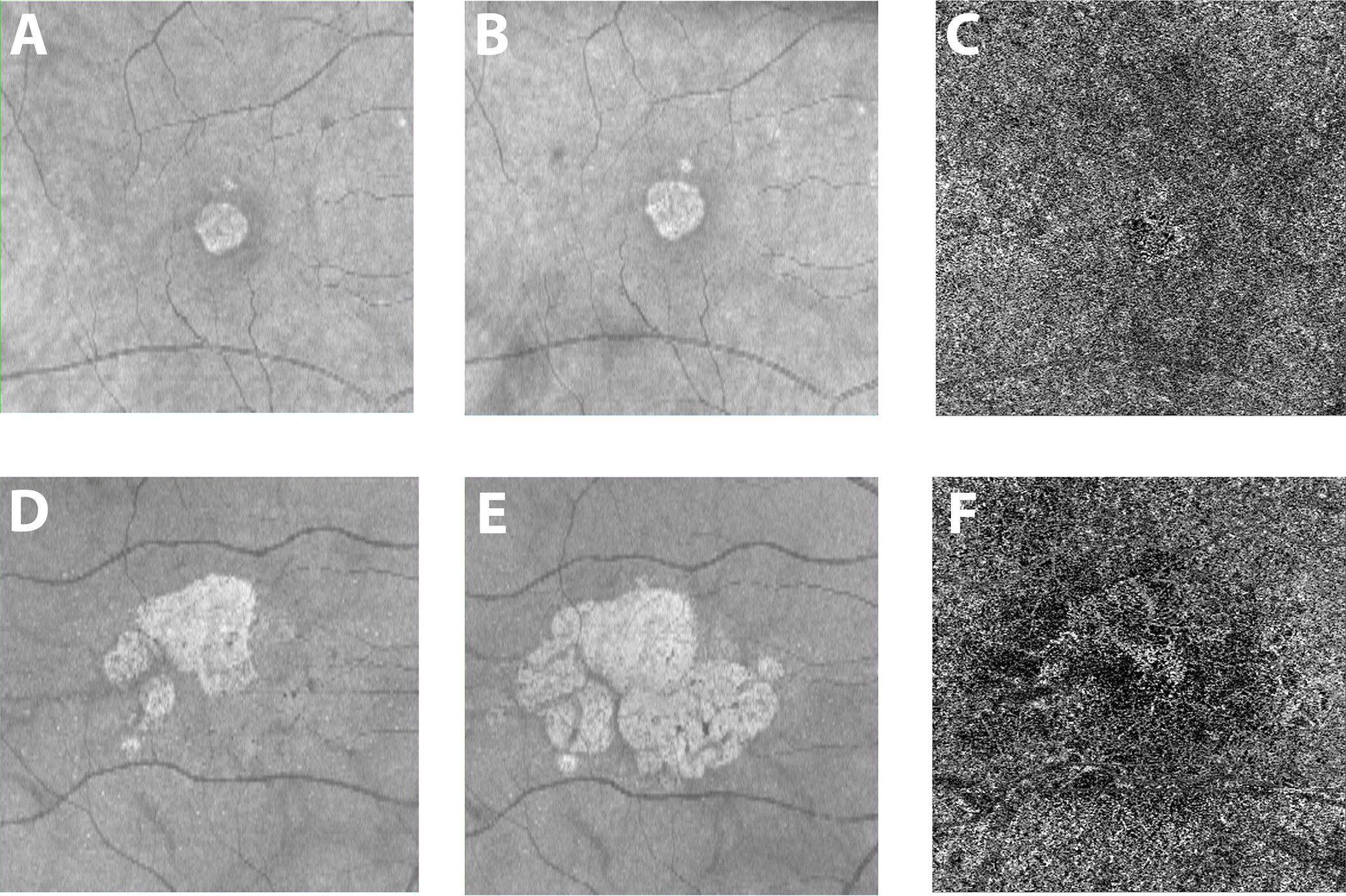
Figure 1: Courtesy of Nassisi et al., 2019 [18]: Geographic atrophy and different growth rates: One eye from two patients with geographic atrophy (GA) are shown in the two rows of images. The brighter central lesion represent the area of atrophy in each patient. Panels A and D show structural (en face) optical coherence tomography (OCT) images at baseline. Panels B and E show the corresponding en face OCT images acquired one year later. The 2 patients show a very different yearly growth rate (0.07 and 0.73 for the first and second row respectively) of the atrophic lesions. Panels C and F show the corresponding OCT angiogram at the level of the choriocapillaris from the baseline visit for these two patients. White areas show choriocapillaris (CC) blood flow, while darker areas show a lack of flow. The images show dramatically different flow surrounding the atrophic lesion, with more severe CC blood flow impairment with OCTA in the patient with more rapid atrophy progression (41.2% versus 53% for the first and second row respectively).
