
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Leigh Greathouse | + 1695 word(s) | 1695 | 2021-04-25 10:06:36 | | | |
| 2 | Rita Xu | Meta information modification | 1695 | 2021-05-07 09:55:12 | | |
Video Upload Options
Tryptophan metabolism, via the kynurenine (Kyn) pathway, and microbial transformation of tryptophan to indolic compounds are fundamental for host health; both of which are altered in colon carcinogenesis.
1. Introduction
Over a century ago (1901), English chemist Frederick Gowland Hopkins made a significant contribution to the field of nutrition by discovering the essential amino acid Tryptophan (Trp) from casein, a milk protein. As an essential amino acid, Trp can only be obtained through diet, mainly meat, dairy, and seeds. Trp is required for protein and niacin biosynthesis and is a precursor of serotonin and melatonin. Apart from these fundamental functions, Trp is appreciated for its influence on both host and microbial metabolism via two distinct pathways: the Kynurenine (Kyn) Pathway and Indolic Pathway. These pathways metabolize Trp into metabolic and neuroactive compounds that influence microbial composition and host physiology in an enzyme-dependent manner. Indole has a bicyclic ring formed by benzene and pyrrole groups. This indole structure is part of Trp and makes it unique in its structure and function. Indole can be kept intact or cleaved from the Trp structure, generating bioactive molecules [1]. Specific taxa among the microbiota also take advantage of this unique property of Trp to produce indoles for signaling and defense [2]. Microbial and dietary indoles can also trigger or suppress immune function thereby maintaining a symbiotic relationship with the host [3][4]. However, both Kyn and indolic processes can become dysfunctional in the pathogenesis of colon cancer.
2. The Four Main Pathways of Human Trp Metabolism
There are four known metabolic pathways that Trp can enter once in the upper gastrointestinal (GI) tract; Kyn pathway, serotonin pathway, indolic pathway (bacterial degradation) and tryptamine pathway [5]. These distinct pathways compete for the available free Trp pool and specifically convert Trp into Kyn, serotonin, indoles, and tryptamine as the end products, respectively [6]. About 90–95% [7][8] of Trp is converted into Kyn and downstream metabolites (Figure 1) via the Kyn pathway. These metabolites include L-kynurine acid, 3-hydroxykynurine, anthralinic acid, quinolinic acid, 3-hydroxyanthralininc acid, picolinic acid, most of which produce NAD+ and ATP in host cells, and play a vital role in inflammation, immune tolerance, and neurotransmission [5][9][10][11].
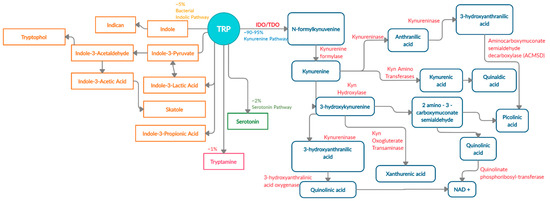
Figure 1. Schematic representation of enzymes and metabolites in the Trp-Kyn pathway.
About 4–6% of unabsorbed Trp then moves along the gastrointestinal tract and is metabolized by the microbiome into indole and indolic compounds including indole-3 pyruvate, indole-3-acetamide, indole-3-acetaldehyde, indole-3-acetic acid, indole-3-lactic acid, and indole-3- propionic acid [8][12][13][14][15][16]. These indolic compounds can bind to pregnane X receptors (PXR) and aryl hydrocarbon receptor (AhR) to promote intestinal homeostasis, enhance barrier function and tight junctions, reduce permeability, regulate intestinal immune tolerance [5][13][17][18]. Additionally, indoles can act as antioxidants and neuroprotective compounds [19][20]. Overall, indole production has an important impact on host health as a result of Trp metabolism by the microbiota [5].
