
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Faheem Sheikh | + 2241 word(s) | 2241 | 2021-04-28 06:10:21 | | | |
| 2 | Rita Xu | Meta information modification | 2241 | 2021-04-29 06:16:07 | | |
Video Upload Options
Nanofibers are considered versatile materials with remarkable potential in tissue engineering and regeneration. In addition to their extracellular matrix-mimicking properties, nanofibers can be functionalized with specific moieties (e.g., antimicrobial nanoparticles, ceramics, bioactive proteins, etc.) to improve their overall performance.
1. Introduction
Nanofibers can be of synthetic or natural origin, which are preferably fabricated through a technique termed electrospinning. Electrospinning affords us 1D nanomaterials in the form of micro and nanofibers. Nanofibers have attained a premier place in the field of tissue engineering and are the main focus of current regenerative medicine owing to their remarkable extracellular matrix-mimicking functional properties [1][2][3]. Nanofibers can be functionalized with additional moieties to impart specific properties in addition to their inherent tissue regenerative properties [4][5][6]. Nanofibers have been studied and successfully evaluated for different uses because of their textured microstructure, which imparts high surface area. The unique structural features and the increased surface area to volume ratio, controllable pore size, biomimetic morphology, and the presence of interconnected pores present in nanofibers suggest efficient delivery of bioactive compounds through nanofiber.
Nanofibers support enzyme immobilization, which can improve enzymes’ performance by increasing surface area, mass transfer resistance, loading efficiency, and the possibility of recycling the enzymes after catalytic performances. The primary emphasis after fabrication of nanofibers so far, however, has been mainly on improving their mechanical properties, cellular adhesion, and biocompatibility. Nevertheless, several studies have used bioactive proteins in nanofibers as moieties for triggering signaling pathways for regulating tissue regeneration [7][8][9]. A unique approach for nanofiber modification is that of enzyme immobilization [10]. As we know, enzymes are versatile biological entities that carry out the catalysis of various reactions to regulate the rate of processes in living organisms, and hence enzymes play a vital role in the optimal working of cellular systems. In tissue repair and regeneration, many enzymes operate to catalyze important reactions for maintaining tissue homeostasis, e.g., collagenases, transglutaminases, metalloproteinases, trypsin, serine proteases, alkyl phosphatase, etc. [11][12][13][14][15]. Inspired by the properties of these natural biocatalysts (i.e., enzymes), several studies have reported enzyme-immobilized nanofibers for improved tissue regeneration applications. These studies show that enzyme-immobilized biocatalytic nanofibers offer a novel approach for the repair and reconstruction of tissues. These nanofibers work by undertaking the catalysis of processes that are essential for the regeneration of tissue, e.g., polymerization of extracellular matrix (ECM) components, increasing gaseous diffusion to assist artificial respiratory devices, etc. Alternatively, these biocatalytic nanofibers help to eradicate certain obstacles that hinder the process of tissue repair or healing, e.g., digestion of components (such as collagen) of a scar to increase its porosity for unrestrained cellular proliferation. Other strategies involve the use of enzymes to facilitate better crosslinking in nanofibers for improved properties and performance. This review provides a comprehensive account of numerous strategies explored for improving the utilization of biocatalytic nanofibers in tissue engineering. In this perspective, here we discuss the applications of biocatalytic nanofibers in wound healing, ECM polymerization, artificial tissue fabrication, bone regeneration, etc.
It is noteworthy to mention that a number of techniques are used to immobilize enzymes on nanofibers utilizing the principles of encapsulation, adsorption, and covalent bonding between the polymers and the enzymes [16][17]. However, encapsulation involves the entrapment of enzymes into the nanofiber network, wherein the enzymes are mixed with the polymer solution, which is later used in nanofiber fabrication. The encapsulation technique increases the enzyme’s stability and ensures that no enzyme leakage occurs from the nanofibers. On the other hand, adsorption involves binding of the enzyme through the formation of weak forces, e.g., electrostatic attraction, hydrophobic and/or Van der Waal’s force. A robust immobilization technique is one through which the formation of covalent bonds between the nanofiber and the enzyme can occur. This covalent bonding-mediated immobilization can occur through the functional groups present on the nanofibers that can interact with enzymes to form bonds, hence arresting the enzyme in the polymer [18]. Physical binding, i.e., adsorption, generally produces weak bonds between the nanofiber and the enzyme, resulting in reversible bond formation. Such type of binding is highly sensitive to process conditions such as pH, temperature, etc. On the other hand, covalent linkage gives rise to highly robust and durable enzyme-immobilized nanofibers but, at the same time, is an expensive method. [19].