OCTA of the Choriocapillaris in Neovascular AMD
OCTA of Macular Neovascularization
Neovascular AMD is characterized by the proliferation of new blood vessels, termed macular neovascularization (MNV). MNV is associated with a high risk of rapid and severe visual decline and may necessitate the frequent intravitreal injection of anti-VEGF agents (a vascular growth factor inhibitor). Given the remarkable visual benefits of antiVEGF therapy of neovascular AMD, the identification and classification of MNV is of critical significance. For these reasons, the assessment of MNV is an important application of OCTA. The advent of OCTA has transformed the diagnostic power of the clinician to detect and image MNV and has provided insights into the pathways of neovascular AMD.
When used in conjunction with its corresponding structural OCT (the OCTA image without information on blood flow), OCTA is a powerful tool for both the diagnosis and classification of MNV. It has higher sensitivity and specificity than fluorescein angiography, the gold standard before the advent of OCTA, or indocyanine green angiography [20][21][22][23], and does not require dye injection. However, OCTA does not provide information about dynamic leakage and may miss components of neovascularization with low blood flow speeds such as polypoidal (i.e. aneurysmal MNV) lesions.
Beyond identification, OCTA facilitates classification of the neovascularization. MNV can be classified by anatomical position: type 1 MNV is located below the RPE and originates from the choroid, type 2 MNV is located in the sub-retinal space and originates from the choroid, and type 3 MNV is located within the neurosensory retina and originates from the deep retinal capillary plexus and is also known as retinal angiomatous proliferation or RAP [24][25]. Within these classifications, MNV can be sub-classified based on the morphology or shape of the vessels. Mature type 1 MNV is characterized by large thick branching vessels with secondary finer capillary branching. Various patterns of large vessel branching have been described, including “medusa,” “seafan” and “tangled” morphologies, but these subclassifications have limited predictive and clinical value [26]. Hypermature type 1 MNV is characterized by larger vessels without the secondary capillary ramification (“dead tree” pattern) [27][28][29][30][31][32] and are more commonly associated with macular atrophy and/or fibrosis. The vascular morphology of Type 2 MNV [33][34] is comparable to that of Type 1 MNV, and thus these two MNV types can only truly be differentiated based on their position relative to the RPE. Type 3 MNV originates from the retinal deep capillary plexus (DCP) within the retina rather than the choriocapillaris below the retina. Nascent Type 3 lesions may show progressive downgrowth towards the RPE [35][36][37]. It is interesting that one study noted worse perfusion of the choriocapillaris in eyes with type 3 MNV compared to fellow non-neovascular eyes, implying that choriocapillaris disease may play a critical role in the development of these lesions [38].
The OCTA morphology of neovascularization can correlate with activity. Fine vessels at the advancing edge of MNV, branching complexity, and a dark halo surrounding the lesion may indicate activity. Conversely, large, “dead tree” like vessels and a paucity of fine branching capillaries may indicate relative quiescence [1][2][3][4][5][6][7][8][9][10][11][12][13][14][15][16][17][18][19][20][21][22][23][39][40][41][42]. These descriptive features, however, do not have predictive value to guide anti-VEGF therapy, nor is it clear whether they can be graded reliably. After anti-VEGF treatment, MNV can show a rapid decrease in the fine capillary vessels typically at the lesion border. This may be because the capillaries at the lesion edge are less protected by vascular support cells called pericytes than the mature feeding or central trunk vessels that are more resistant to anti-VEGF treatment [43][44][45]. MNV may also temporarily decrease in size after anti-VEGF therapy but subsequently increase in size after two weeks [46]. With repeated anti-VEGF injections, often only the mature, “tree trunk-like” pericyte protected vessels remain, after which lesions are referred to as “mature” or “hypermature” [47][48]. Chronic anti-VEGF therapy of mature type 1 MNV is associated with progressive growth in the lesion area after 1 year in the majority of cases [48]. Lesions with extensive vascularity are typically associated with good visual acuity and lesions with low vascularity are associated with poor acuity.
