
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Marta Ruíz-Bermejo | + 1421 word(s) | 1421 | 2021-04-20 10:44:46 | | | |
| 2 | Vicky Zhou | Meta information modification | 1421 | 2021-04-29 11:02:29 | | |
Video Upload Options
HCN-derived polymers are a heterogeneous group of complex substances synthesized from pure HCN; from its salts; from its oligomers, specifically its trimer and tetramer, aminomalononitrile (AMN) and diaminomaleonitrile (DAMN), respectively; or from its hydrolysis products, such as formamide, under a wide range of experimental conditions. The characteristics and properties of HCN-derived polymers depend directly on the synthetic conditions used for their production and, by extension, their potential applications. These puzzling systems have been known mainly in the fields of prebiotic chemistry and in studies on the origins of life and astrobiology since the first prebiotic production of adenine by Oró in the early years of the 1960s. However, the first reference regarding their possible role in prebiotic chemistry was mentioned in the 19th century by Pflüger. Currently, HCN-derived polymers are considered keys in the formation of the first and primeval protometabolic and informational systems, and they may be among the most readily formed organic macromolecules in the solar system. In addition, HCN-derived polymers have attracted a growing interest in materials science due to their potential biomedical applications as coatings and adhesives; they have also been proposed as valuable models for multifunctional materials with emergent properties such as semiconductivity, ferroelectricity, catalysis and photocatalysis, and heterogeneous organo-synthesis. However, the real structures and the formation pathways of these fascinating substances have not yet been fully elucidated. Several models based on either computational approaches or spectroscopic and analytical techniques have endeavored to shed light on their complete nature.
1. Introduction
HCN-derived polymers, commonly simply called HCN polymers, comprise a heterogeneous family of complex organic substances synthesized from pure HCN or soluble cyanide salts (e.g., NaCN, KCN or NH4CN); from the oligomers, trimers and tetramers of HCN, including aminomalononitrile (AMN) and diaminomaleonitrile (DAMN); or from the hydrolysis products of HCN such as formamide, under a wide range of experimental conditions. They are heterogeneous solids ranging in color from yellow or orange to brown or black depending on the degree of polymerization and/or cross-linking. A brief summary of their syntheses is shown in Scheme 1.
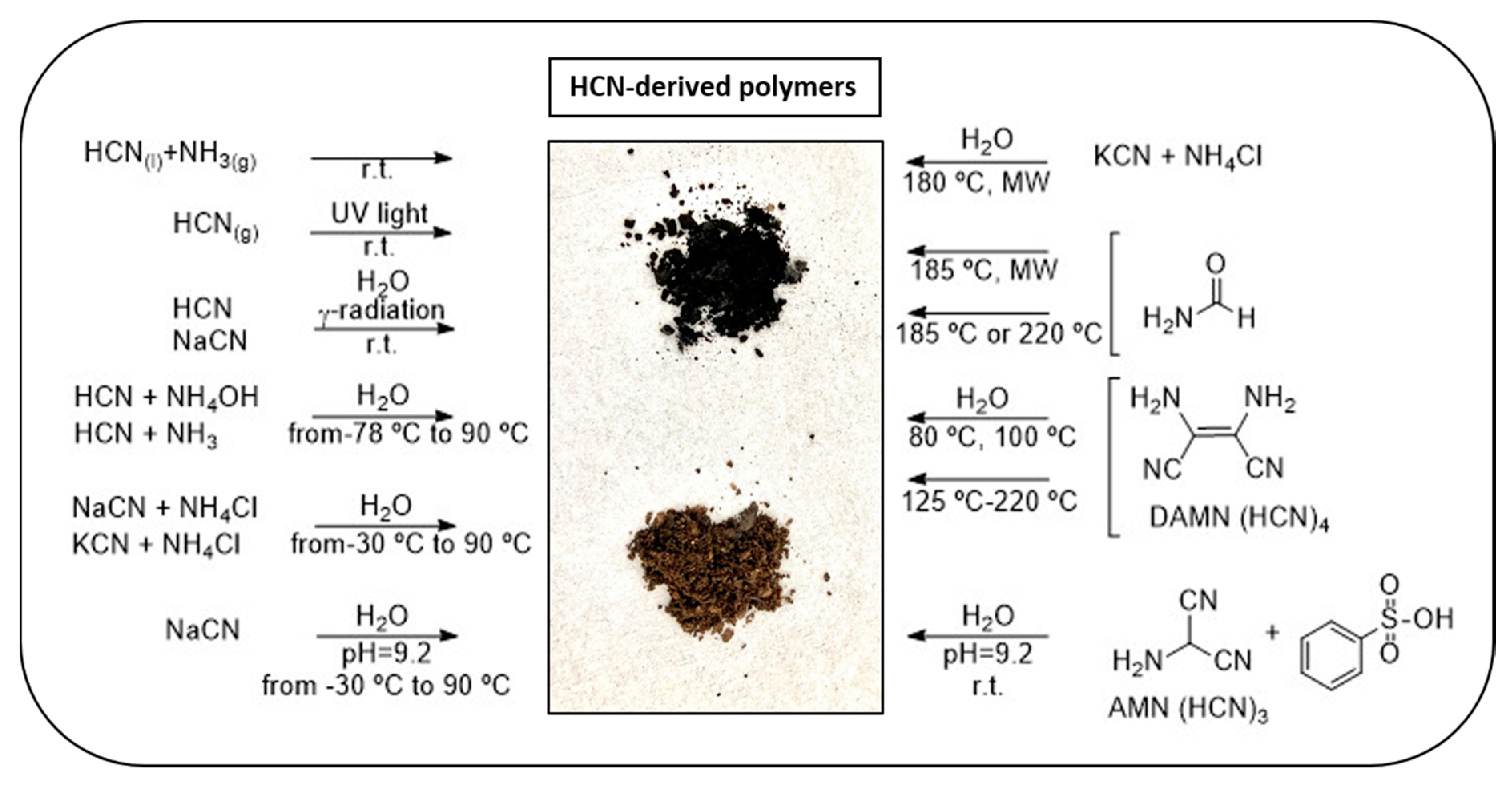
Scheme 1. Summary of the main routes for the production of HCN-derived polymers.