2. ECM Digestion
The ECM in the musculoskeletal system’s fibrous connective tissue comprises of aligned collagen fiber bundles that function as microstructural reinforcement to impart mechanical strength to the whole system [20][21][22]. The ECM imparts tissue resistance against external stresses along the fiber length and provides the strength to withstand the external load. During an injury to such tissues, the ECM–collagen microstructure gets disrupted, and hence the mechanical strength and load-bearing capacity of the tissue is altered. During the course of self-healing at the injury site, the tissue develops a scar mass that consists of a disordered high-density collagen fiber structure [22]. The application of a tissue-engineered scaffold at that site is limited because of the already deposited dense ECM of the scar. This is because the naturally existing high-density ECM bears less porosity, which hinders cell proliferation, adhesion, and migration. Hence, it acts as the barrier for endogenous tissue repair, resulting in inadequate healing. In this regard, researchers have suggested the use of the collagen digestion approach as an effective strategy for managing the efficient working of a nanofibrous implant. In this course, several studies have shown effective wound healing while applying digestion of wound edges using matrix-digesting enzymes that are immobilized on nanofibers. Examples of such enzymes that many researchers use as biocatalysts for tissue repair are collagenase, trypsin, hyaluronidase, etc. [23][24][25].
An excellent illustration of the application of this strategy to tissue repair is the one demonstrated by Qu et al. for restoration after knee meniscal injury [26]. It is well known that tearing in joint menisci leads to the destruction of collagen microstructure and the development of dense collagen scar that often results in degenerative osteoarthritis [27][28]. The primary treatment for meniscal injuries is meniscectomy or resection of the affected meniscal area through the surgery. However, the recovery rate during such a procedure is relatively low, and the meniscectomy imparts only temporary pain relief. Several patients need a second surgery, resulting in increased morbidity rates. Furthermore, the surgical procedures may damage articular cartilage and meniscal fibrocartilage, requiring total arthroplasty in some cases [29][30].
The basic idea behind employing a biocatalytic approach for such tissue repair is to decrease the density of meniscal scar to reduce the impedance in the healing process and allow easy cell migration, proliferation, and matrix deposition. Qu et al. fabricated collagenase-incorporated nanofibers for the repair of knee meniscal injury, employing the biocatalysis of collagen degradation in the dense matrix of wound scar. The nanofiber scaffolds were fabricated by electrospinning using 8% w/v polyethylene solution (in 1:1 ethanol/water) or 40–50% w/v polyethylene oxide/poly(ε-caprolactone) solution containing 1.25% w/v collagenase for 10–15 min onto glass coverslips. The in vitro release studies indicated that the nanofibers released the collagenase enzyme in a controllable manner. The scaffolds were evaluated in vitro for meniscal repair in juvenile and adult bovine menisci, as indicated in Figure 1. The scaffolds were placed in the meniscus defects, which significantly reduced the matrix integrity, increased the porosity, and reduced the levels of proteoglycans and collagen at the wound edge. This resulted in an increase in the cell infiltration and integration of fibrils, leading to the closure of more than 90% of the wound within four weeks. The annulus-core boundary of the meniscus displayed relatively higher cell density.
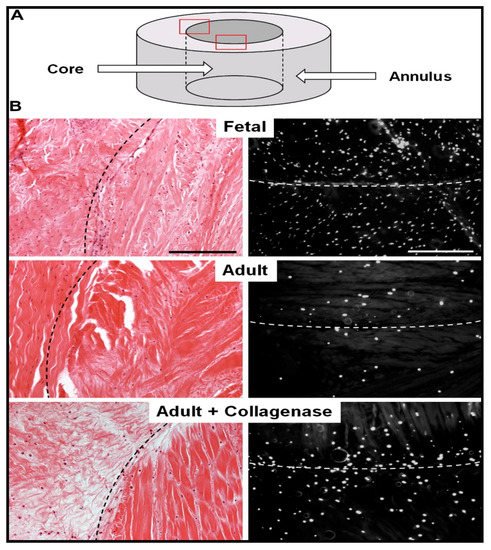
Figure 1. Reduction in the adult bovine meniscal matrix density and cellularity increase through collagenase-immobilized nanofiber treatment. (A) Schematic showing meniscal annulus and core. (B) Histological analysis of repair constructs using hematoxylin and eosin staining (left) and 4′,6-diamidino-2-phenylindole (right) staining. Reproduced from [26]. Copyright (2013) Elsevier Ltd.