OCTA of the Choriocapillaris in Neovascular AMD
Beyond the direct study of the neovascular membrane, OCTA studies show that the choriocapillaris is impaired in the environment surrounding the MNV. MNV is commonly encircled by a “dark-halo” on OCTA, an area devoid of blood flow. This may represent a “vascular steal” phenomenon [49] because of flow diverted through the neovascular membrane [50] or may be the result of inner choroidal ischemia [51][52]. This dark halo may in fact represent a marker of neovascular activity. The choriocapillaris immediately surrounding MNV shows worse flow deficits than other areas of the macula [51][53][54], indicating choriocapillaris impairment. This association holds true even when analyzing only the choriocapillaris immediately outside of the dark halo in eyes that have not yet received anti-VEGF treatment (Figure 2) [52], indicating that the choriocapillaris immediately surrounding the MNV is truly diseased. This suggests that choriocapillaris disease may cause a lack of oxygen in the RPE, which may then drive the release of VEGF, an angiogenic growth factor, leading to the development of MNV. It cannot, however, exclude the possibility that the choriocapillaris disease is instead caused by the MNV. It is interesting that choriocapillaris flow deficits may be more severe around active versus inactive or quiescent (i.e. nonexudative) MNV and future applications of OCTA may become important to determine which MNV lesions require anti-VEGF therapy [52]. Surprisingly, the choriocapillaris in the peripheral macula far from the MNV lesion is similar to age-matched normal eyes. This is in contrast to eyes with advanced non-neovascular atrophic AMD, which show significant, more severe choriocapillaris flow deficits throughout the macula. This has led to the hypothesis that the choriocapillaris in eyes with atrophic AMD may be so severely impaired that it is no longer capable of creating an MNV response. These concepts will need to be validated in future prospective studies.
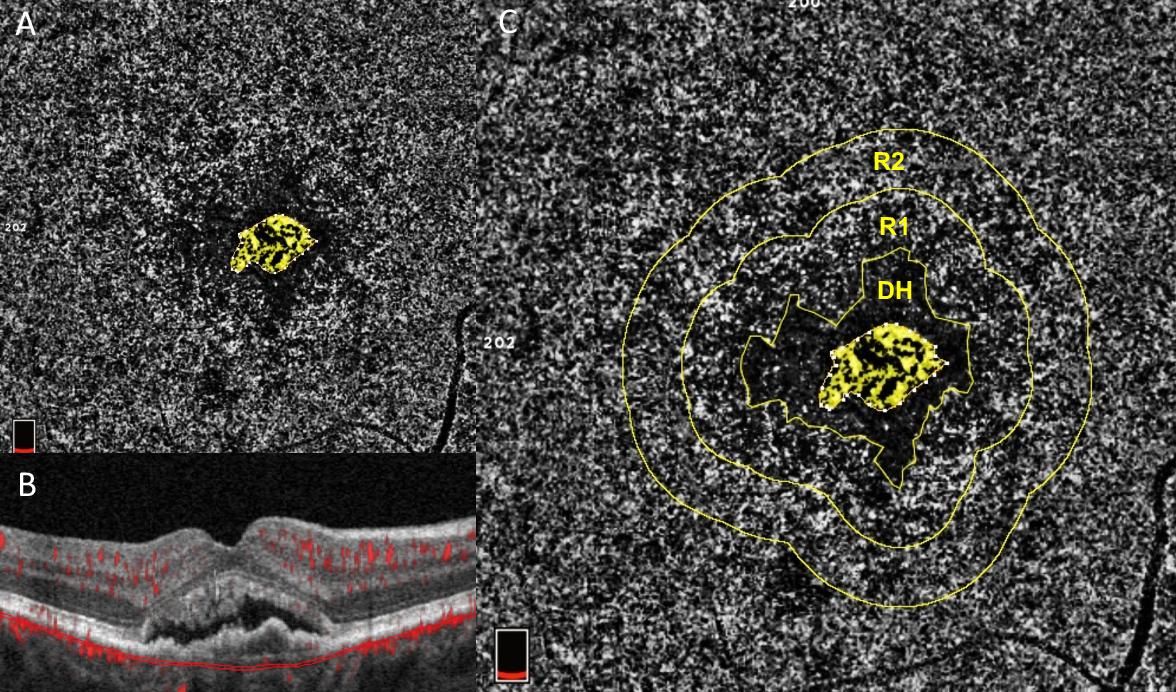
Figure 2: Courtesy of Scharf et al., 2020 [52]: Choriocapillaris flow deficits around macular neovascularization: One eye from a patient with neovascular AMD who had never received anti-VEGF treatment. Panels A and C show an OCTA image of the choriocapillaris. Panel B shows a cross sectional, structural OCT image of the retina with macular neovascularization (MNV) at its center and overlying subretinal fluid. The OCTA images in A and C are captured from the area between the two thin red lines in panel B, the area of the choriocapillaris. Choriocapillaris angiograms were analyzed for the amount of choriocapillaris flow deficits (dark areas) in two concentric rings (R1) and (R2) around the peri-lesional dark halo (DH). These areas represent disease of the choriocapillaris. The ring closer to the MNV (R1) exhibits significantly more severe flow deficits than the more peripheral ring. Both rings exhibit significantly greater flow deficits than the same areas analyzed with OCTA in age-matched normal controls, according to the study by Scharf et al. This suggests that choriocapillaris disease in the environment around MNV may drive its growth.
