
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Jacques Desbrieres | + 1275 word(s) | 1275 | 2021-04-27 10:33:10 | | | |
| 2 | Catherine Yang | Meta information modification | 1275 | 2021-04-30 05:10:09 | | |
Video Upload Options
A strong trend in dental applications is to apply nanotechnology and smart nanomaterials such as nanoclays, nanofibers, nanocomposites, nanobubbles, nanocapsules, solid-lipid nanoparticles, nanospheres, metallic nanoparticles, nanotubes, and nanocrystals.
1. Introduction
The evolution of dental materials has passed through several stages, being determined by the complexity of existing technologies at the time. For centuries, the scientific world has constantly sought to find materials that are able to provide properties considered essential for use in oral cavities. For this reason, the materials used in dentistry must meet several physical, chemical, and mechanical requirements (Figure 1), including the following: (1) They must be strong and fracture-resistant; (2) they should be permanently attached to the tooth or bone structure; (3) they should have dimensional stability when exposed to solvents or changes of temperature; (4) they should have a similar appearance to the tooth structure and possess properties similar to tooth enamel and dentin; (5) they should be aesthetic; (6) they should exhibit minimal conduction; (7) the density and abrasion resistance should be close or equal to that of the natural tooth; (8) they should have a sufficient elasticity, low fragility, and transparency; (9) they should be easy to manufacture and process; (10) they should adhere to tissues; (11) they should be tasteless and odorless; (12) they should be easily maintained or fixed; and (13) they should have a reasonable cost [1][2][3][4][5][6].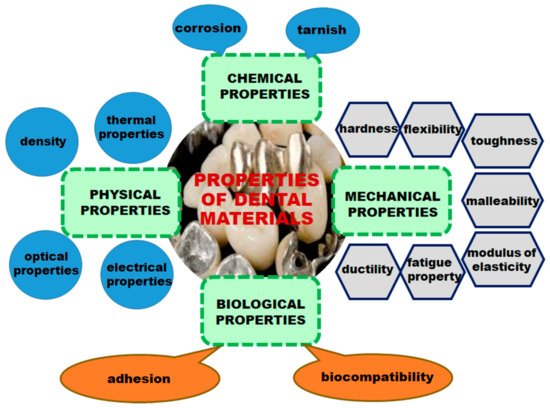 Figure 1. Properties of dental materials.When choosing a dental material, both mechanical and physical properties, as well as biological properties, must be considered. When a biomaterial is placed in contact with tissues and fluids of the human body, there are several types of interactions between the material and biological environment [7].
Figure 1. Properties of dental materials.When choosing a dental material, both mechanical and physical properties, as well as biological properties, must be considered. When a biomaterial is placed in contact with tissues and fluids of the human body, there are several types of interactions between the material and biological environment [7].
2. Smart Nanoparticles in Dentistry
The introduction of the term nanotechnology by Norio Taniguchi in 1974 [8] represented a huge opportunity for the development of new dental products that could be applied in restorative dentistry, periodontology, implant odontology, biomineralization, oral cancers, endodontics, and adhesive dentistry [9]. Based on their morphologies, nanomaterials with dental applications can be classified as follows: Nanocomposites; nanobubbles; nanocapsules; solid-lipid nanoparticles; nanospheres; metallic nanoparticles; nanotubes; nanocrystals; nanoclays; and nanofibers [10].The association between the two most frequently employed terms used in the last periods, consisting of “smart” and “nanoparticles”, led to smart nanoparticles, which have received much interest for their uses in the medical field and in particular, in dental applications, including dental implants, polishing of the enamel surface, the prevention of dental cariers, antisensitivity agents, and teeth whitening toothpaste.The benefits of nanoparticles used in dental applications can be highlighted by their properties:
- They ensure a large surface area [9][11];
- They can present antimicrobial, antiviral, and antifungal properties and as a consequence, can prevent biofilm formation when nanoparticles loaded with an antimicrobial agent are incorporated in resin composites [12];
- They enhance the mechanical properties of dental material, especially in restorative dentistry;
- They improve the bond strength between dentin and biomaterial;
- They prevent crack propagation and white spot lesions;
- They improve the fracture toughness of porcelain restorations [13].