The HCN is a ubiquitous molecule in the Universe [1][2]. It has been detected in interstellar clouds, star-forming regions, planetary nebulae, interplanetary dust, comets [3], meteorites and atmospheres of satellites and planets such as Titan and Pluto [4][5][6]. In a terrestrial context, HCN can be identified in volcanic eruptions [7] and hydrothermal vents [8][9] and may have been relatively abundant in the atmosphere of the early Earth [10][11]. In addition, HCN polymers are considered the oldest organic substances of the solar system [12][13]. On the other hand, the synthetic story of HCN polymers began long ago. In the lab, HCN was prepared for the first time by Scheele in the second part of the 18th century by heating blood with KOH and charcoal. He obtained a mixture that he called “Blutlage”, which he distilled with sulfuric acid [14]. A few years later, Proust observed the oligomerization of HCN in the early 19th century [15]. In the last part of that century, Wipperman reported his research on the conversion of aqueous HCN into its trimer (amino-malonic acid dinitrile), which was subsequently hydrolyzed and decarboxylated to produce glycine [16], and Pflüger published one of the earliest chemical speculations concerning the origin of “living proteins” from cyano compounds [17].
However, the aqueous chemistry of HCN only achieved its current importance in studies about the origin of life from the first prebiotic synthesis of adenine by Oró [18]. Since then, the reaction of HCN polymerization has generally been considered the preferential prebiotic route for the synthesis of purines and pyrimidine derivatives. Thus, it has been suggested that HCN polymers may be important substances in the first stages of the chemical evolution of life. Indeed, currently, HCN chemistry is considered key in new proposals regarding scenarios and hypotheses related to the first stages of increasing molecular complexity that led to the rise of life. In addition, beyond the interest in HCN polymers in the fields of astrochemistry, prebiotic chemistry and astrobiology, these fascinating substances are inspiring new materials and present interesting properties that can lead to promising applications. Despite their being of great interest in various fields of knowledge, the relationships between the structures and properties of HCN-derived polymers are not sufficiently clear due to a lack of full characterization and because their properties are highly sensitive to experimental synthesis conditions. By extension, their corresponding pathways of formation are also unclear, although several structural models and hypothetical pathways have been proposed that consider both experimental data and computational analyses.