Future Directions
OCTA study of the choriocapillaris is a remarkable advancement in the field of retinal imaging and AMD. As a relatively novel imaging modality, there is much progress still to be made in both image acquisition and choriocapillaris analysis. Faster scan speeds and advancements in software for image acquisition and artifact correction have the potential to improve image quality and consistency. Progress in choriocapillaris quantification algorithms may improve the reliability and reproducibility of these measurements and allow for automated analysis which could be clinically applicable. New image analysis algorithms like variable interscan time analysis (VISTA), which provides information on relative blood flow speed [55], continue to expand the capabilities of OCTA. With continued OCTA hardware and software innovation, advancements in clinical applications and pathophysiologic discoveries in AMD are set to follow.
[1][2][3][4][5][6][7][8][9][10][11][12][13][14][15][16][17][18][19][20][21][22][23][24][25][26][27][28][29][30][31][32][33][34][35][36][37][38][39]
References
- W.L. Wong, X. Su, X. Li, C.M. Cheung, R. Klein, C.Y. Cheng and T.Y. Wong. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health 2014, 2, e106-16. 10.1016/S2214-109X(13)70145-1.
- R.E.K. Man, A.T.L. Gan, E.K. Fenwick, K.Y.C. Teo, A.C.S. Tan, G.C.M. Cheung, Z.L. Teo, N. Kumari, T.Y. Wong, C.Y. Cheng and E.L. Lamoureux. Impact of incident age-related macular degeneration and associated vision loss on vision-related quality of life. Br J Ophthalmol 2021, 10.1136/bjophthalmol-2020-318269.
- A. Biesemeier, T. Taubitz, S. Julien, E. Yoeruek and U. Schraermeyer. Choriocapillaris breakdown precedes retinal degeneration in age-related macular degeneration. Neurobiology of Aging 2014, 35, 2562-2573. 10.1016/j.neurobiolaging.2014.05.003.
- D.S. McLeod, M. Taomoto, T. Otsuji, W.R. Green, J.S. Sunness and G.A. Lutty. Quantifying changes in RPE and choroidal vasculature in eyes with age-related macular degeneration. Invest Ophthalmol Vis Sci 2002, 43, 1986-93.
- A. Abdelsalam, L. Del Priore and M.A. Zarbin. Drusen in age-related macular degeneration: pathogenesis, natural course, and laser photocoagulation-induced regression. Surv Ophthalmol 1999, 44, 1-29. 10.1016/s0039-6257(99)00072-7.
- R.F. Spaide. Choriocapillaris Flow Features Follow a Power Law Distribution: Implications for Characterization and Mechanisms of Disease Progression. American Journal of Ophthalmology 2016, 170, 58-67. 10.1016/j.ajo.2016.07.023.
- E. Borrelli, Y. Shi, A. Uji, S. Balasubramanian, M. Nassisi, D. Sarraf and S.R. Sadda. Topographic Analysis of the Choriocapillaris in Intermediate Age-related Macular Degeneration. American Journal of Ophthalmology 2018, 196, 34-43. 10.1016/j.ajo.2018.08.014.
- M. Nassisi, T. Tepelus, G. Corradetti and S.R. Sadda. Relationship between choriocapillaris flow and scotopic microperimetry in early and intermediate age related macular degeneration. Am J Ophthalmol 2020, 10.1016/j.ajo.2020.04.018.
- M. Nassisi, T. Tepelus, M.G. Nittala and S.R. Sadda. Choriocapillaris flow impairment predicts the development and enlargement of drusen. Graefe's Archive for Clinical and Experimental Ophthalmology 2019, 257, 2079-2085. 10.1007/s00417-019-04403-1.