In addition to Kyn pathway and microbial degradation, 1–2% of Trp can be converted into 5-hydroxytryptamine (serotonin) in the enterochromaffin cells of the intestinal mucosa. Serotonin is primarily involved in regulating gut motility, vasodilation, and maintenance of mood [21]. A minor contribution of Trp (about 1%) is towards the production of tryptamine, which can be initiated by both the host and microbiota [22]. Microbial tryptamine, a ligand for serotonin receptors, can also affect gut motility and transit time, given the abundance of such receptors in the GI tract [23]. Contribution of the gut microbiota towards serotonin and tryptamine production is an understudied field of research and will need further investigation in the context of carcinogenesis.
3. The Kynurenine Pathway Is the Major Tryptophan Degradation Pathway
The Kyn pathway (90–95% of ingested Trp) is the major Trp degradation pathway that produces Kyn and other neuroactive metabolites either in the liver or in the extrahepatic tissue. In addition to neuroactive metabolites, the Kyn pathway also regulates systemic Trp levels, availability of Trp for serotonin synthesis, vitamin B3, and hepatic heme synthesis [9]. About 90% of Trp metabolized via the Kyn pathway takes place in the liver where all the enzymes for complete transformation of Trp into NAD+ are present. The hepatic Kyn pathway is mainly induced by signaling from glucocorticoids, as well as estrogen and glucagon [5][24][25]. The remaining 10% of the Trp transformation takes place via the Kyn pathway in the extrahepatic tissues, and is induced by cytokines (Interferon-γ (IFN-γ), Interleukin-1 (IL-1), Interluekin-6 (IL-6)), lipopolysaccharides, prostaglandins and amyloid peptides [7]. However, the metabolites produced in the extrahepatic tissues do not contain all the enzymes for complete conversion of Trp into NAD+. Availability, or lack thereof, of all enzymes to completely transform Trp determines the intermediates that are produced, ultimately affecting the functional outcome [9][26][27][28].
The array of metabolites produced via the Kyn pathway have led many researchers to investigate Trp and the role of these metabolically active metabolites in immune function, behavioral disorders including mood, anxiety and depression, inflammatory conditions, gut homeostasis, inflammatory bowel disease (IBD), neurodegenerative disorders such as Alzheimer’s disease, and cancer. All these disease states appear to have an altered or amplified Kyn pathway activity [1][10][29][30][31][32][33][34]. Under inflammatory conditions, common to most of these disease states, Trp is shunted into the Kyn pathway, especially in the extrahepatic tissue (mostly immune cells), while the hepatic pathway activity is reduced [9][25][35]. This shunting of Trp into Kyn pathway, reduces Trp availability for other pathways especially serotonin and indolic pathways; the two pathways which have shown to be beneficial for host health in the presence of Trp [34][36][37]. Furthermore, reduced Trp and increased Kyn, alters the activation and balance of innate and adaptive immune cells towards a tolerogenic milieu [37]. Therefore, Kyn pathway activity is amplified under inflammatory conditions, in part due to an NAD+ requirement for increased energy demands in the immune cells and to implement immunomodulatory functions [35].
3.1. Kynurenine Pathway and the First-Rate Limiting Enzyme IDO1
The first and rate-limiting enzymes in Trp catabolism are Tryptophan 2,3-dioxygenase (TDO) and Indoleamine 2,3-dioxygenase (IDO1), which are present in the liver and in the extrahepatic tissues, respectively. TDO regulates systemic levels of Trp. Under normal physiological conditions, the majority of Trp is metabolized in the liver to produce Kyn, C -reactive proteins, haptoglobin, and fibrinogen [25]. IDO1 is expressed by various different tissue and cells types including lungs, placenta, GI tract, immune cells (e.g., monocytes, macrophages, dendritic cells, antigen-presenting cells) and epithelial cells. The function of IDO1 in extrahepatic cells is the same, which is the conversion of Trp into Kyn [38].