2. Promising Applications in Materials Science
There is a well-established research line driving the role of HCN in chemical evolution processes from inorganic chemistry to primitive biology. Beyond this prebiotic approach to HCN chemistry, HCN polymers have recently received growing attention in the fields of materials and surface science towards the development of multifunctional systems [19] (Figure 1).
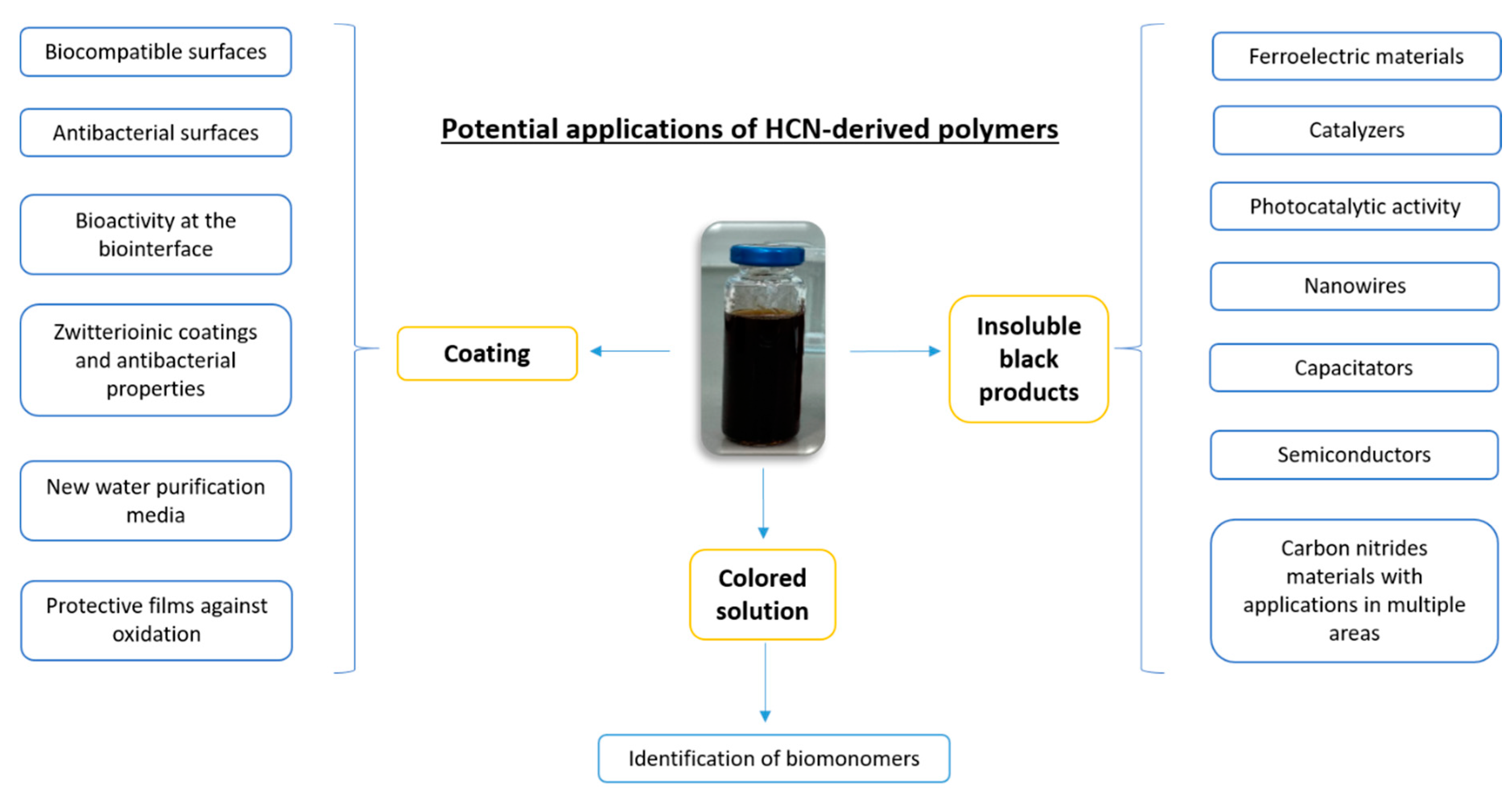
Figure 1. Current stage of the potential applications of HCN-derived polymers. The properties of HCN-derived polymers are directly dependent on the synthetic experimental conditions as well as on the chosen phase from the whole polymerization.