- G. Corradetti, L. Tiosano, M. Nassisi, A.R. Alagorie, F. Corvi, M.G. Nittala and S. Sadda. Scotopic microperimetric sensitivity and inner choroid flow deficits as predictors of progression to nascent geographic atrophy. Br J Ophthalmol 2020, 10.1136/bjophthalmol-2020-316893.
- F. Corvi, L. Tiosano, G. Corradetti, M.G. Nittala, S. Lindenberg, A.R. Alagorie, J.A. McLaughlin, T.K. Lee and S.R. Sadda. Choriocapillaris Flow Deficit as a risk factor for progression of Age-Related Macular Degeneration. Retina 2020, 10.1097/IAE.0000000000002990.
- R.S. Ramrattan, T.L. van der Schaft, C.M. Mooy, W.C. de Bruijn, P.G. Mulder and P.T. de Jong. Morphometric analysis of Bruch's membrane, the choriocapillaris, and the choroid in aging. Invest Ophthalmol Vis Sci 1994, 35, 2857-64.
- E. Corbelli, R. Sacconi, A. Rabiolo, S. Mercuri, A. Carnevali, L. Querques, F. Bandello and G. Querques. Optical Coherence Tomography Angiography in the Evaluation of Geographic Atrophy Area Extension. Invest Ophthalmol Vis Sci 2017, 58, 5201-5208. 10.1167/iovs.17-22508.
- A. Kvanta, M. Casselholm de Salles, U. Amren and H. Bartuma. Optical Coherence Tomography Angiography of the Foveal Microvasculature in Geographic Atrophy. Retina 2017, 37, 936-942. 10.1097/IAE.0000000000001248.
- A.R. Alagorie, A. Verma, M. Nassisi and S.R. Sadda. Quantitative Assessment of Choriocapillaris Flow Deficits in Eyes with Advanced Age-Related Macular Degeneration Versus Healthy Eyes. American Journal of Ophthalmology 2019, 205, 132-139. 10.1016/J.AJO.2019.04.037.
- E.M. Moult, N.K. Waheed, E.A. Novais, W. Choi, B. Lee, S.B. Ploner, E.D. Cole, R.N. Louzada, C.D. Lu, P.J. Rosenfeld, J.S. Duker and J.G. Fujimoto. Swept-Source Optical Coherence Tomography Angiography Reveals Choriocapillaris Alterations in Eyes with Nascent Geographic Atrophy and Drusen-Associated Geographic Atrophy. Retina 2016, 36 Suppl 1, S2-S11. 10.1097/IAE.0000000000001287.
- M. Thulliez, Q. Zhang, Y. Shi, H. Zhou, Z. Chu, L. de Sisternes, M.K. Durbin, W. Feuer, G. Gregori, R.K. Wang and P.J. Rosenfeld. Correlations between Choriocapillaris Flow Deficits around Geographic Atrophy and Enlargement Rates Based on Swept-Source OCT Imaging. Ophthalmol Retina 2019, 3, 478-488. 10.1016/j.oret.2019.01.024.
- M. Nassisi, E. Baghdasaryan, E. Borrelli, M. Ip and S.R. Sadda. Choriocapillaris flow impairment surrounding geographic atrophy correlates with disease progression. PLoS One 2019, 14, e0212563. 10.1371/journal.pone.0212563.
- A.R. Alagorie, M. Nassisi, A. Verma, M. Nittala, G. Corradetti, S. Velaga and S.R. Sadda. Relationship between proximity of choriocapillaris flow deficits and enlargement rate of geographic atrophy. Graefes Arch Clin Exp Ophthalmol 2020, 258, 995-1003. 10.1007/s00417-020-04615-w.
- G.J. Coscas, M. Lupidi, F. Coscas, C. Cagini and E.H. Souied. Optical Coherence Tomography Angiography versus traditional multimodal imaging in assessing the activity of exudative age-related macular degeneration. Retina 2015, 35, 2219-2228. 10.1097/IAE.0000000000000766.
- M. Inoue, J.J. Jung, C. Balaratnasingam, K.K. Dansingani, E. Dhrami-Gavazi, M. Suzuki, T.E. de Carlo, A. Shahlaee, M.A. Klufas, A. El Maftouhi, J.S. Duker, A.C. Ho, M.Q. Maftouhi, D. Sarraf, K.B. Freund and C.-S. Group. A Comparison Between Optical Coherence Tomography Angiography and Fluorescein Angiography for the Imaging of Type 1 Neovascularization. Invest Ophthalmol Vis Sci 2016, 57, OCT314-23. 10.1167/iovs.15-18900.