Feini et al. adopted the same approach for the fabrication of biocatalytic nanofibers that incorporated a chemotactic agent in addition to the immobilized collagenase [31]. These researchers hypothesized that in addition to reducing the stiffness and density of native ECM, it was essential to guide the adult repairing cells to migrate to the wound site. Therefore, a chemotactic agent (i.e., platelet-derived growth factor-AB) was used to encapsulate in the nanofibers. These nanofibers were fabricated by electrospinning using 15% hyaluronic acid, 35% poly(ethylene oxide), and 50% poly(ε-caprolactone). Various nanofibrous scaffolds of different compositions that were evaluated are shown in Figure 2.
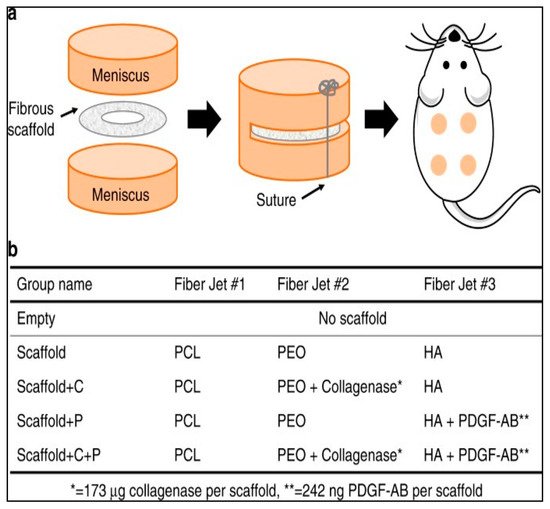
Figure 2. Nanofibrous scaffolds used for in vivo evaluation of collagenase-immobilized nanofibers. (a) Animal model for tissue repair evaluation: scaffolds placed subcutaneously in rats; (b) composition of various scaffolds used by Feini et al. in their study. PCL: poly(ε-caprolactone); PEO: poly(ethylene oxide); HA: hyaluronic acid; PDGF-AB: platelet-derived growth factor-AB. Reproduced from [31]. Copyright (2017), Feini Qu et al.
The nanofibers were able to successfully release the collagenase with an initial 20% showing a burst release and the remaining 80% released in a controlled manner over a period of 5 h in a phosphate buffer saline. On the other hand, 67% of platelet-derived growth factor-AB was released over a period of 12 days. The nanofibers were evaluated for their tissue repairing application in meniscal defects. The results showed increased cellularity at the wound site and integration with the adjoining tissues. The nanofibers were assessed for their meniscal repair potential in vivo in athymic rats by inserting the nanofibrous scaffolds subcutaneously. This revealed the role of the dense extracellular matrix in hindering the healing process of the wound. The degradation of the wound matrix collagen increased significantly with the use of the novel collagenase scaffold, while allowing for the formation of its own cellular matrix. This was evident from the appearance of interconnected thin collagen fibers that were found to bridge the scaffold with the wound tissue within four weeks (Figure 3).
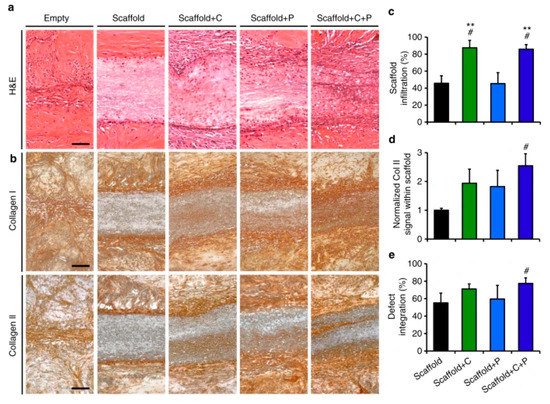
Figure 3. Effect of collagenase-immobilized, platelet-derived growth factor-AB nanofibrous scaffolds on tissue integration, matrix formation, and cellularity. (a) Histological images of hematoxylin and eosin-stained wound after scaffold fixation. (b) Histological images of the wound after immunostaining showing collagen I and II; (c–e). The cell infiltration, normalized collagen II signal within the scaffold and % defect integration (% integrated tissue at the wound site) for various scaffolds are also presented. # = p < 0.05 vs. Scaffold, ** = p < 0.05 vs. Scaffold + P. These figures are reproduced from [31]. Copyright (2017), Feini Qu et al.
