
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Yun Kim | + 2837 word(s) | 2837 | 2021-04-26 05:23:58 | | | |
| 2 | Peter Tang | Meta information modification | 2837 | 2021-04-28 08:15:53 | | |
Video Upload Options
Sarcopenia is characterized by a skeletal muscle disorder with progressive and generalized loss of muscle mass and function, and it increases the risk of adverse outcomes with considerable prevalence in patients with chronic liver disease. Sarcopenia in chronic liver disease underlies complicated and multifactorial mechanisms for pathogenesis, including alterations in protein turnover, hyperammonemia, energy disposal, hormonal changes, and chronic inflammation. The key contribution to sarcopenia in patients with chronic liver diseases can be the hyperammonemia-induced upregulation of myostatin, which causes muscle atrophy via the expression of atrophy-related genes.
1. Introduction
Sarcopenia is characterized by a skeletal muscle disorder with progressive and generalized loss of muscle mass, strength, and function, thus increasing the risk of adverse outcomes, such as physical disability and higher rates of hospitalization and mortality [1]. The term “sarcopenia” was first used in the 1980s to refer to age-related skeletal muscle decline [2]. The definition of sarcopenia has changed from muscle-wasting conditions with low muscle mass into a term involving muscle function in the current concept of sarcopenia [3][4]. The evolution of the definition is attributed to the fact that muscle function has been shown to be a more influential clinical biomarker than muscle mass alone [5][6]. The overall prevalence of sarcopenia was estimated at 10% in both men and women, which indicates that a considerable proportion of the elderly, even in a healthy population, has sarcopenia [7]. Moreover, sarcopenia is generally known to be associated with various chronic inflammatory states, including chronic liver disease [6]. It has been shown to be a significant risk factor for non-alcoholic fatty liver disease (NAFLD), regardless of obesity or metabolic syndrome [8][9][10]. This relationship was convincing because similar pathological factors, including insulin resistance and inflammation, exist between sarcopenia and NAFLD [11][12][13]. Notably, the prevalence rate of sarcopenia is assumed to be 30–70% in cirrhotic patients, with a higher rate among men than in women (61.6% vs. 36%, respectively) [14][15][16]. Minimal hepatic encephalopathy, a complication of liver cirrhosis, was significantly associated with the presence of either muscle mass loss or strength loss (60.9% vs. 37.7%, respectively) [17]. In addition, sarcopenia commonly develops in patients with end-stage liver disease, for which the prevalence of sarcopenia ranges from 14 to 78% and from 30 to 100% in patients before and after liver transplantation, respectively [18]. Furthermore, sarcopenia can be a clinically significant predictor of higher rates of mortality and infection [16][19], longer hospitalization [20], and increased economic burden [21], thus reducing the quality of life [22]. However, challenges arise because the mechanisms of sarcopenia in chronic liver diseases are poorly understood and no approved and effective therapeutics to counteract sarcopenia are available.
2. Etiology of Sarcopenia in Chronic Liver Diseases
Sarcopenia in chronic liver disease is a complicated and multifactorial disease with several main drivers, such as impairment in protein turnover, malnutrition, hyperammonemia, chronic inflammation, and hormonal changes (Figure 1). Understanding the hypotheses of sarcopenia development would play a key role in overcoming the therapeutic limitations.
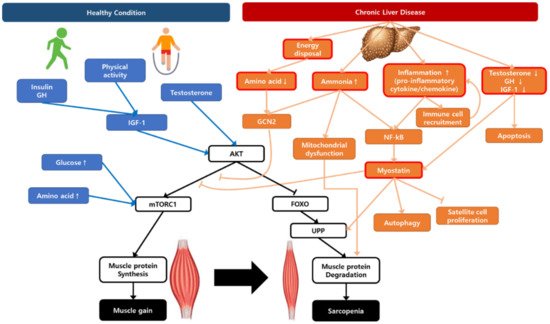
Figure 1. A schematic representation illustrating the regulation of muscle protein synthesis and degradation with the contribution of chronic liver disease to sarcopenia. AKT, protein kinase B; FOXO, Forkhead box O; GCN2, general control non-depressed 2; GH, growth hormone; IGF-1, insulin growth factor-1; mTORC1, mammalian target of rapamycin complex 1; NF-κB, nuclear factor-κB; UPP, ubiquitin–proteasome pathway.