- F. Corvi, M. Cozzi, A. Invernizzi, L. Pace, S.R. Sadda and G. Staurenghi. Optical coherence tomography angiography for detection of macular neovascularization associated with atrophy in age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol 2020, 10.1007/s00417-020-04821-6.
- M.A. Bonini Filho, T.E. de Carlo, D. Ferrara, M. Adhi, C.R. Baumal, A.J. Witkin, E. Reichel, J.S. Duker and N.K. Waheed. Association of Choroidal Neovascularization and Central Serous Chorioretinopathy With Optical Coherence Tomography Angiography. JAMA Ophthalmol 2015, 133, 899-906. 10.1001/jamaophthalmol.2015.1320.
- R.F. Spaide, G.J. Jaffe, D. Sarraf, K.B. Freund, S.R. Sadda, G. Staurenghi, N.K. Waheed, U. Chakravarthy, P.J. Rosenfeld, F.G. Holz, E.H. Souied, S.Y. Cohen, G. Querques, K. Ohno-Matsui, D. Boyer, A. Gaudric, B. Blodi, C.R. Baumal, X. Li, G.J. Coscas, A. Brucker, L. Singerman, P. Luthert, S. Schmitz-Valckenberg, U. Schmidt-Erfurth, H.E. Grossniklaus, D.J. Wilson, R. Guymer, L.A. Yannuzzi, E.Y. Chew, K. Csaky, J.M. Mones, D. Pauleikhoff, R. Tadayoni and J. Fujimoto. Consensus Nomenclature for Reporting Neovascular Age-Related Macular Degeneration Data: Consensus on Neovascular Age-Related Macular Degeneration Nomenclature Study Group. Ophthalmology 2020, 127, 616-636. 10.1016/j.ophtha.2019.11.004.
- K.B. Freund, S.A. Zweifel and M. Engelbert. Do we need a new classification for choroidal neovascularization in age-related macular degeneration? Retina 2010, 30, 1333-49. 10.1097/IAE.0b013e3181e7976b.
- L. Mendonça, R. Perrott-Reynolds, R. Schwartz, H.A. Madi, N. Cronbach, I. Gendelman, A. Muldrew, F. Bannon, K. Balaskas, C.M.G. Cheung, A.A. Fawzi, D. Ferrara, K.B. Freund, J.G. Fujimoto, M.R. Munk, G. Querques, R. Ribeiro, P.J. Rosenfeld, S.R. Sadda, J. Sahni, D. Sarraf, R. Spaide, U. Schmidt-Erfurth, E. Souied, G. Staurenghi, R. Tadayoni, R. Wang, U. Chakravarthy and N.K. Waheed. Deliberations of an international panel of experts on OCTA Nomenclature of nAMD. Ophthalmology 2020,
- L. Kuehlewein, M. Bansal, T.L. Lenis, N.A. Iafe, S.R. Sadda, M.A. Bonini Filho, T.E. De Carlo, N.K. Waheed, J.S. Duker and D. Sarraf. Optical Coherence Tomography Angiography of Type 1 Neovascularization in Age-Related Macular Degeneration. Am J Ophthalmol 2015, 160, 739-48 e2. 10.1016/j.ajo.2015.06.030.
- K.K. Dansingani and K.B. Freund. Optical Coherence Tomography Angiography Reveals Mature, Tangled Vascular Networks in Eyes With Neovascular Age-Related Macular Degeneration Showing Resistance to Geographic Atrophy. Ophthalmic Surg Lasers Imaging Retina 2015, 46, 907-12. 10.3928/23258160-20151008-02.
- L. Mastropasqua, L. Toto, E. Borrelli, P. Carpineto, L. Di Antonio and R. Mastropasqua. Optical Coherence Tomography Angiography Assessment of Vascular Effects Occurring after Aflibercept Intravitreal Injections in Treatment-Naive Patients with Wet Age-Related Macular Degeneration. Retina 2017, 37, 247-256. 10.1097/IAE.0000000000001145.
- A. Miere, P. Butori, S.Y. Cohen, O. Semoun, V. Capuano, C. Jung and E.H. Souied. Vascular Remodeling of Choroidal Neovascularization after Anti-Vascular Endothelial Growth Factor Therapy Visualized on Optical Coherence Tomography Angiography. Retina 2019, 39, 548-557. 10.1097/IAE.0000000000001964.