Under normal conditions, IDO1 expression regulates T cell proliferation to prevent tissue damage and reduce oxidative stress [25]. During inflammation, the expression of TDO is reduced and IDO1 expression is increased. Once IDO1 is activated upon signaling from cytokines such as IFN-γ, TNFα, prostaglandins and lipopolysaccrides, it converts tryptophan into N-formylkynurenine, followed by a rapid transformation into Kyn, the first stable catabolite in the pathway [5][39][40]. Kyn is then acted upon by various enzymes in a tissue-dependent manner and produces downstream neuroactive and immunoactive metabolites which regulate immune cell activity [41].
Extrahepatic Kyn production governs immune homeostasis [42]. This action occurs by reduction of activated T-cells, dendritic cells and, natural killer cells and induction of Th1 cell apoptosis to control excessive inflammation [11][40]. Each downstream Kyn metabolites perform specific functions. Kynurenic acid elicits an anti-inflammatory response through its anti-oxidant properties, while picolinic acid exhibits anti-tumor activity by suppressing T-cell and c-Myc activation [11][43][44][45]. Furthermore, 3-hydroxyanthralinic acid and quinolinic acid can act as neurotoxins in certain disease states including chronic brain injury, osteoporosis, coronary heart disease, Huntington’s disease, stroke, depression, and colon cancer [40][46][47]. Collectively, these downstream metabolites modulate immune homeostasis in part by activating the ligand-dependent transcription factor aryl hydrocarbon receptor (AhR).
3.2. Kynurenine Pathway and AhR Activation
AhR is a transcription factor that belongs to the family of basic helix-loop-helix transcription factors that control genes containing xenobiotic response elements (XREs), as well as through non-XRE response elements; estrogen receptor and retinoic acid receptors [48]. In an inactive state, AhR is bound to chaperone proteins. Upon ligand binding, the chaperone protein disassociates with the complex and cytoplasmic AhR translocate into the nucleus heterodimerizing with AhR nuclear translocator (ARNT), leading to transcription of CYP1A1, CYP1B1, IDO1, TDO, IL-22, GSTA and AhRR (Table 1) [49][50]. Several of these genes control metabolism of xenobiotic and environmental chemicals including dioxins, polycyclic aromatic hydrocarbons, benzathracens and halogenated aromatic hydrocarbons through regulation of cytochrome P-450 [51][52][53][54][55]. Furthermore, AhR can interact with additional transcription factors, including pro-inflammatory nuclear factor-kB, and epigenetic regulators, as well as, acting as an E3 ubiquitin ligase. In the intestinal epithelial cells, AhR works to control immune homeostasis, epithelial barrier function, and symbiosis with the microbiota [48].
Table 1. Classes of Ahr ligands.
| Class of Molecule | Metabolites | Genes Activated | Effects (Ligand Dependent) |
|---|---|---|---|
| Tryptophan Metabolites | Kynurenine Kynurenic Acid Xanthurenic Acid Anthralinic Acid Quinolinic Acid Pinolinic Acid Cinnabarinic Acid 3-hydroxykynurenine 3-hydroxyanthralinic acid |
CYP1A1/CYP1B1 AHRR IL-6 VEGFA PTGS2 IL-22 IDO1 TDO |
Neurotoxic Effects B-cell & T-cell differentiation Development of intraepithelial lymphocytes Immune tolerance Enhanced epithelial barrier function Anti-inflammatory Intestinal homeostasis Gut motility Microbial composition Antimicrobial affects Mucosal barrier function Serotonin modulation |
| Dioxins | Polycyclic Aromatic hydrocarbons Benzatheracues Halogenated aromatic hydrocarbons |
||
| Dietary compounds | 3-3-diindolemethane (DIM) Indole-3-carbinol Indole-3-acetonitrile Indole [3,2-b] carbazole 2-(indole-3-ylmethyl)-3,3-diindolymethane Herbs- Ginseng, gingko biloba, licorice |
||
| Microbial metabolites | Indole 3-hydroxyindole Indolealdehyde Tryptamine Indole acetic acid Tryptanthrin 3-methyl indole Indirubin Indigo Indole sulphate Malassezin |
||
| Photo-oxidative Trp metabolite | 6-formylindole [3,2-b] Carbazole (FICZ) |
AhR activation is a crucial mediator for intestinal immunity and intestinal homeostasis through the development and regulation of intraepithelial lymphocytes and innate lymphoid cells [13][56][57][58][59][60]. AhR activation can be induced by distinct classes of endogenous ligands including Trp metabolites, dietary compounds, microbial Trp metabolites, bilirubin, arachidonic acid, prostaglandins, and cytokines (Table 1). Depending upon the ligand, AhR activation can result in different molecular and physiological responses including histone modification, lipid synthesis, energy, xenobiotic metabolism, immune function, epithelial barrier function and cell migration [56][57][58][59][60][61]. Therefore, AhR is involved in multiple pathways that regulate endogenous and exogeneous signals that controls the immune response.