During the aqueous polymerization of HCN, cyanide, AMN or DAMN can often be observed in three phases from the reaction medium: (i) a colored solution from yellow to dark brown; (ii) an insoluble black residue that can be collected by filtration or centrifugation; and (iii) a film deposited on the reaction vessel wall. Traditionally, in the field of prebiotic chemistry, the main focus has been the study of solutions for the identification of biomonomers and related compounds by chromatography and spectroscopy techniques. The insoluble black residues have been analysed under this point of view due to the likely similar nature of the HCN polymers and Titan’s tholins. In this line, the polymorphism and electronic structure of polyimines have been investigated for their potential impacts on the understanding of the prebiotic chemistry of Titan [20] since He et al. showed that 75% of an HCN-based polymer formed in laboratory experiments consisted of a polyimine [21]. Due to the characteristics found for this polyimine, consisting formally of hydrogen isocyanide units, it was proposed as a valuable model for functional material design, such as for ferroelectric materials, nanowires, semiconductors and catalysts [20]. Moreover, nanofibers of poly-hydrogen cyanide synthesized from the heating of formamide present photocatalytic activities [22], and some DAMN polymers might be used as capacitators [23]. On the other hand, NH4CN and the DAMN polymers described recently present structural features very similar to those of extensively studied carbon nitrides [24][23][25], which are known to be used as materials with applications in multiple areas [26].
Finally, the films were properly studied for their potential biomedical applications. The most extensively investigated films are the AMN-based film coatings [27]. AMN spontaneously polymerizes yielding coatings similar to polydopamine coatings, providing biocompatible surfaces [28]. Recent studies have demonstrated that water solutions of AMN produce adhesive coatings that can be deposited on a wide range of substrates and provide excellent cell attachment as well as the ability to bind metals for the generation of antibacterial surfaces [29][30]. The copolymerization of AMN with 3,4-di- and 3,4,5-trihydroxybenzaldehyde leads to the production of bone-contacting medical devices with excellent bioactivity at the bio-interfaces [31]. The copolymerization of AMN with sulfo-betaine methacrylate and 2-aminoethyl methacrylate via free radicals forms zwitterionic coatings, which reduce biofouling and foreign body responses [32] and also have antibacterial properties [33]. In this line of antimicrobial activity, zeolite substrates were coated with copolymers based on AMN and 3,4,5-trihydroxybenzaldehyde for the passive ultrafiltration of stormwater/greywater, providing new water purification media [34]. Moreover, the antioxidant activities of films obtained based on AMN polymers have been recently reported [35].
3. Conclusions and Outlooks
HCN chemistry has been comprehensively revised considering its traditional high interest for several fields, such as prebiotic chemistry, astrobiology and cosmochemistry, as well as taking into account recent possibilities for the design and development of new and advanced functional materials. It has been clearly shown that the synthetic conditions have a significant influence on the final properties of HCN-derived polymers. Nevertheless, we are still far from a fundamental understanding of the underlying structure-property relationships of this singular and complex system, which might form the basis for a “molecular engineering” approach to electronic, optoelectronic, and photonic polymers. This is due to their intractability and insolubility in organic solvents, which impede the appropriate characterization of their molecular structure and physical properties as well as exploration of their applications. These conclusive facts open a broad area of research into new insights regarding either chemical evolution or materials and surface science.
