
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Michele Manoni | + 1530 word(s) | 1530 | 2021-04-20 12:27:48 | | | |
| 2 | Lindsay Dong | -2 word(s) | 1528 | 2021-04-29 11:40:36 | | |
Video Upload Options
The milk fat fraction is a rich source of nutrients and bioactive factors. This fraction is composed of milk fat globules (MFGs) surrounded by the milk fat globule membrane (MFGM). In this review we revise the literature that deals two minor topics that characterize the milk fat fraction. The first topic is the MFGM proteome, which has several bioactive properties and shows similarities and variations among species and phases of lactation. The second topic is the content of essential nutrients among MFGs and MFGM, named minerals and lipophilic vitamins, in order to assess the nutrifunctional role of the milk fat fraction.
1. Introduction
Milk is nature's most complete food because it is an essential source of nutrients and bioactive compounds such as immunoglobulins, antimicrobial proteins, oligosaccharides, phospholipids, hormones, minerals and vitamins [1][2]. Milk is an oil-in-water emulsion, and milk lipids are organized as lipid droplets composed of a hydrophobic core surrounded by a hydrophilic membrane. These two components are called milk fat globule (MFG) core and milk fat globule membrane (MFGM), respectively [3][4][5]. Milk lipids exert many bioactive functions in newborns and acts as a “natural carrier” for the milk compounds that retain these bioactive properties [6]. These compounds are mainly phospholipids, sphingolipids and proteins of the MFGM, lipophilic vitamins, minerals and cholesterol.
In the last decades, research has focused on the characterization of MFGM and on the exploitation of its properties to design and produce value-added products. Alongside the progress in this area, the research has been less concerned both with the industrial exploitation of the MFGM proteome and with the comprehension of the content of minor components of the milk lipid fraction. In this review we discussed the similarities and differences regarding the composition of the MFGM proteome of various species, highlighting the properties of the human and bovine MFGM proteome. Then, we revised the existing literature about the content and the distribution of micronutrients in the milk lipid fraction of human and bovine milk. The aim of the review is to provide an overview of the minor constituents of the milk lipid fraction, discussing their bioactive properties and suggesting innovative attempts of implementing the findings present in the literature about these topics.
2. MFGM Proteome and Comparative Proteomics
The use of proteomics techniques enabled a better comprehension of the role of MFGM proteome in milk and allowed to identify a higher number of MFGM proteins across all species. In addition, comparative proteomics analyses were performed to elucidate the variations and the similarities of MFGM proteome among species and phases of lactation [7].
Human and cow MFGM proteomes show several similarities. In terms of quantity, human and cow proteomes share the highest number of MFGM proteins and the highest linear correlation coefficient (166 proteins, R = 0.71) if compared to the MFGM proteome of goat (95 proteins, R = 0.60) and yak (76 proteins, R = 0.62) [7]. These data are also reported in Figure 1.
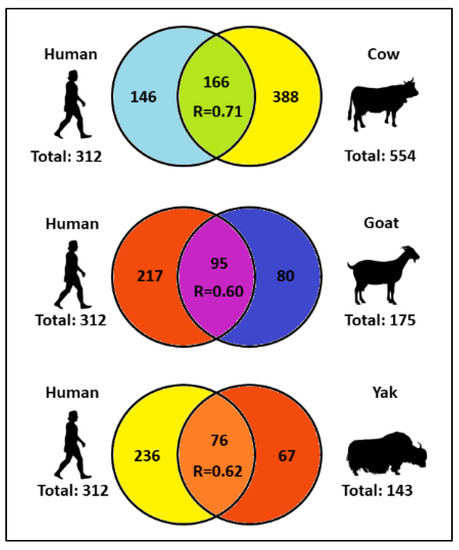
Figure 1. Number of individual and common milk fat globule membrane (MFGM) proteins in human, cow, goat and yak, and Pearson correlation coefficient (R) between the MFGM proteomes of human–cow, human–goat and human–yak. Adapted from [8].