Several reports have shown the importance of AhR ligands in modulating gastrointestinal homeostasis via mucosal immune cells. However, the exact mechanism of this modulation is unclear due to the multitude of different ligands and transcriptional factors involved in controlling physiological effects [50]. AhR-modulated intestinal homeostasis can be achieved in one of two ways 1) it can exert an anti-inflammatory response through the activation of Tregs; 2) it can enhance intestinal mucosal integrity through the activation of Th17 cells and induction of IL-22 [13][50][62][63]. Therefore, AhR activation is critical in maintaining intestinal homeostasis. Thus, disruption in AhR expression or activity can lead to altered intestinal homeostasis and carcinogenesis.
References
- Palego, L.; Betti, L.; Rossi, A.; Giannaccini, G. Tryptophan Biochemistry: Structural, Nutritional, Metabolic, and Medical Aspects in Humans. J. Amino Acids 2016, 2016.
- Lee, J.-H.; Lee, J. Indole as an intercellular signal in microbial communities. FEMS Microbiol. Rev. 2010, 34, 426–444.
- Mandrich, L.; Caputo, E. Brassicaceae-Derived Anticancer Agents: Towards a Green Approach to Beat Cancer. Nutrients 2020, 12, 868.
- Powell, D.N.; Swimm, A.; Sonowal, R.; Bretin, A.; Gewirtz, A.T.; Jones, R.M.; Kalman, D. Indoles from the commensal microbiota act via the AHR and IL-10 to tune the cellular composition of the colonic epithelium during aging. Proc. Natl. Acad. Sci. USA 2020, 117, 21519–21526.
- Cervenka, I.; Agudelo, L.Z.; Ruas, J.L. Kynurenines: Tryptophan’s metabolites in exercise, inflammation, and mental health. Science 2017, 357.
- Rabbani, M.A.G.; Barik, S. 5-Hydroxytryptophan, a major product of tryptophan degradation, is essential for optimal replication of human parainfluenza virus. Virology 2017, 503, 46–51.
- Dehhaghi, M.; Panahi, H.K.S.; Guillemin, G.J. Microorganisms, Tryptophan Metabolism, and Kynurenine Pathway: A Complex Interconnected Loop Influencing Human Health Status. Int. J. Tryptophan Res. 2019, 12, 117864691985299.
- Gao, J.; Xu, K.; Liu, H.; Liu, G.; Bai, M.; Peng, C.; Li, T.; Yin, Y. Impact of the Gut Microbiota on Intestinal Immunity Mediated by Tryptophan Metabolism. Front. Cell. Infect. Microbiol. 2018, 8.
- Badawy, A.A.-B. Kynurenine Pathway of Tryptophan Metabolism: Regulatory and Functional Aspects. Int. J. Tryptophan Res. IJTR 2017, 10.