3. Current and Emerging Treatment Options for Sarcopenia in Chronic Liver Disease
In recent decades, several drugs have been investigated in clinical trials to counteract sarcopenia, but no pharmacologically effective therapeutics have been approved to date. However, current and emerging treatment options for sarcopenia have been reported to be under development. In February 2021, a manual search from the Clinicaltrials.gov database (accessed on 1 February 2021) yielded several published interventional clinical trials related to sarcopenia and the potential expanded use with mostly late-phase studies (Table S1). In addition, the list of clinical trials studying therapeutic interventions for sarcopenia in chronic liver disease is provided in Table 1, which mostly consists of dietary supplements and/or behavioral interventions. Unfortunately, there is only one registered interventional clinical trial of a pharmacological treatment option using testosterone, indicating that the development of a new drug is urgently needed (Table 1). The following section discusses an update on the potential pharmacological treatment options for the treatment of sarcopenia with chronic liver diseases.
Table 1. Current and emerging therapeutic interventions for sarcopenia with chronic liver diseases observed in clinical trials.
|
Target or Mechanism of Action |
Intervention |
Sponsor/Collaborator |
Clinical Phase |
Indication |
Status |
NCT Number |
Year |
Title |
|
|---|---|---|---|---|---|---|---|---|---|
|
Start |
End |
||||||||
|
Testosterone |
Testosterone undecanoate |
Institute of Liver and Biliary Sciences, India |
NA |
Liver cirrhosis |
Recruiting |
NCT03995251 |
2019 |
2020 |
Efficacy and Safety of Testosterone Therapy in Improving Sarcopenia in Men with Cirrhosis: A Randomized Controlled Trial |
|
Behavior |
Exercise |
University of California, San Francisco/Johns Hopkins University, Duke University |
NA |
End-stage liver disease, sarcopenia, liver cirrhosis |
Completed |
NCT02367092 |
2016 |
2019 |
Exercise Intervention in Liver Transplant Patients |
|
Behavior |
Exercise |
Memorial Hospital Groups |
NA |
End-stage liver disease, chronic liver failure, sarcopenia |
Completed |
NCT04546048 |
2018 |
2019 |
The Early Strength Training Exercise Therapy in Liver Recipients: Protocol for an Observational Feasibility Trial |
|
Behavior |
Pulmonary rehabilitation exercise, home-based exercise |
Mayo Clinic |
NA |
End-stage liver disease |
Recruiting |
NCT03266575 |
2018 |
Ongoing |
Does Pulmonary Rehabilitation Improve Frailty and Sarcopenia in End-Stage Liver Disease? |
|
Dietary supplement |
Amino acid infusion |
Rigshospitalet, Denmark/Hvidovre University Hospital |
NA |
Cirrhosis |
Completed |
NCT02132962 |
2014 |
2015 |
Sarcopenia and Cirrhosis |
|
Dietary supplement |
BCAA |
Puerta de Hierro University Hospital |
NA |
Sarcopenia |
Completed |
NCT04073693 |
2017 |
2019 |
Characterization of the Nutritional Status in the Patient with Liver Cirrhosis and Impact of a Nutritional Intervention with Nutritional Supplements with BCAA vs. Standard Treatment in the Subgroup of Patients with Sarcopenia |
|
Dietary supplement |
BCAA |
Dayanand Medical College and Hospital |
4 |
Liver cirrhosis |
Recruiting |
NCT03633279 |