Two main strategies for the preparation of nanomaterials are depicted: top-down and bottom-up methods. The difference between the techniques consists of the fact that the top-down approach is a destructive method that refers to a decrease in bulk materials to nano-scale particles (mechanical milling, nano-litography (photolitography, electron beam, ion beam, and X-ray litography), laser ablation, sputtering, and thermal decomposition), while the bottom-up approach is a constructive method that refers to the generation of material from atoms to clusters to nanomaterials (chemical vapor deposition, sol-gel method, self-assembly, spinning, pyrolysis, and biosynthesis) [6].The structural parts of the teeth are as follows:
- Enamel: The very hard, thin, and translucent layer that covers the surface of the dental crown;
- Dentin: A calcified tissue of the tooth situated inside the enamel and cementum;
- Cementum: Specialized calcified substance that is a part of the periodontium and covers the root of a tooth;
- Dental pulp: Unmineralized oral tissue situated in the center of the tooth that is composed of soft connective tissue, blood vessels, lymph vessels, and nervous elements.
2.1. Smart Nanoparticles as Drug Delivery Systems
Good oral hygiene is very important for human health because poor oral hygiene can lead to dental diseases, such as gum diseases, infection, bone loss, heart diseases, and strokes.Millions of bacterial cells are found in the oral environment. Some of them are beneficial, while others are very harmful, causing many oral diseases, such as the following:
- Bad breath (halitosis) is caused by gum diseases, dental caries, oral cancer, dry mouth, and bacteria on the tongue;
- Dental caries appear when acids, bacteria, and food form a plaque that covers the teeth [14];
- Gum diseases (gingivitis and periodontitis) represent an inflammation of the gums, as well as an infection of the tissues caused by the formation of a sticky, colorless plaque on teeth [15];
- Oral cancers, which include any malignant lesions on the gums, tongue, lips, cheek, floor of the mouth, and hard and soft palate [16];
- Mouth sores, which are inflammatory disorders characterized by small lesions that develop on the soft tissues in the mouth or at the base of the gums. The following disorders belong to this category: Canker sores (aphtous ulcers); fever blisters or cold sores caused by the Herpes simplex; oral thrush or candidiasis; angular cheilosis; fibrous inflammatory hyperplasia; and inflammatory papillary hyperplasia [17];
- Tooth erosion;
- Tooth sensitivity;
- Toothaches and dental emergencies.
As drug delivery systems, smart nanoparticles present superior properties, including a small size; a wider therapeutic window; the ability to overcome multiple drug resistance; multifunctionality; long circulation drugs; the ability to minimize off-target effects; a pH-triggered on/off switchable system; a superior efficacy; a decreased toxicity; improved pharmacokinetics; the feasibility of incorporation of both hydrophilic and hydrophobic active principles; controlled/sustained release; an enhanced permeability and retention; and the feasibility of variable routes of administration, including oral and parenteral administration and inhalation [18][19][20][21].