In 2011, Hettinga and collaborators [9] observed slight variations in the number of proteins responsible for various molecular functions in human and cow MFGM proteomes. The authors observed that the number of MFGM proteins related to the immune defense was comparable between cows (44) and humans (51). The cow’s MFGM proteome was enriched in antibacterial proteins, named cathelicidins and mucins, related to innate immunity. Instead, the human MFGM proteome was enriched in four proteins involved in the mucosal immune system: IgA, CD14, lactoferrin and lysozyme [9].
Moreover, colostral MFGM proteome contains a higher number of proteins related to the immune defense than milk of later phases of lactation [10][11][12]. A recent study [12] identified a total of 411 MFGM proteins in human and bovine colostrum. Of the 26 differentially expressed proteins, 9 were upregulated and 17 downregulated in humans if compared to bovine colostrum. These 9 proteins, such as neutrophil defensin 1, protein S100, immunoglobulin K and lactadherin, were mostly involved in the innate and adaptive immune defense. The authors assessed that the MFGM proteome of human colostrum was more enriched in immune-related proteins than bovine colostrum [12].
All the results reported lead to two main conclusions: (i) the immune-related proteins are high in human colostral MFGM proteome and (ii) the molecular function and protein expression levels vary among species and different phases of lactation.
3. Milk Minerals and Vitamins
Milk and dairy products are crucial to the intake of minerals and vitamins. In fact, they provide 20–40% of total dietary intake of vitamins and 10–20% of total dietary intake of minerals in Western countries [13][14].
3.1 Minerals
The major minerals in milk are calcium, magnesium, phosphorus, sodium, zinc and potassium [13]. The various minerals are distributed in milk according to their chemical characteristics. The milk lipid fraction contains mainly iron, copper, zinc and calcium, even though some of these minerals are present in trace amounts (Table 1).
Table 1. Distribution of minerals in whole milk and in milk lipid fraction of human and cow’s milk. Data from Zamberlin et al. (2011) [13]. Mulder and Walstra (1974) [15] , Fransson and Lonnerdal (1983) [16], Soliman (2005) [17], Walton and Flynn (2013) [18], Chassaing et al. (2016) [19] and Pietrzak-Fie´cko and Kamelska-Sadowska (2020) [20].
| Minerals | Whole milk | Milk lipid fraction | ||
| Human | Cow | Human | Cow | |
| Calcium (mg/100 g) | 22–41 | 107–133 | 3.5–6.6 (16%) | 0.2–0.4 (1%) |
| Iron (μg/100 g) | 40–50 | 30-70 | <20 (40%) | <1 (14%) |
| Copper (μg/100 g) | 30–50 | 12-17 | <8 (15%) | <0.3 (2%) |
| Zinc (μg/100 g) | 145–165 | 350-400 | <30 (18%) | <4 (1%) |
| Magnesium (mg/100 g) | 3–3.5 | 9-16 | 0.06–0.07 (2%) | - |
| Phosphorus (mg/100 g) | 12–17 | 90-102 | - | - |
It is important to underline that milk usually undergoes several industrial processes before consumption. For what concerns minerals, their content is unaffected by heating because minerals are both very heat-stable, and in milk they are usually bound with other components, such as enzymes [21].
Since this fraction is rich in bioactive polar lipids and proteins, and it also contains certain amounts of minerals, the inclusion into value-added products could be beneficial.
3.2 Vitamins
Milk contains both hydrophilic and lipophilic vitamins. Hydrophilic vitamins are vitamins C and those belonging to the B family, whereas lipophilic vitamins are A, D, E and K. According to their chemical features, they are distributed either in the aqueous phase or in the milk lipid fraction [2][22]. The milk lipid fraction is a major source of lipophilic vitamins (Figure 2).
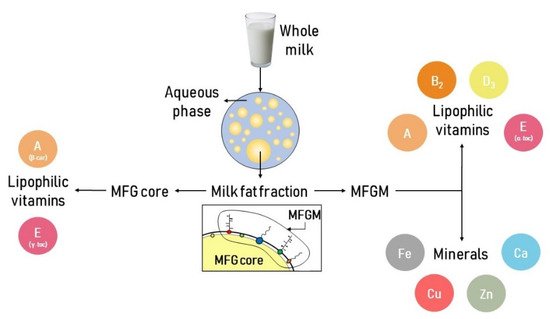
Figure 2. Schematic representation of the major minerals and lipophilic vitamins present in the milk lipid fraction.