2018 |
2020 |
Treatment of Sarcopenia Improves the Muscle Mass and Muscle Strength of Patients with Liver Cirrhosis—Child C: A Randomized Double Blind Control Trial |
|
Dietary supplement |
BCAA |
Institute of Liver and Biliary Sciences, India |
NA |
Chronic liver disease |
Recruiting |
NCT04246918 |
2020 |
Ongoing |
Effect of Branched Chain Amino Acids Supplementation on Muscle Mass, Muscle Quality, and Molecular Markers of Muscle Regeneration in Patients With Chronic Liver Disease: A Randomized Controlled Trial |
|
Dietary supplement |
HMB |
University of Roma La Sapienza |
NA |
Sarcopenia |
Completed |
NCT03234920 |
2015 |
2018 |
Effects of β-Hydroxy-β-methylbutyrate (HMB) Supplementation after Liver Transplantation: Randomized and Controlled Pilot Study |
|
Dietary supplement |
CaHMB |
Shanghai Zhongshan Hospital |
NA |
Sarcopenia, liver cirrhosis |
Unknown |
NCT03605147 |
2018 |
2019 |
The Effect of Calcium β-Hydroxy-β-methylbutyrate Supplementation in Sarcopenia in Liver Cirrhosis: A Randomized Double-Blind Controlled Trial |
|
Dietary supplement |
HMB |
University of Roma La Sapienza |
NA |
Sarcopenia, liver cirrhosis |
Recruiting |
NCT03892070 |
2019 |
2020 |
β-Hydroxy-β-methylbutyrate Supplementation and Physical Activity in Liver Cirrhosis: A Controlled Trial |
|
Dietary supplement |
Ensure Plus Advance, Ensure High Protein |
Instituto Aragones de Ciencias de la Salud/Refbio2: Trans-Pyrenean Cooperation Network for Biomedical Research |
NA |
Sarcopenia, liver cirrhosis |
Active, not recruiting |
NCT03285217 |
2017 |
2019 |
HMB for Denutrition in Patients with Cirrhosis (HEPATIC) |
|
Dietary supplement |
Fresubin energy (dietary protein energy supplement) |
Medical University of Graz |
NA |
Sarcopenia, liver cirrhosis |
Recruiting |
NCT03080129 |
2017 |
Ongoing |
Microbiome and Sarcopenia in Patients with Liver Cirrhosis: A Prospective Controlled Cohort Study |
|
Dietary supplement |
Medically tailored meals, protein supplements |
University of Michigan |
NA |
Sarcopenia, liver cirrhosis, hepatic encephalopathy, ascites |
Recruiting |
NCT04675775 |
2021 |
Ongoing |
Medically Tailored Meals to Prevent Recurrent Hepatic Encephalopathy: The BRAINFOOD Pilot Trial |
|
Multifactorial intervention |
Home exercise, BCAA supplements, multispecies probiotic |
Fundació Institut de Recerca de l’Hospital de la Santa Creu i Sant Pau |
NA |
Sarcopenia, liver cirrhosis, frailty syndrome (FS) |
Recruiting |
NCT04243148 |
2020 |
Ongoing |
Frailty in Patients with Cirrhosis: Prognostic Value of the Phase Angle in Hospitalized Patients and Effect of a Multifactorial Intervention (Home Exercise, Branched-chain Amino Acids, and Probiotics) |
|
Multifactorial intervention |
Physical training program, behavioral modification therapy, nutritional consultation |
University of Arkansas |
NA |
End-stage liver disease, liver transplant, sarcopenia, cirrhosis |
Completed |
NCT02776553 |
2016 |
2020 |
A Physical Activity Program in End-Stage Liver Disease: Pilot Study Assessing Changes in Physical Fitness, Sarcopenia, and the Metabolic Profile |
Data were presented in the Clinicaltrials.gov on 1 February 2021. Abbreviation: BCAA, branched chain amino acid; HMB, β-hydroxy-β-methylbutyrate; NA, not applicable.