In these results, the cell proliferation and new collagen formation were more evident in the case of collagenase-immobilized scaffolds. An important aspect of these novel scaffolds is that the collagen degradation takes place only up to 300 µm from the wound edge. The study further revealed endogenous cell-mediated wound closure by recruitment was possible through the chemotactic property of the scaffolds.
Another target for extracellular digestion as a fast tissue repair facilitation strategy is the chondroitin sulfate proteoglycan digestion. The formation of a glial scar is considered as one of the major factors restricting the nerve regeneration process during the healing of a spinal cord injury. Therefore, a glial scar remains a chief therapeutic target for the treatment of spinal cord injury and efforts are being put forward to devise methods for eradicating glial scar to facilitate adequate reconstruction. Because the significant component of such a scar is chondroitin sulfate proteoglycans, several researchers have used a biocatalytic approach to eradicate these glial scars associated with spinal cord injury by using the enzyme chondroitinase as the biocatalyst. There is not much literature available demonstrating this application of the biocatalytic nanofibers; however, Liu et al. have applied this approach to the treatment of spinal cord injury [32]. Presently, intrathecal injection of chondroitinase ABC (lasting for weeks) is the central treatment approach for spinal cord injury, which is quite invasive, apart from chondroitinase ABC being thermal sensitive and susceptible to degradation in the host. Additionally, the use of intrathecal chondroitinase ABC injection is restricted because it overflows beyond the injection site, reducing the amount reaching the actual injury site. Liu et al. fabricated collagen scaffolds containing neurotrophin-3 and chondroitinase ABC for the repair of spinal cord injury. These nanofibers were able to release both neurotrophin-3 and chondroitinase ABC in a sustained manner, thus avoiding the rapid clearance from the site of action, which generally happens with conventional drugs. A similar combination for spinal cord injury treatment has already been attempted by other researchers who demonstrated an enhanced locomotor function and sensory axon growth in rats [33]. However, hydrogels are generally isotropic and weak. Furthermore, hydrogels exhibit poor mechanical and morphological properties, which are pivotal for cell alignment, migration, and proliferation. On the other hand, nanofibers fulfill all these requirements and provide an excellent alternative as a scaffold for neurotrophin-3 and chondroitin ABC delivery for neuronal repair in the treatment of spinal cord injury. Nanofibers’ excellent topographical properties enable their use in spinal cord injury due to their ability to increase cells, as demonstrated by Chew et al. using Schwann cells [34]. Further, when incorporating neurotrophic factors in the topographically favorable nanofibers, an increase in nerve regeneration was observed [35]. As demonstrated by Liu et al., nanofibers have been used in the treatment of acute spinal cord injury in rats [36].
The nanofibers fabricated by Liu et al. provided sustained release of both neurotrophin-3 and chondroitin ABC, minimizing the chances of losing therapeutic agents. These researchers used rat tail extracted collagen scaffolds for immobilization of neurotrophin-3 and chondroitin ABC. The immobilization was achieved by crosslinking through microbial transglutaminase crosslinking. In addition to this, heparin was added to increase the protective effect on chondroitin ABC. This was evident because the chondroitin ABC showed 32% bioactivity after 32 days compared with the 1.9% action after 22 days.
References
- Dahlin, R.L.; Kasper, F.K.; Mikos, A.G. Polymeric Nanofibers in Tissue Engineering. Tissue Eng. Part B Rev. 2011, 17, 349–364.
- Liao, S.; Li, B.; Ma, Z.; Wei, H.; Chan, C.; Ramakrishna, S. Biomimetic electrospun nanofibers for tissue regeneration. Biomed. Mater. 2006, 1, R45–R53.