The main lipophilic vitamins in milk are present either in the MFGM or in the MFG core. Vitamin A, vitamin D3, α-tocopherol and riboflavin are mainly located in the MFGM, whereas β-carotene and γ-tocopherol are mainly located in the MFG core (Figure 2). Table 2 reports the content of lipophilic vitamins in human and cow milk lipid fractions.
Table 5. Content of lipophilic vitamins in human and cow milk. Data from Baldi and Pinotti (2008) [6], Öste et al. (1997) [23], Park (2007) [24], Fantuz et al. (2016) [25] and Biadala and Konieczny (2018) [26].
| Vitamins | Human Milk | Cow’s Milk |
| Vitamin A + all-trans- -carotene (mg/100 g) | 0.05–0.06 | 0.04 |
| Vitamin D (μg/100 g) | 0.04–0.07 | 0.05–0.06 |
| Vitamin E (mg/100 g) | 0.24–0.28 | 0.10–0.13 |
| Vitamin K (μg/100 g) | 0.3–0.5 | 1.1 |
Regarding the effect of heating on the milk vitamin content, there is a distinction between hydrophilic and lipophilic vitamins. The levels of the hydrophilic vitamins B1, B12 and C decrease by 10–20% after pasteurization. Instead, vitamin B2 is unaffected, and this is valid also for the lipophilic vitamins. Indeed, vitamins A, D, E and -carotene appear to suffer no loss after pasteurization or microwave heating [21][27].
4. Prospective Opportunities for the Future
The well-established higher content of immune-related proteins in MFGM of colostrum across all species could be used to enhance the immune functions of specific subjects by potentially decreasing the number of infective episodes in newborns [28]. Moreover, the human MFGM proteome is enriched in proteins capable of protecting the integrity of the mucosal immune system, thus decreasing the risk of pathogen adhesion and infection [9]. The industrial exploitation of this knowledge could lead to the design of glycoprotein-rich MFGM mixtures that exert an antiadhesive effect at the intestinal mucosal level [29] or an antiviral effect, for example against rotavirus infection [30].
Recently, the isolation and separation procedures of MFGM moved forward [31][32], increasing the possibility to isolate and separate specific bioactive lipid or protein components. The human milk lipid fraction has a higher content of minerals if compared to cow’s milk. This suggests that the human milk lipid fraction is more effective as being a “natural solvent” for micronutrients, especially for minerals. This feature could be crucial to face new necessities, such as deficient conditions regarding the intake of certain minerals or vitamins. The innovative element of this application of the milk lipid fraction lies in the fact that the bioactive role of micronutrients would be associated with the bioactive role of MFGM phospholipids and the MFGM proteome. The affinity between MFMG and vitamins can be exploited to design and produce stable delivery systems for molecules.
5. Conclusions
The complex architecture of MFGs ensures a stable dispersion for the bioactive compounds of milk. The MFGM proteome varies among species but retains its antimicrobial and antiadhesive properties. Further, the MFGM proteome can act as a scaffold for many of the micronutrients such as minerals and lipophilic vitamins, which can indirectly join the
milk lipid fraction. The presence of minerals and lipophilic vitamins enhances the bioactive role of the milk lipid fraction.
References
- Young W. Park; Overview of Bioactive Components in Milk and Dairy Products. Bioactive Components in Milk and Dairy Products 2009, 1, 3-14, 10.1002/9780813821504.ch1.
- Frédéric Gaucheron; Milk and Dairy Products: A Unique Micronutrient Combination. Journal of the American College of Nutrition 2011, 30, 400S-409S, 10.1080/07315724.2011.10719983.
- V.L. Spitsberg; Invited Review: Bovine Milk Fat Globule Membrane as a Potential Nutraceutical. Journal of Dairy Science 2005, 88, 2289-2294, 10.3168/jds.s0022-0302(05)72906-4.
- E. Arranz; M. Corredig; Invited review: Milk phospholipid vesicles, their colloidal properties, and potential as delivery vehicles for bioactive molecules. Journal of Dairy Science 2017, 100, 4213-4222, 10.3168/jds.2016-12236.