3.1. Hormonal Treatment
Since testosterone deficiency is a common feature in advanced liver diseases, a previous study reported that testosterone treatment can reduce fat mass and hemoglobin A1c and can increase muscle and bone mass, along with hemoglobin elevation in patients with cirrhosis [23]. Testosterone treatment particularly increases the expression of androgen receptors, resulting in muscle cell growth and the differentiation for muscle protein synthesis [24]. In addition, testosterone drives the upregulation of IGF-1 via the Akt pathway to enhance beneficial effects on muscle growth via the proliferation of satellite cells [25][26]. By means of another pathway, testosterone replacement therapy contributes to the myostatin downregulation, further suppressing apoptosis in skeletal muscles [25]. However, clear molecular evidence of testosterone treatment should be obtained via further research, and adverse events, such as cardiovascular diseases, fluid retention, gynecomastia, sleep apnea, and the progression of prostatic diseases, need to be cautiously monitored [27]. Therefore, long-term confirmatory studies are needed to prove its efficacy and safety in sarcopenic patients with chronic liver diseases [28].
As previously mentioned, GH deficiency is correlated with the development of chronic liver diseases, which supports the hypothesis that GH replacement treatment can improve muscle mass by increasing serum IGF-1 levels and IGF binding protein 3 with the activation of the mTORC1 signaling pathway [29][30][31]. GH replacement therapy may also involve antioxidant defenses through the activation of mitochondrial biogenesis pathways [31]. However, GH supplementation may cause a high rate of adverse reactions, including worsening ascites and edema, with limited applicability due to its high cost [31][32]. Therefore, the clinical utility of GH replacement treatment needs to be confirmed in further studies to identify its safety and efficacy in clinical use.
3.2. Myostatin and Activin Receptor
Myostatin is an important target in various studies because of its detrimental effects on muscle protein synthesis [33]. Myostatin inhibits the differentiation and growth of skeletal muscle cells by binding to the activin type IIB (ACVRIIB) receptor, which subsequently inhibits the differentiation of myoblasts and the mTORC1 signaling pathway [34]. Stamulumab (MYO-029), a myostatin inhibitor studied in human trials, is a recombinant human antibody that neutralizes myostatin, which inhibits its binding to ACVRIIB. However, further development was stopped due to the limited efficacy on muscle strength in phase 2 clinical trials in patients with muscular dystrophy. Landogrozumab (LY-2495655), another myostatin inhibitor as a humanized monoclonal antibody under review, also binds to myostatin and neutralizes its activity. Landogrozumab has been shown to increase total lean body mass with a fat mass reduction in older weak fallers and to improve general physical performance [35]. Clinical trials of landogrozumab treatment for muscle atrophy in patients with hip arthroplasty identified improvement in muscle mass; however, the lean body mass did not meet the threshold [36]. Trevogrumab (REGN1033) is another human monoclonal antibody that targets myostatin for the treatment of sarcopenia, where its safety and efficacy are still being assessed after the completion of phase 2 clinical trials [37].
Ramatercept (ACE-031), an ACVRIIB/Fc recombinant fusion protein, binds to the ligands (e.g., myostatin, activins, and growth differentiation factor 11) of ACVRIIB to inhibit the endogenous receptor binding. Despite its award of orphan designation and accelerated review by the U.S. Food and Drug Administration (FDA), further development for the treatment of muscular dystrophy was stopped after completion in 2011 because of safety concerns, such as minor nosebleeds, gum bleeding, and/or small dilated blood vessels within the skin [38][39]. ACE-083, as an alternative form of ACE-031, is a locally acting and follistatin-based fusion protein that binds and acts by neutralizing myostatin, activins, and growth differentiation factor 11 [40]. Follistatin is known to improve muscle growth and function by preventing ligands from binding to receptors [41]. A first-in-human phase I clinical trial of ACE-083 demonstrated that it was well tolerated and produced increased muscle volume in healthy volunteers, which provides evidence for the potential treatment of various neuromuscular disorders, along with the need for further investigation of its efficacy and safety [42].