- Y. Jia, S.T. Bailey, T.S. Hwang, S.M. McClintic, S.S. Gao, M.E. Pennesi, C.J. Flaxel, A.K. Lauer, D.J. Wilson, J. Hornegger, J.G. Fujimoto and D. Huang. Quantitative optical coherence tomography angiography of vascular abnormalities in the living human eye. Proc Natl Acad Sci U S A 2015, 112, E2395-402. 10.1073/pnas.1500185112.
- F. Sulzbacher, A. Pollreisz, A. Kaider, S. Kickinger, S. Sacu, U. Schmidt-Erfurth and C. Vienna Eye Study. Identification and clinical role of choroidal neovascularization characteristics based on optical coherence tomography angiography. Acta Ophthalmol 2017, 95, 414-420. 10.1111/aos.13364.
- L. Kuehlewein, S.R. Sadda and D. Sarraf. OCT angiography and sequential quantitative analysis of type 2 neovascularization after ranibizumab therapy. Eye (Lond) 2015, 29, 932-5. 10.1038/eye.2015.80.
- M.L. Farecki, M. Gutfleisch, H. Faatz, K. Rothaus, B. Heimes, G. Spital, A. Lommatzsch and D. Pauleikhoff. Characteristics of type 1 and 2 CNV in exudative AMD in OCT-Angiography. Graefes Arch Clin Exp Ophthalmol 2017, 255, 913-921. 10.1007/s00417-017-3588-y.
- L. Kuehlewein, K.K. Dansingani, T.E. de Carlo, M.A. Bonini Filho, N.A. Iafe, T.L. Lenis, K.B. Freund, N.K. Waheed, J.S. Duker, S.R. Sadda and D. Sarraf. Optical Coherence Tomography Angiography of Type 3 Neovascularization Secondary to Age-Related Macular Degeneration. Retina 2015, 35, 2229-35. 10.1097/IAE.0000000000000835.
- A.C. Tan, K.K. Dansingani, L.A. Yannuzzi, D. Sarraf and K.B. Freund. Type 3 Neovascularization Imaged with Cross-Sectional and En Face Optical Coherence Tomography Angiography. Retina 2017, 37, 234-246. 10.1097/IAE.0000000000001343.
- R. Sacconi, D. Sarraf, S. Garrity, K.B. Freund, L.A. Yannuzzi, O. Gal-Or, E. Souied, A. Sieiro, E. Corbelli, A. Carnevali, L. Querques, F. Bandello and G. Querques. Nascent Type 3 Neovascularization in Age-Related Macular Degeneration. Ophthalmol Retina 2018, 2, 1097-1106. 10.1016/j.oret.2018.04.016.
- E. Borrelli, E.H. Souied, K.B. Freund, G. Querques, A. Miere, O. Gal-Or, R. Sacconi, S.R. Sadda and D. Sarraf. Reduced Choriocapillaris Flow in Eyes with Type 3 Neovascularization and Age-Related Macular Degeneration. Retina 2018, 38, 1968-1976. 10.1097/IAE.0000000000002198.
- M. Al-Sheikh, N.A. Iafe, N. Phasukkijwatana, S.R. Sadda and D. Sarraf. Biomarkers of Neovascular Activity in Age-related Macular Degeneration Using Optical Coherence Tomography Angiography. Retina 2018, 38, 220-230. 10.1097/IAE.0000000000001628.
- M.A. Wirth, F. Freiberg, M. Pfau, J. Wons, M.D. Becker and S. Michels. Optical coherence tomography angiography in age-related macular degeneration: persistence of vascular network in quiescent choroidal neovascularization. Acta Ophthalmologica 2017, 95, 428-430. 10.1111/aos.13226.
- E.D. Cole, D. Ferrara, E.A. Novais, R.N. Louzada and N.K. Waheed. Clinical Trial Endpoints for Optical Coherence Tomography Angiography in Neovascular Age-Related Macular Degeneration. Retina 2016, 36 Suppl 1, S83-S92. 10.1097/IAE.0000000000001338.
- A. El Ameen, S.Y. Cohen, O. Semoun, A. Miere, M. Srour, M. Quaranta-El Maftouhi, H. Oubraham, R. Blanco-Garavito, G. Querques and E.H. Souied. Type 2 Neovascularization Secondary to Age-Related Macular Degeneration Imaged by Optical Coherence Tomography Angiography. Retina 2015, 35, 2212-8. 10.1097/IAE.0000000000000773.
- B. Lumbroso, M. Rispoli and M.C. Savastano. Longitudinal Optical Coherence Tomography-Angiography Study of Type 2 Naive Choroidal Neovascularization Early Response after Treatment. Retina 2015, 35, 2242-51. 10.1097/IAE.0000000000000879.