- Taleb, S. Tryptophan Dietary Impacts Gut Barrier and Metabolic Diseases. Front. Immunol. 2019, 10, 2113.
- Venkateswaran, N.; Conacci-Sorrell, M. Kynurenine: An oncometabolite in colon cancer. Cell Stress 2020, 4, 24–26.
- Chimerel, C.; Emery, E.; Summers, D.K.; Keyser, U.; Gribble, F.M.; Reimann, F. Bacterial Metabolite Indole Modulates Incretin Secretion from Intestinal Enteroendocrine L Cells. Cell Rep. 2014, 9, 1202–1208.
- Hubbard, T.D.; Murray, I.A.; Perdew, G.H. Indole and Tryptophan Metabolism: Endogenous and Dietary Routes to Ah Receptor Activation. Drug Metab. Dispos. 2015, 43, 1522–1535.
- Krishnan, S.; Ding, Y.; Saedi, N.; Choi, M.; Sridharan, G.V.; Sherr, D.H.; Yarmush, M.L.; Alaniz, R.C.; Jayaraman, A.; Lee, K. Gut Microbiota-Derived Tryptophan Metabolites Modulate Inflammatory Response in Hepatocytes and Macrophages. Cell Rep. 2018, 23, 1099–1111.
- Nyangale, E.P.; Mottram, D.S.; Gibson, G.R. Gut Microbial Activity, Implications for Health and Disease: The Potential Role of Metabolite Analysis. J. Proteome Res. 2012, 11, 5573–5585.
- Shimada, Y.; Kinoshita, M.; Harada, K.; Mizutani, M.; Masahata, K.; Kayama, H.; Takeda, K. Commensal Bacteria-Dependent Indole Production Enhances Epithelial Barrier Function in the Colon. PLoS ONE 2013, 8.
- Alexeev, E.E.; Lanis, J.M.; Kao, D.J.; Campbell, E.L.; Kelly, C.J.; Battista, K.D.; Gerich, M.E.; Jenkins, B.R.; Walk, S.T.; Kominsky, D.J.; et al. Microbiota-Derived Indole Metabolites Promote Human and Murine Intestinal Homeostasis through Regulation of Interleukin-10 Receptor. Am. J. Pathol. 2018, 188, 1183–1194.
- Garg, A.; Zhao, A.; Erickson, S.L.; Mukherjee, S.; Lau, A.J.; Alston, L.; Chang, T.K.H.; Mani, S.; Hirota, S.A. Pregnane X Receptor Activation Attenuates Inflammation-Associated Intestinal Epithelial Barrier Dysfunction by Inhibiting Cytokine-Induced Myosin Light-Chain Kinase Expression and c-Jun N-Terminal Kinase 1/2 Activation. J. Pharmacol. Exp. Ther. 2016, 359, 91–101.
- Negatu, D.A.; Gengenbacher, M.; Dartois, V.; Dick, T. Indole Propionic Acid, an Unusual Antibiotic Produced by the Gut Microbiota, With Anti-inflammatory and Antioxidant Properties. Front. Microbiol. 2020, 11.
- Konopelski, P. Indoles—Gut Bacteria Metabolites of Tryptophan with Pharmacotherapeutic Potential. Curr. Drug Metab. 2018, 19, 883–890.
- Mawe, G.M.; Hoffman, J.M. Serotonin signalling in the gut—Functions, dysfunctions and therapeutic targets. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 473–486.
- Badawy, A.A.-B. Tryptophan Metabolism: A Versatile Area Providing Multiple Targets for Pharmacological Intervention. Egypt. J. Basic Clin. Pharmacol. 2019, 9.
- Bhattarai, Y.; Williams, B.B.; Battaglioli, E.J.; Whitaker, W.R.; Till, L.; Grover, M.; Linden, D.R.; Akiba, Y.; Kandimalla, K.K.; Zachos, N.C.; et al. Gut Microbiota-Produced Tryptamine Activates an Epithelial G-Protein-Coupled Receptor to Increase Colonic Secretion. Cell Host Microbe 2018, 23, 775–785.e5.