ACVRIIB is another potentially effective target for the development of treatments for sarcopenia. Bimagrumab (BYM-338), a human monoclonal antibody targeting ACVRIIB, was designed to competitively bind to ACVRIIB with higher affinity than its ligands. Breakthrough therapy designation was granted to bimagrumab in 2013 by the FDA for sporadic inclusion body myositis, which is characterized by inflammatory myopathy and progressive skeletal muscle atrophy. A preclinical study showed that bimagrumab increased the differentiation of myoblasts and inhibited the activity of myostatin or activin A, thus resulting in the improvement of skeletal muscle mass in mice [43]. A phase 2 clinical trial of bimagrumab in elderly patients with sarcopenia and limited mobility showed that bimagrumab improved muscle growth/function and mobility [44]. However, other late phases of clinical trials for bimagrumab treatment increased skeletal muscle mass in one study but observed no significant effects on functional capacity in sarcopenic patients with chronic obstructive pulmonary disease or sporadic inclusion body myositis [45][46]. In addition, the clinical use of ACVRIIB inhibitors may cause several adverse events, including muscle spasms, diarrhea, and acne [44][45][46]. Although there are many past and ongoing studies showing that the inhibition of the myostatin/ACVRIIB signaling pathway may counteract sarcopenia, the combination approaches with nutritional and/or physical activity could be a more promising and effective treatment for sarcopenia [37][47]. While this therapeutic approach has not yet been studied in sarcopenic patients with chronic liver diseases, the current status of research indicates that myostatin/ACVRIIB signaling inhibition can be an emerging treatment option for muscular dystrophy.
3.3. Ammonia-Lowering Treatment
As previously discussed, hyperammonemia is a feature of patients with cirrhosis that contributes to abnormal skeletal muscle proteostasis. Although the clinical utility of ammonia-lowering treatment is expected to be effective, whether this therapeutic approach can improve proteostasis and reverse sarcopenia in chronic liver disease is uncertain. A preclinical study showed that ammonia-lowering treatment significantly increased lean body mass and improved grip strength and skeletal muscle growth [48]. Perturbed molecular actions due to hyperammonemia were also improved with the reduction of myostatin expression and autophagy markers and with the reversal of GCN2/eIF2α phosphorylation [48]. L-ornithine L-aspartate can be an adequate option for ammonia-lowering treatment for patients with cirrhosis suffering from hepatic encephalopathy through the improvement of skeletal muscle growth and function, as supported by several randomized clinical trials and meta-analyses [49]. In addition, nutraceuticals, such as BCAA, L-carnitine, omega-3 polyunsaturated fatty acids, zinc, and vitamin D, may provide a promising standard of care with beneficial improvements in muscle homeostasis for sarcopenia in chronic liver disease [50]. Confirmation of these ammonia-lowering approaches for the treatment of sarcopenia in chronic liver disease is necessary for powered and well-controlled clinical trials to provide further evidence of efficacy.
3.4. Clinical Nutrition
In cirrhotic patients, the rates of both hepatic glucose production and oxidation are decreased owing to a depletion of hepatic glycogen, although gluconeogenesis is increased [51][52]. Thus, patients are susceptible to an accelerated state of starvation after an overnight fast [53]. Furthermore, impaired protein turnover and decreased plasma levels of essential fatty acids are observed in cirrhosis [54][55]. Therefore, the European Society for Clinical Nutrition and Metabolism (ESPEN) guideline recommends that the starvation period be kept short in cirrhosis to ameliorate protein turnover by taking 3–5 meals/day and a late evening snack [56]. A late evening snack has been shown to improve the nitrogen balance and decreased lipid oxidation, regardless of the composition or type of formulation used [57][58]. It is also suggested that cirrhotic patients with sarcopenia should include an optimal energy intake of 30–35 kcal/kg/day and a target protein intake of 1.2–1.5 g/kg/day [56]. To overcome protein depletion in cirrhotic patients with sarcopenia, including those with sarcopenic obesity, increased protein intake can improve protein anabolism and the status of total body protein [56][59][60]. In addition, in cirrhotic patients, including those with advanced cirrhosis and a previous episode of hepatic encephalopathy, a long-term BCAA supplementation (0.20–0.25 g/kg/day) had beneficial effects on protein metabolism, resulting in improved muscle mass, as well as minimal hepatic encephalopathy [61][62][63][64]. Since a specific nutritional intervention is needed in sarcopenic patients with chronic liver disease, multidisciplinary nutrition care should be implemented in the metabolic management of patients to achieve nutritional goals.