2.2. Smart Materials in Restorative Dentistry
Restorative dental materials are synthetic components used to repair or replace the tooth structure [22]. Dental restoration materials have an ancient history dating back to 600 B.C., when a paste made of silver, tin, and mercury was used for dental restoration in China, being considered the precursor of modern amalgams. A real advance in restorative dentistry was the synthesis of composite diacrylic resins, which revolutionized the working techniques and changed the treatment schemes [22]. Restorative materials can be used for short-time or long-time applications and can be classified as direct restorative materials and indirect restorative materials. Direct tooth restoration represents a simple method in which the bonding materials are placed, shaped, and hardened during a simple procedure. Direct restoration involves the filling of cavities using amalgam, composite resin, glass ionomer cement, resin-modified glass-ionomer cement, compomers, and cermets [23]. Indirect dental restorations require multiple and more complex procedures, including the fabrication of crowns, bridges, dental implants, inlays, onlays, and veneers [24].The ideal restoration materials must have the following properties: Plasticity—the material must have a plastic setting phase and a plastic working phase; a good chemical-volumetric stability in a sense that it must not expand or contract or dissolve in the mouth; a good bonding strength to the tooth; a low thermal conductivity, in order to stop heat transfer to the pulpal organ; resistance to attrition, abrasion, and chemical erosion; a color that must be identical or close to that of natural teeth; setting time = 3–5 min; a dental ablation process that must be performed as easily as possible; and ease in the handling, application, and finishing of restoration materials [25]. The use of nanotechnology in restorative dentistry presents several advantages, including an improved diametral strength, enhancement of the tensile strength, an increase in the flexural strength, a high fracture toughness, and an increase in the shear bond strength [26]. The nanomaterials used for restorative dentistry are dental nanocomposites, glass-ionomer cements, endodontic sealers, and tooth regeneration nanomaterials [27].
References
- Johnston, W.M.; Kao, E.C. Assessment of appearance by visual observation and clinical colorimetry. J. Dent. Res. 1989, 68, 819–822.
- Peralta, S.L.; de Leles, S.B.; Dutra, A.L.; Guimares, V.B.S.; Piva, E.; Lund, R.G. Evaluation of physical-mechanical properties, antibacterial effect and cytotoxicity of temporary restorative materials. J. Appl. Oral Sci. 2018, 26, e20170562.
- Peskeroy, C.; Culha, O. Comparative evolution of mechanical proprieties of dental nanomaterials. J. Nanomater. 2017, 6171578.
- Pecho, O.E.; Ghinea, R.; Navarro do Amaral, E.A.; Cardona, J.C.; Della Bona, A.; Perez, M.M. Relevant optical properties for direct restorative materials. Dent. Mater. 2016, 32, e105–e112.
- Pulgar, R.; Lucena, C.; Espinar, C.; Pecho, O.E.; Ruiz-Lopez, J.; Della Bona, A.; Perez, M.M. Optical and colorimetric evaluation of a multi-color polymer infiltrated ceramic-network material. Dent. Mater. 2019, 35, e131–e139.
- Anusavice, K.J. Phillip’s Science of Dental Materials, 11th ed.; Saunders Co., Elsevier Science Ltd.: Bethesda, MD, USA, 2003.
- Robertson, T.M.; Heyman, H.O.; Swift, J.E.J., Jr. Studervant’s Art and Science of Operative Dentistry, 4th ed.; Mosby, Elsevier Science Ltd.: St. Louis, MO, USA, 2002; pp. 16–36.
- Bayda, S.; Adeel, M.; Tuccinardi, T.; Corolani, M.; Rizzolio, F. The history of nanoscience and nanotechnology: From vhemical-physical applications to nanomedicine. Molecules 2020, 25, 112.
- Foong, L.K.; Foroughi, M.M.; Mirhosseini, A.F.; Safaei, M.; Jahani, S.; Mostafavi, M.; Ebrahimpoor, N.; Sharifi, M.; Varma, S.R.; Khatami, M. Applications of nano-materials in diverse dentistry regimes. RSC Adv. 2020, 10, 15430–15460.
- Pinon-Segundo, E.; Mendoza-Munoz, M.; Quintanar-Guerrero, D. Nanoparticles as dental drug-delivery systems. In Nanobiomaterials in Clinical Dentistry, 2nd ed.; Subramani, K., Ahmed, W., Hartsfield, J.K., Jr., Eds.; Elsevier Science Ltd.: Amsterdam, The Netherlands, 2019; Chapter 23; pp. 567–593.
- Peola, J.E. Nanodentistry: The benefits of nanotechnology in dentistry and its impact on oral health. J. Stud. Sci. Technol. 2017, 10, 45–50.