- Laurence Bernard; Muriel Bonnet; Carole Delavaud; Mylène Delosière; Anne Ferlay; Hélène Fougère; Benoît Graulet; Milk Fat Globule in Ruminant: Major and Minor Compounds, Nutritional Regulation and Differences Among Species. European Journal of Lipid Science and Technology 2018, 120, 1700039, 10.1002/ejlt.201700039.
- Antonella Baldi; Luciano Pinotti; Lipophilic Microconstituents of Milk. Chemistry and Biology of Pteridines and Folates 2007, 606, 109-125, 10.1007/978-0-387-74087-4_3.
- Maria Cavaletto; Maria G Giuffrida; Amedeo Conti; The proteomic approach to analysis of human milk fat globule membrane. Clinica Chimica Acta 2004, 347, 41-48, 10.1016/j.cccn.2004.04.026.
- Jing Lu; Xinyu Wang; Weiqing Zhang; Lu Liu; Xiaoyang Pang; Shuwen Zhang; Jiaping Lv; Comparative proteomics of milk fat globule membrane in different species reveals variations in lactation and nutrition. Food Chemistry 2016, 196, 665-672, 10.1016/j.foodchem.2015.10.005.
- Kasper Hettinga; Hein Van Valenberg; Sacco De Vries; Sjef Boeren; Toon Van Hooijdonk; Johan Van Arendonk; Jacques Vervoort; The Host Defense Proteome of Human and Bovine Milk. PLOS ONE 2011, 6, e19433, 10.1371/journal.pone.0019433.
- Mei Yang; Wei Deng; Xueyan Cao; Lijie Wang; Na Yu; Yan Zheng; Junrui Wu; Rina Wu; Xiqing Yue; Quantitative Phosphoproteomics of Milk Fat Globule Membrane in Human Colostrum and Mature Milk: New Insights into Changes in Protein Phosphorylation during Lactation. Journal of Agricultural and Food Chemistry 2020, 68, 4546-4556, 10.1021/acs.jafc.9b06850.
- Mallory C. Honan; Megan J. Fahey; Amanda J. Fischer-Tlustos; Michael A. Steele; Sabrina L. Greenwood; Shifts in the Holstein dairy cow milk fat globule membrane proteome that occur during the first week of lactation are affected by parity. Journal of Animal Science and Biotechnology 2020, 11, 1-15, 10.1186/s40104-020-00478-7.
- Mei Yang; Xiuming Peng; Junrui Wu; Ri-Na Wu; Biao Liu; Wenhui Ye; Xin Xu; Xiqing Yue; Differential proteomic analysis of milk fat globule membrane proteins in human and bovine colostrum by iTRAQ-coupled LC-MS/MS. European Food Research and Technology 2016, 243, 901-912, 10.1007/s00217-016-2798-6.
- Zamberlin, Š.; Antunac, N.; Havranek, J.; Samaržija, D.; Mineral elements in milk and dairy products. Mljekarstvo 2011, 62, 111-125.
- Alida Melse-Boonstra; Bioavailability of Micronutrients From Nutrient-Dense Whole Foods: Zooming in on Dairy, Vegetables, and Fruits. Frontiers in Nutrition 2020, 7, 101, 10.3389/fnut.2020.00101.
- G. Mieth; H. Mulder und P. Walstra: The Milk Fat Globule. Emulsion science as applied to milk products and comparable foods. 8 Bildtafeln, 103 Abb. und 40 Tab. Commonwealth Agricultural Bureaux Farnham Royal, Bucks., England, und Centre for Agricultural Publishing and Documentation Wageningen, Niederlande 1974. Preis: 7 £. Food / Nahrung 1975, 19, 380-381, 10.1002/food.19750190414.
- Gun-Britt Fransson; Bo Lönnerdal; Distribution of Trace Elements and Minerals in Human and Cow's Milk. Pediatric Research 1983, 17, 912-915, 10.1203/00006450-198311000-00015.
- Soliman, Z.A.; Comparison of chemical and mineral content of milk from human, cow, buffalo, camel and goat in Egypt. Egypt J. Hosp. Med 2005, 21, 116-130.