References
- Bacchus-Montabonel, M.-C. Theoretical Investigation of Proton Collisions on Prebiotic Candidates: Hydrogen Cyanide Polymers. Phys. Chem. Chem. Phys. 2017, 19, 19566–19572.
- Rannou, P.; West, R. Supersaturation on Pluto and Elsewhere. Icarus 2018, 312, 36–44.
- Hänni, N.; Altwegg, K.; Pestoni, B.; Rubin, M.; Schroeder, I.; Schuhmann, M.; Wampfler, S. First in Situ Detection of the CN Radical in Comets and Evidence for a Distributed Source. Mon. Not. R. Astron. Soc. 2020, 498, 2239–2248.
- Mandt, K.; Luspay-Kuti, A.; Hamel, M.; Jessup, K.-L.; Hue, V.; Kammer, J.; Filwett, R. Photochemistry on Pluto: Part II HCN and Nitrogen Isotope Fractionation. Mon. Not. R. Astron. Soc. 2017, 472, 118–128.
- Wirström, E.S.; Charnley, S.B. Revised Models of Interstellar Nitrogen Isotopic Fractionation. Mon. Not. R. Astron. Soc. 2018, 474, 3720–3726.
- Pearce, B.K.D.; Molaverdikhani, K.; Pudritz, R.E.; Henning, T.; Hébrard, E. HCN Production in Titan’s Atmosphere: Coupling Quantum Chemistry and Disequilibrium Atmospheric Modeling. Astrophys. J. 2020, 901, 110.
- Hill, R.D. An Efficient Lightning Energy Source on the Early Earth. Orig. Life Evol. Biosph. 1992, 22, 277–285.
- Holm, N.G.; Neubeck, A. Reduction of Nitrogen Compounds in Oceanic Basement and Its Implications for HCN Formation and Abiotic Organic Synthesis. Geochem. Trans. 2009, 10, 9.
- Huber, C.; Kraus, F.; Hanzlik, M.; Eisenreich, W.; Wächtershäuser, G. Elements of Metabolic Evolution. Chem. Eur. J. 2012, 18, 2063–2080.
- Ferus, M.; Kubelík, P.; Knížek, A.; Pastorek, A.; Sutherland, J.; Civiš, S. High Energy Radical Chemistry Formation of HCN-Rich Atmospheres on Early Earth. Sci. Rep. 2017, 7, 6275.
- Airapetian, V.S.; Glocer, A.; Gronoff, G.; Hébrard, E.; Danchi, W. Prebiotic Chemistry and Atmospheric Warming of Early Earth by an Active Young Sun. Nat. Geosci. 2016, 9, 452–455.
- Matthews, C.N. Dark Matter in the Solar System: Hydrogen Cyanide Polymers. Orig. Life Evol. Biosph. 1991, 21, 421–434.
- Matthews, C.N. Hydrogen Cyanide Polymers: From Laboratory to Space. Planet. Space Sci. 1995, 43, 1365–1370.
- Bauer, H. Die Ersten Organisch-Chemischen Synthesen. Naturwissenschaften 1980, 67, 1–6.
- Proust, J.L. Gehlen’s J. Chem. Physik, 3, 384; 1806. Ann. Chim. Physique 1807, 60, 233.
- Wippermann, R. Ueber Tricyanwasserstoff, Eine Der Blausäure Polymere Verbindung. Eur. J. Inorg. Chem. 1874, 7, 767–772.
- Pflüger, E. Nachtrag zu meinem Aufsatz: Ueber die physiologische Verbrennung in den lebendigen Organismen. Pflüger Arch. 1875, 10, 641–644.
- Oró, J. Synthesis of Adenine from Ammonium Cyanide. Biochem. Biophys. Res. Commun. 1960, 2, 407–412.
- d’Ischia, M.; Manini, P.; Moracci, M.; Saladino, R.; Ball, V.; Thissen, H.; Evans, R.A.; Puzzarini, C.; Barone, V. Astrochemistry and Astrobiology: Materials Science in Wonderland? Int. J. Mol. Sci. 2019, 20, 4079.