- Tanaka, T.; Knox, W.E. The Nature and Mechanism of the Tryptophan Pyrrolase (Peroxidase-Oxidase) Reaction of Pseudomonas and of Rat Liver. J. Biol. Chem. 1959, 234, 1162–1170.
- Le Floc’H, N.; Otten, W.; Merlot, E. Tryptophan metabolism, from nutrition to potential therapeutic applications. Amino Acids 2011, 41, 1195–1205.
- Murray, I.A.; Patterson, A.D.; Perdew, G.H. AH receptor ligands in cancer: Friend and foe. Nat. Rev. Cancer 2014, 14, 801–814.
- Stone, T.W.; Stoy, N.; Darlington, L.G. An expanding range of targets for kynurenine metabolites of tryptophan. Trends Pharmacol. Sci. 2013, 34, 136–143.
- DiNatale, B.C.; Murray, I.A.; Schroeder, J.C.; Flaveny, C.A.; Lahoti, T.S.; Laurenzana, E.M.; Omiecinski, C.J.; Perdew, G.H. Kynurenic Acid Is a Potent Endogenous Aryl Hydrocarbon Receptor Ligand that Synergistically Induces Interleukin-6 in the Presence of Inflammatory Signaling. Toxicol. Sci. 2010, 115, 89–97.
- Ding, X.; Bin, P.; Wu, W.; Chang, Y.; Zhu, G. Tryptophan Metabolism, Regulatory T Cells, and Inflammatory Bowel Disease: A Mini Review. Mediat. Inflamm. 2020, 2020, 1–10.
- Tryptophan Metabolism: Implications for Biological Processes, Health and Disease; Engin, A.; Engin, A.B. (Eds.) Springer International Publishing: Cham, Switzerland, 2015.
- Gupta, N.K.; Thaker, A.I.; Kanuri, N.; Riehl, T.E.; Rowley, C.W.; Stenson, W.F.; Ciorba, M.A. Serum Analysis of Tryptophan Catabolism Pathway: Correlation With Crohn’s Disease Activity. Inflamm. Bowel Dis. 2012, 18, 1214–1220.
- Santhanam, S.; Alvarado, D.M.; Ciorba, M.A. Therapeutic targeting of inflammation and tryptophan metabolism in colon and gastrointestinal cancer. Transl. Res. 2016, 167, 67–79.
- Sorgdrager, F.J.H.; Naudé, P.J.W.; Kema, I.P.; Nollen, E.A.; de Deyn, P.P. Tryptophan Metabolism in Inflammaging: From Biomarker to Therapeutic Target. Front. Immunol. 2019, 10.
- Zhang, H.-L.; Zhang, A.-H.; Miao, J.-H.; Sun, H.; Yan, G.-L.; Wu, F.-F.; Wang, X.-J. Targeting regulation of tryptophan metabolism for colorectal cancer therapy: A systematic review. RSC Adv. 2019, 9, 3072–3080.
- Moffett, J.R.; Arun, P.; Puthillathu, N.; Vengilote, R.; Ives, J.A.; Badawy, A.A.-B.; Namboodiri, A.M. Quinolinate as a Marker for Kynurenine Metabolite Formation and the Unresolved Question of NAD+ Synthesis During Inflammation and Infection. Front. Immunol. 2020, 11.
- Crotti, S.; D’Angelo, E.; Bedin, C.; Fassan, M.; Pucciarelli, S.; Nitti, D.; Bertazzo, A.; Agostini, M. Tryptophan metabolism along the kynurenine and serotonin pathways reveals substantial differences in colon and rectal cancer. Metabolomics 2017, 13, 148.