- Nemati, S.; Kim, S.-J.; Shin, Y.M.; Shin, H. Current progress in application of polymeric nanofibers to tissue engineering. Nano Converg. 2019, 6, 36.
- Shrestha, B.K.; Shrestha, S.; Tiwari, A.P.; Kim, J.-I.; Ko, S.W.; Kim, H.-J.; Park, C.H.; Kim, C.S. Bio-inspired hybrid scaffold of zinc oxide-functionalized multi-wall carbon nanotubes reinforced polyurethane nanofibers for bone tissue engineering. Mater. Des. 2017, 133, 69–81.
- Udomluck, N.; Koh, W.-G.; Lim, D.-J.; Park, H. Recent Developments in Nanofiber Fabrication and Modification for Bone Tissue Engineering. Int. J. Mol. Sci. 2020, 21, 99.
- Karuppuswamy, P.; Venugopal, J.R.; Navaneethan, B.; Laiva, A.L.; Sridhar, S.; Ramakrishna, S. Functionalized hybrid nanofibers to mimic native ECM for tissue engineering applications. Appl. Surf. Sci. 2014, 322, 162–168.
- Udomluck, N.; Lee, H.; Hong, S.; Lee, S.-H.; Park, H. Surface functionalization of dual growth factor on hydroxyapatite-coated nanofibers for bone tissue engineering. Appl. Surf. Sci. 2020, 520, 146311.
- Shin, Y.C.; Kim, J.; Kim, S.E.; Song, S.-J.; Hong, S.W.; Oh, J.-W.; Lee, J.; Park, J.-C.; Hyon, S.-H.; Han, D.-W. RGD peptide and graphene oxide co-functionalized PLGA nanofiber scaffolds for vascular tissue engineering. Regen. Biomater. 2017, 4, 159–166.
- Junka, R.; Valmikinathan, C.M.; Kalyon, D.M.; Yu, X. Laminin Functionalized Biomimetic Nanofibers for Nerve Tissue Engineering. J. Biomater. Tissue Eng. 2013, 3, 494–502.
- Wang, Z.-G.; Wan, L.-S.; Liu, Z.-M.; Huang, X.-J.; Xu, Z.-K. Enzyme immobilization on electrospun polymer nanofibers: An overview. J. Mol. Catal. B Enzym. 2009, 56, 189–195.
- Sun, Y.; Weber, K.T. Angiotensin converting enzyme and myofibroblasts during tissue repair in the rat heart. J. Mol. Cell. Cardiol. 1996, 28, 851–858.
- Nawaz, M.; Shah, N.; Zanetti, B.R.; Maugeri, M.; Silvestre, R.N.; Fatima, F.; Neder, L.; Valadi, H. Extracellular Vesicles and Matrix Remodeling Enzymes: The Emerging Roles in Extracellular Matrix Remodeling, Progression of Diseases and Tissue Repair. Cells 2018, 7, 167.
- Chan, E.C.; Jiang, F.; Peshavariya, H.M.; Dusting, G.J. Regulation of cell proliferation by NADPH oxidase-mediated signaling: Potential roles in tissue repair, regenerative medicine and tissue engineering. Pharmacol. Ther. 2009, 122, 97–108.
- Mignatti, P.; Rifkin, D.B.; Welgus, H.G.; Parks, W.C. Proteinases and tissue remodeling. In The Molecular and Cellular Biology of Wound Repair; Clark., R.A.F., Ed.; Springer: Boston, MA, USA, 1988; pp. 427–474.
- Telci, D.; Griffin, M. Tissue transglutaminase (TG2)—A wound response enzyme. Front. Biosci. 2006, 2006. 11, 867–882.
- Nguyen, H.H.; Kim, A.M. An Overview of Techniques in Enzyme Immobilization. Appl. Sci. Converg. Technol. 2017, 26, 157–163.
- Sirisha, V.; Jain, A.; Jain, A. Enzyme immobilization. Adv. Food Nutr. Res. 2016, 79, 179–211.
- Smith, S.; Goodge, K.; Delaney, M.; Struzyk, A.; Tansey, N.; Frey, M. A Comprehensive Review of the Covalent Immobilization of Biomolecules onto Electrospun Nanofibers. Nanomaterials 2020, 10, 2142.