- Rahm, M.; Lunine, J.I.; Usher, D.A.; Shalloway, D. Polymorphism and Electronic Structure of Polyimine and Its Potential Significance for Prebiotic Chemistry on Titan. Proc. Natl. Acad. Sci. USA 2016, 113, 8121–8126.
- He, C.; Lin, G.; Upton, K.T.; Imanaka, H.; Smith, M.A. Structural Investigation of HCN Polymer Isotopomers by Solution-State Multidimensional NMR. J. Phys. Chem. A 2012, 116, 4751–4759.
- Zhou, X.; Fang, Y.; Su, Y.; Ge, C.; Jin, B.; Li, Z.; Wu, S. Preparation and Characterization of Poly-Hydrogen Cyanide Nanofibers with High Visible Light Photocatalytic Activity. Catal. Commun. 2014, 46, 197–200.
- Ruiz-Bermejo, M.; de la Fuente, J.L.; Carretero-González, J.; García-Fernández, L.; Aguilar, M.R. A Comparative Study on HCN Polymers Synthesized by Polymerization of NH4CN or Diaminomaleonitrile in Aqueous Media: New Perspectives for Prebiotic Chemistry and Materials Science. Chem. Eur. J. 2019, 25, 11437–11455.
- Hortal, L.; Pérez-Fernández, C.; de la Fuente, J.L.; Valles, P.; Mateo-Martí, E.; Ruiz-Bermejo, M. A Dual Perspective on the Microwave-Assisted Synthesis of HCN Polymers towards the Chemical Evolution and Design of Functional Materials. Sci. Rep. 2020, 10, 22350.
- Mas, I.; de la Fuente, J.L.; Ruiz-Bermejo, M. Temperature Effect on Aqueous NH4CN Polymerization: Relationship between Kinetic Behaviour and Structural Properties. Eur. Polym. J. 2020, 132, 109719.
- Inagaki, M.; Tsumura, T.; Kinumoto, T.; Toyoda, M. Graphitic Carbon Nitrides (g-C3N4) with Comparative Discussion to Carbon Materials. Carbon 2019, 141, 580–607.
- Thissen, H.; Evans, R.A.; Ball, V. Films and Materials Derived from Aminomalononitrile. Processes 2021, 9, 82.
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-Inspired Surface Chemistry for Multifunctional Coatings. Science 2007, 318, 426–430.
- Thissen, H.; Koegler, A.; Salwiczek, M.; Easton, C.D.; Qu, Y.; Lithgow, T.; Evans, R.A. Prebiotic-Chemistry Inspired Polymer Coatings for Biomedical and Material Science Applications. NPG Asia Mater. 2015, 7, e225.
- Ball, V.; Toh, R.J.; Voelcker, N.H.; Thissen, H.; Evans, R.A. Electrochemical Deposition of Aminomalonitrile Based Films. Colloids Surf. A Physicochem. Eng. Asp. 2018, 552, 124–129.
- Menzies, D.J.; Ang, A.; Thissen, H.; Evans, R.A. Adhesive Prebiotic Chemistry Inspired Coatings for Bone Contacting Applications. ACS Biomater. Sci. Eng. 2017, 3, 793–806.
- Chen, W.-H.; Liao, T.-Y.; Thissen, H.; Tsai, W.-B. One-Step Aminomalononitrile-Based Coatings Containing Zwitterionic Copolymers for the Reduction of Biofouling and the Foreign Body Response. ACS Biomater. Sci. Eng. 2019, 5, 6454–6462.
- Liao, T.-Y.; Easton, C.D.; Thissen, H.; Tsai, W.-B. Aminomalononitrile-Assisted Multifunctional Antibacterial Coatings. ACS Biomater. Sci. Eng. 2020, 6, 3349–3360.
- Jung, J.; Menzies, D.J.; Thissen, H.; Easton, C.D.; Evans, R.A.; Henry, R.; Deletic, A.; McCarthy, D.T. New Prebiotic Chemistry Inspired Filter Media for Stormwater/Greywater Disinfection. J. Hazard. Mater. 2019, 378, 120749.
- Ball, V. Antioxidant Activity of Films Inspired by Prebiotic Chemistry. Mater. Lett. 2021, 285, 129050.