- Routy, J.-P.; Routy, B.; Graziani, G.M.; Mehraj, V. The Kynurenine Pathway is a Double-Edged Sword in Immune-Privileged Sites and in Cancer: Implications for Immunotherapy. Int. J. Tryptophan Res. 2016, 9, 67–77.
- Nikolaus, S.; Schulte, B.; Al-Massad, N.; Thieme, F.; Schulte, D.M.; Bethge, J.; Rehman, A.; Tran, F.; Aden, K.; Häsler, R.; et al. Increased Tryptophan Metabolism Is Associated With Activity of Inflammatory Bowel Diseases. Gastroenterology 2017, 153, 1504–1516.
- Nguyen, N.T.; Enakahama, T.; Le, D.H.; van Son, L.; Chu, H.H.; Ekishimoto, T. Aryl Hydrocarbon Receptor and Kynurenine: Recent Advances in Autoimmune Disease Research. Front. Immunol. 2014, 5.
- Puccetti, P.; Fallarino, F.; Italiano, A.; Soubeyran, I.; MacGrogan, G.; Debled, M.; Velasco, V.; Bodet, D.; Eimer, S.; Veldhoen, M.; et al. Accumulation of an Endogenous Tryptophan-Derived Metabolite in Colorectal and Breast Cancers. PLoS ONE 2015, 10.
- Savitz, J. The kynurenine pathway: A finger in every pie. Mol. Psychiatry 2020, 25, 131–147.
- Ye, Z.; Yue, L.; Shi, J.; Shao, M.; Wu, T. Role of IDO and TDO in Cancers and Related Diseases and the Therapeutic Implications. J. Cancer 2019, 10, 2771–2782.
- Venkateswaran, N.; Lafita-Navarro, M.C.; Hao, Y.-H.; Kilgore, J.A.; Perez-Castro, L.; Braverman, J.; Borenstein-Auerbach, N.; Kim, M.; Lesner, N.P.; Mishra, P.; et al. MYC promotes tryptophan uptake and metabolism by the kynurenine pathway in colon cancer. Genes Dev. 2019, 33, 1236–1251.
- Prodinger, J.; Loacker, L.J.; Schmidt, R.L.J.; Ratzinger, F.; Greiner, G.; Witzeneder, N.; Hoermann, G.; Jutz, S.; Pickl, W.F.; Steinberger, P.; et al. The tryptophan metabolite picolinic acid suppresses proliferation and metabolic activity of CD4+T cells and inhibits c-Myc activation. J. Leukoc. Biol. 2016, 99, 583–594.
- Walczak, K.; Turski, W.A.; Rajtar, G. Kynurenic acid inhibits colon cancer proliferation in vitro: Effects on signaling pathways. Amino Acids 2014, 46, 2393–2401.
- Chen, Y.; Guillemin, G.J. Kynurenine Pathway Metabolites in Humans: Disease and Healthy States. Int. J. Tryptophan Res. 2009, 2, IJTR.S2097.
- Darlington, L.G.; Forrest, C.M.; Mackay, G.M.; Smith, R.A.; Smith, A.J.; Stoy, N.; Stone, T.W. On the Biological Importance of the 3-hydroxyanthranilic Acid: Anthranilic Acid Ratio. Int. J. Tryptophan Res. 2010, 3, 51–59.
- Rothhammer, V.; Quintana, F.J. The aryl hydrocarbon receptor: An environmental sensor integrating immune responses in health and disease. Nat. Rev. Immunol. 2019, 19, 184–197.
- Bock, K.W. Aryl hydrocarbon receptor (AHR): From selected human target genes and crosstalk with transcription factors to multiple AHR functions. Biochem. Pharmacol. 2019, 168, 65–70.
- Lamas, B.; Natividad, J.M.; Sokol, H. Aryl hydrocarbon receptor and intestinal immunity. Mucosal Immunol. 2018, 11, 1024–1038.
- Murray, I.A.; Perdew, G.H. Ligand activation of the Ah receptor contributes to gastrointestinal homeostasis. Curr. Opin. Toxicol. 2017, 2, 15–23.