- J.P. Marques, J.F. Costa, M. Marques, M.L. Cachulo, J. Figueira and R. Silva. Sequential Morphological Changes in the CNV Net after Intravitreal Anti-VEGF Evaluated with OCT Angiography. Ophthalmic Res 2016, 55, 145-51. 10.1159/000442671.
- N.W. Muakkassa, A.T. Chin, T. de Carlo, K.A. Klein, C.R. Baumal, A.J. Witkin, J.S. Duker and N.K. Waheed. Characterizing the Effect of Anti-Vascular Endothelial Growth Factor Therapy on Treatment-Naive Choroidal Neovascularization Using Optical Coherence Tomography Angiography. Retina 2015, 35, 2252-9. 10.1097/IAE.0000000000000836.
- B. Lumbroso, M. Rispoli, M.C. Savastano, Y. Jia, O. Tan and D. Huang. Optical Coherence Tomography Angiography Study of Choroidal Neovascularization Early Response after Treatment. Dev Ophthalmol 2016, 56, 77-85. 10.1159/000442782.
- R.F. Spaide. Optical Coherence Tomography Angiography Signs of Vascular Abnormalization With Antiangiogenic Therapy for Choroidal Neovascularization. Am J Ophthalmol 2015, 160, 6-16. 10.1016/j.ajo.2015.04.012.
- D. Xu, J.P. Davila, M. Rahimi, C.B. Rebhun, A.Y. Alibhai, N.K. Waheed and D. Sarraf. Long-term Progression of Type 1 Neovascularization in Age-related Macular Degeneration Using Optical Coherence Tomography Angiography. Am J Ophthalmol 2018, 187, 10-20. 10.1016/j.ajo.2017.12.005.
- J. Forster, W. Harriss-Phillips, M. Douglass and E. Bezak. A review of the development of tumor vasculature and its effects on the tumor microenvironment. Hypoxia 2017, Volume 5, 21-32. 10.2147/HP.S133231.
- M. Rispoli, M.C. Savastano and B. Lumbroso. Quantitative Vascular Density Changes in Choriocapillaris Around CNV After Anti-VEGF Treatment: Dark Halo. Ophthalmic Surg Lasers Imaging Retina 2018, 49, 918-924. 10.3928/23258160-20181203-02.
- A.D. Treister, P.L. Nesper, A.E. Fayed, M.K. Gill, R.G. Mirza and A.A. Fawzi. Prevalence of subclinical CNV and choriocapillaris nonperfusion in fellow eyes of unilateral exudative AMD on OCT angiography. Translational Vision Science and Technology 2018, 10.1167/tvst.7.5.19.
- J.M. Scharf, G. Corradetti, A.R. Alagorie, C. Grondin, A. Hilely, D. Wang, S. Sadda and D. Sarraf. Choriocapillaris Flow Deficits and Treatment-Naive Macular Neovascularization Secondary to Age-Related Macular Degeneration. Invest Ophthalmol Vis Sci 2020, 61, 11. 10.1167/iovs.61.11.11.
- E.M. Moult, A.Y. Alibhai, C. Rebhun, B. Lee, S. Ploner, J. Schottenhamml, L. Husvogt, C.R. Baumal, A.J. Witkin, A. Maier, J.S. Duker, P.J. Rosenfeld, N.K. Waheed and J.G. Fujimoto. Spatial distribution of choriocapillaris impairment in eyes with choroidal neovascularization secondary to age-related macular degeneration. Retina 2019, 1-1. 10.1097/IAE.0000000000002556.
- A.R. Alagorie, A. Verma, M. Nassisi, M. Nittala, S. Velaga, L. Tiosano and S.R. Sadda. Quantitative Assessment of Choriocapillaris Flow Deficits Surrounding Choroidal Neovascular Membranes. Retina 2020, 40, 2106-2112. 10.1097/IAE.0000000000002878.
- S.B. Ploner, E.M. Moult, W. Choi, N.K. Waheed, B. Lee, E.A. Novais, E.D. Cole, B. Potsaid, L. Husvogt, J. Schottenhamml, A. Maier, P.J. Rosenfeld, J.S. Duker, J. Hornegger and J.G. Fujimoto. TOWARD QUANTITATIVE OPTICAL COHERENCE TOMOGRAPHY ANGIOGRAPHY: Visualizing Blood Flow Speeds in Ocular Pathology Using Variable Interscan Time Analysis. Retina 2016, 36 Suppl 1, S118-S126. 10.1097/IAE.0000000000001328.