- Kasraei, S.; Sami, L.; Hendi, S.; Arkani, M.Y.; Rezaci-Soufi, L.; Khamverdi, Z. Antibacterial properties of composite resins incorporating silver and zinc oxide nanoparticles on Streptococcus mutans and Lactobacillus. Restor. Dent. Endod. 2014, 39, 109–114.
- Uno, M.; Kurachi, M.; Wakamatsu, N.; Doi, Y. Effects of adding silver nanoparticles on the toughening of dental porcelain. J. Prosthet. Dent. 2013, 109, 242–247.
- Hanning, M.; Hanning, C. Nanotechnology and its role in caries therapy. Adv. Dent. Res. 2012, 24, 53–57.
- Sindhura Reddy, N.; Sowmya, S.; Bumgardner, J.D.; Chennazlei, K.P.; Biswas, R.; Jayakumar, R. Tetracycline nanoparticles loaded calcium sulfate composite beads for periodontal management. Biochim. Biophys. Acta Gen. Subj. 2014, 1840, 2080–2090.
- Koopaie, M. Nanoparticulate systems for dental drug delivery. In Nanoengineered Biomaterials for Advanced Drug Delivery, 1st ed.; Mozafaei, M., Ed.; Elsevier Science Ltd.: Amsterdam, The Netherlands, 2020; pp. 525–559.
- Kupp, L.I.; Sheridan, P.J. Denture sore mouth. Dermatol. Clin. 2003, 21, 115–122.
- Kalaydina, R.V.; Bajwa, K.; Qorri, B.; Decarlo, A.; Szewczuk, M.R. Recent advances in “smart” delivery systems for extended drug release in cancer therapy. Int. J. Nanomed. 2018, 13, 4727–4745.
- Desai, P.; Thumma, N.J.; Wagh, P.R.; Zhan, S.; Ann, D.; Wang, J.; Prabhu, S. Cancer chemoprevention using nanotechnology-based approaches. Front. Pharmacol. 2020, 11, 323.
- Galperina, S.; Kisich, K.; Iseman, M.D.; Heifets, L. The potential advantages of nanoparticle drug delivery systems in chemotherapy of tuberculosis. Am. J. Respir. Crit. Care Med. 2005, 172, 1487–1490.
- Aleya, L.; Abdel-Daim, M.M. Nanoparticles and its biomedical applications in health and diseases: Special focus on drug delivery. Environ. Sci. Pollut. Res. 2020, 27, 19151–19168.
- Anusavice, K.J. Biocompatibility. In Phillip’s Science of Dental Materials, 12th ed.; Anusavice, K.J., Shen, C., Rawls, H.R., Eds.; Saunders Co., Elsevier Science Ltd.: Bethesda, MD, USA, 2013; Chapter 7; pp. 111–147.
- Azeem, R.A.; Sureshbabu, N.M. Clinical performance of direct versus indirect composite restorations in posterior teeth: A systematic review. J. Conserv. Dent. 2018, 21, 2–9.
- Antonelli da Veiga, A.M.; Carneiro Cunha, A.; Ferreira, D.M.T.P.; da Silva Fidalgo, T.K.; Chianca, T.K.; Rodrigues Reis, K.; Cople Maia, L. Longetivity of direct and indirect resin composite restorations in permanent posterior teeth: A systematic review and meta-analysis. J. Dent. 2016, 54, 1–12.
- Phillips, R.W.; Skinner, E.W. Skinner’s Science of Dental Materials, 9th ed.; Saunders Co., Elsevier Science Ltd.: Philadelphia, PA, USA, 1991; pp. 61–67.
- Malek, M.; Farzaneh, F.; Samani, Y.; Pachenari, F.; Pachenari, H. The applications of nanotechnology in restorative dentistry: A review study. Nanomed. J. 2019, 6, 241–249.
- Khurshid, Z.; Zafar, M.; Qasim, S.; Shahab, S.; Naseem, M.; AbuReqaiba, A. Advances in nanotechnologies for restorative dentistry. Materials 2015, 8, 717–731.