- Homaei, A.A.; Sariri, R.; Vianello, F.; Stevanato, R. Enzyme immobilization: An update. J. Chem. Biol. 2013, 6, 185–205.
- Gillies, A.R.; Lieber, R.L. Structure and function of the skeletal muscle extracellular matrix. Muscle Nerve 2011, 2011. 44, 318–331.
- Kjaer, M. Role of Extracellular Matrix in Adaptation of Tendon and Skeletal Muscle to Mechanical Loading. Physiol. Rev. 2004, 84, 649–698.
- Subramanian, A.; Schilling, T.F. Tendon development and musculoskeletal assembly: Emerging roles for the extracellular matrix. Development 2015, 142, 4191–4204.
- Bravenboer, J.V.D.B.; Der Maur, C.D.I.; Bos, P.K.; Feenstra, L.; Verhaar, J.A.N.; Weinans, H.; Van Osch, G.J.V.M. Improved cartilage integration and interfacial strength after enzymatic treatment in a cartilage transplantation model. Arthritis Res. 2004, 6, R469.
- Obradovic, B.; Martin, I.; Padera, R.F.; Treppo, S.; Freed, L.E.; Vunjak-Navakovic, G. Integration of engineered cartilage. J. Orthop. Res. 2001, 19, 1089–1097.
- Janssen, L.M.; Der Maur, C.D.I.; Bos, P.K.; Hardillo, J.A.; Van Osch, G.J.V.M. Short-Duration Enzymatic Treatment Promotes Integration of a Cartilage Graft in a Defect. Ann. Otol. Rhinol. Laryngol. 2006, 115, 461–468.
- Qu, F.; Lin, J.-M.G.; Esterhai, J.L.; Fisher, M.B.; Mauck, R.L. Biomaterial-mediated delivery of degradative enzymes to improve meniscus integration and repair. Acta Biomater. 2013, 9, 6393–6402.
- Makris, E.A.; Hadidi, P.; Athanasiou, K.A. The knee meniscus: Structure–function, pathophysiology, current repair techniques, and prospects for regeneration. Biomaterials 2011, 32, 7411–7431.
- Tu, K.; Cole, B.J.; Freedman, K.B. Augmentation of meniscus repair. Oper. Tech. Sports Med. 2003, 11, 127–133.
- Aagaard, H.; Verdonk, R. Function of the normal meniscus and consequences of meniscal resection. Scand. J. Med. Sci. Sports 2007, 9, 134–140.
- Englund, M. Meniscal tear—A feature of osteoarthritis. Acta Orthop. Scand. 2004, 75 (Suppl. 312), 1–45.
- Qu, F.; Holloway, J.L.; Esterhai, J.L.; Burdick, J.A.; Mauck, R.L. Programmed biomolecule delivery to enable and direct cell migration for connective tissue repair. Nat. Commun. 2017, 8, 1780.
- Liu, T.; Xu, J.; Chan, B.P.; Chew, S.Y. Sustained release of neurotrophin-3 and chondroitinase ABC from electrospun collagen nanofiber scaffold for spinal cord injury repair. J. Biomed. Mater. Res. Part A 2012, 100, 236–242.
- Lee, H.; McKeon, R.J.; Bellamkonda, R.V. Sustained delivery of thermostabilized chABC enhances axonal sprouting and functional recovery after spinal cord injury. Proc. Natl. Acad. Sci. USA 2010, 107, 3340–3345.
- Chew, S.Y.; Mi, R.; Hoke, A.; Leong, K.W. The effect of the alignment of electrospun fibrous scaffolds on Schwann cell maturation. Biomaterials 2008, 29, 653–661.
- Chew, S.Y.; Mi, R.; Hoke, A.; Leong, K.W. Aligned Protein-Polymer Composite Fibers Enhance Nerve Regeneration: A Potential Tissue-Engineering Platform. Adv. Funct. Mater. 2007, 17, 1288–1296.
- Liu, T.; Houle, J.D.; Xu, J.; Chan, B.P.; Chew, S.Y. Nanofibrous Collagen Nerve Conduits for Spinal Cord Repair. Tissue Eng. Part A 2012, 18, 1057–1066.