- Natividad, J.M.; Agus, A.; Planchais, J.; Lamas, B.; Jarry, A.C.; Martin, R.; Michel, M.-L.; Chong-Nguyen, C.; Roussel, R.; Straube, M.; et al. Impaired Aryl Hydrocarbon Receptor Ligand Production by the Gut Microbiota Is a Key Factor in Metabolic Syndrome. Cell Metab. 2018, 28, 737–749.e4.
- Opitz, C.A.; Litzenburger, U.M.; Sahm, F.; Ott, M.; Tritschler, I.; Trump, S.; Schumacher, T.; Jestaedt, L.; Schrenk, D.; Weller, M.; et al. An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature 2011, 478, 197–203.
- Tsay, J.-C.J.; Tchou-Wong, K.-M.; Greenberg, A.K.; Pass, H.; Rom, W.N. Aryl hydrocarbon receptor and lung cancer. Anticancer Res. 2013, 33, 1247–1256.
- Yue, T.; Sun, F.; Yang, C.; Wang, F.; Luo, J.; Yang, P.; Xiong, F.; Zhang, S.; Yu, Q.; Wang, C.-Y. The AHR Signaling Attenuates Autoimmune Responses During the Development of Type 1 Diabetes. Front. Immunol. 2020, 11.
- Proietti, E.; Rossini, S.; Grohmann, U.; Mondanelli, G. Polyamines and Kynurenines at the Intersection of Immune Modulation. Trends Immunol. 2020, 41, 1037–1050.
- Zelante, T.; Iannitti, R.G.; Fallarino, F.; Gargaro, M.; de Luca, A.; Moretti, S.; Bartoli, A.; Romani, L. Tryptophan Feeding of the IDO1-AhR Axis in Host-Microbial Symbiosis. Front. Immunol. 2014, 5.
- Qiu, J.; Heller, J.J.; Guo, X.; Chen, Z.-M.E.; Fish, K.; Fu, Y.-X.; Zhou, L. The Aryl Hydrocarbon Receptor Regulates Gut Immunity through Modulation of Innate Lymphoid Cells. Immunity 2012, 36, 92–104.
- Kiss, E.A.; Vonarbourg, C.; Kopfmann, S.; Hobeika, E.; Finke, D.; Esser, C.; Diefenbach, A. Natural Aryl Hydrocarbon Receptor Ligands Control Organogenesis of Intestinal Lymphoid Follicles. Science 2011, 334, 1561–1565.
- Li, Y.; Innocentin, S.; Withers, D.R.; Roberts, N.A.; Gallagher, A.R.; Grigorieva, E.F.; Wilhelm, C.; Veldhoen, M. Exogenous Stimuli Maintain Intraepithelial Lymphocytes via Aryl Hydrocarbon Receptor Activation. Cell 2011, 147, 629–640.
- Cheng, Y.; Jin, U.-H.; Allred, C.D.; Jayaraman, A.; Chapkin, R.S.; Safe, S. Aryl Hydrocarbon Receptor Activity of Tryptophan Metabolites in Young Adult Mouse Colonocytes. Drug Metab. Dispos. 2015, 43, 1536–1543.
- Monteleone, I.; Rizzo, A.; Sarra, M.; Sica, G.; Sileri, P.; Biancone, L.; Macdonald, T.T.; Pallone, F.; Monteleone, G. Aryl Hydrocarbon Receptor-Induced Signals Up-regulate IL-22 Production and Inhibit Inflammation in the Gastrointestinal Tract. Gastroenterology 2011, 141, 237–248.e1.
- Stockinger, B.; di Meglio, P.; Gialitakis, M.; Duarte, J.H. The Aryl Hydrocarbon Receptor: Multitasking in the Immune System. Annu. Rev. Immunol. 2014, 32, 403–432.