- Walton, J.; Flynn, A.; Nutritional adequacy of diets containing growing up milks or unfortified cow’s milk in Irish children (aged 12–24 months).. Food Nutr. Res. 2013, 57, 21836.
- Chantal Chassaing; Cécile Sibra; Jože Verbič; Odd Magne Harstad; Jaroslav Golecký; Bruno Martin; Anne Ferlay; Isabelle Constant; Carole Delavaud; Catherine Hurtaud; et al.Vida Žnidaršič PongracClaire Agabriel Mineral, vitamin A and fat composition of bulk milk related to European production conditions throughout the year. Dairy Science & Technology 2016, 96, 715-733, 10.1007/s13594-016-0300-7.
- Renata Pietrzak-Fiećko; Anna M. Kamelska-Sadowska; The Comparison of Nutritional Value of Human Milk with Other Mammals’ Milk. Nutrients 2020, 12, 1404, 10.3390/nu12051404.
- Assefa Bezie; The Effect of Different Heat Treatment on the Nutritional Value of Milk and Milk Products and Shelf-Life of Milk Products. A Review. Journal of Dairy & Veterinary Sciences 2019, 11, 555822, 10.19080/jdvs.2019.11.555822.
- Hanna Górska-Warsewicz; Krystyna Rejman; Wacław Laskowski; Maksymilian Czeczotko; Milk and Dairy Products and Their Nutritional Contribution to the Average Polish Diet. Nutrients 2019, 11, 1771, 10.3390/nu11081771.
- Öste, R.; Jägerstad, M.; Andersson, I.. Vitamins in Milk and Milk Products ; Fox, P.F., Eds.; Springer: Boston, MA, USA, 1997; pp. 347–402.
- Y. W. Park; Impact of goat milk and milk products on human nutrition.. CAB Reviews: Perspectives in Agriculture, Veterinary Science, Nutrition and Natural Resources 2007, 2, 1-19, 10.1079/pavsnnr20072081.
- F. Fantuz; E. Salimei; P. Papademas; Macro- and Micronutrients in Non-cow Milk and Products and Their Impact on Human Health. Non-Bovine Milk and Milk Products 2016, 1, 209-261, 10.1016/b978-0-12-803361-6.00009-0.
- Agata Biadała; Goat’s milk-derived bioactive components - a review. Mljekarstvo 2018, 68, 239-253, 10.15567/mljekarstvo.2018.0401.
- Robert Sieber; Pius Eberhard; Doris Fuchs; Peter Urs Gallmann; Walter Strahm; Effect of microwave heating on vitamins A, E, B1, B2 and B6 in milk. Journal of Dairy Research 1996, 63, 169-172, 10.1017/s0022029900031642.
- M. Zahar; D.E. Smith; F. Martín; Vitamin A Distribution Among Fat Globule Core, Fat Globule Membrane, and Serum Fraction in Milk. Journal of Dairy Science 1995, 78, 498-505, 10.3168/jds.s0022-0302(95)76660-7.
- Jerry A. Peterson; Stuart Patton; Margit Hamosh; Glycoproteins of the Human Milk Fat Globule in the Protection of the Breast-Fed Infant against Infections. Neonatology 1998, 74, 143-162, 10.1159/000014020.
- K.L. Fuller; T.B. Kuhlenschmidt; M.S. Kuhlenschmidt; R. Jiménez-Flores; S.M. Donovan; Milk fat globule membrane isolated from buttermilk or whey cream and their lipid components inhibit infectivity of rotavirus in vitro. Journal of Dairy Science 2013, 96, 3488-3497, 10.3168/jds.2012-6122.
- Wolfgang Holzmüller; Ulrich Kulozik; Isolation of milk fat globule membrane (MFGM) material by coagulation and diafiltration of buttermilk. International Dairy Journal 2016, 63, 88-91, 10.1016/j.idairyj.2016.08.002.
- Vitaly L. Spitsberg; Liza Ivanov; Vladimir Shritz; Recovery of milk fat globule membrane (MFGM) from buttermilk: effect of Ca-binding salts. Journal of Dairy Research 2019, 86, 374-376, 10.1017/s002202991900061x.