3.5. Regenerative Therapeutic Approach: Mitochondrial Restoration and Anti-Inflammation
Since the current pharmaceutical options for sarcopenia in chronic liver diseases may be ineffective and restricted in terms of the available clinical evidence, novel therapeutic approaches are necessary to improve mitochondrial function, reduce chronic inflammation, and induce muscle tissue regeneration, thus leading to increased muscle growth and function. Considering the abovementioned etiology of sarcopenia, regenerative medicine and stem cell therapy are potential alternatives for sarcopenia alleviation because of their ability to change the proinflammatory microenvironment into regenerating and reinnervating conditions by producing anti-inflammatory cytokines [65]. Mesenchymal stem cell transplantation has been shown to modulate immunological effects through the production of anti-inflammatory cytokines, including IL-10 and IL-13, and to stimulate neurosupportive effects by secreting factors including basic fibroblast growth factor and vascular endothelial growth factor [66][67][68][69][70]. In addition, mesenchymal stem cells may restore mitochondrial function in skeletal muscle via the mediation of mitochondrial transplantation [71]. However, stem cell transplantation has many restrictive hurdles to overcome (e.g., controversial safety and efficacy, ethics, pharmaceutical manufacturing process, and quality control); the secretome of stem cells that houses the important anti-inflammatory agents may provide a more promising option than the direct use of stem cells [65]. Nevertheless, extensive research through preclinical and clinical studies with a larger patient population is still required to determine its efficacy and safety as a potential therapeutic option for sarcopenia in chronic liver disease.
References
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyere, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 601.
- Rosenberg, I.H. Sarcopenia: Origins and clinical relevance. J. Nutr. 1997, 127, 990S–991S.
- Morley, J.E.; Abbatecola, A.M.; Argiles, J.M.; Baracos, V.; Bauer, J.; Bhasin, S.; Cederholm, T.; Coats, A.J.; Cummings, S.R.; Evans, W.J.; et al. Sarcopenia with limited mobility: An international consensus. J. Am. Med. Dir. Assoc. 2011, 12, 403–409.
- Chen, L.K.; Liu, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Bahyah, K.S.; Chou, M.Y.; Chen, L.Y.; Hsu, P.S.; Krairit, O.; et al. Sarcopenia in Asia: Consensus report of the Asian Working Group for Sarcopenia. J. Am. Med. Dir. Assoc. 2014, 15, 95–101.
- Delmonico, M.J.; Harris, T.B.; Visser, M.; Park, S.W.; Conroy, M.B.; Velasquez-Mieyer, P.; Boudreau, R.; Manini, T.M.; Nevitt, M.; Newman, A.B.; et al. Longitudinal study of muscle strength, quality, and adipose tissue infiltration. Am. J. Clin. Nutr. 2009, 90, 1579–1585.
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423.
- Shafiee, G.; Keshtkar, A.; Soltani, A.; Ahadi, Z.; Larijani, B.; Heshmat, R. Prevalence of sarcopenia in the world: A systematic review and meta- analysis of general population studies. J. Diabetes Metab. Disord. 2017, 16, 21.
- Lee, Y.H.; Jung, K.S.; Kim, S.U.; Yoon, H.J.; Yun, Y.J.; Lee, B.W.; Kang, E.S.; Han, K.H.; Lee, H.C.; Cha, B.S. Sarcopaenia is associated with NAFLD independently of obesity and insulin resistance: Nationwide surveys (KNHANES 2008–2011). J. Hepatol. 2015, 63, 486–493.
- Hong, H.C.; Hwang, S.Y.; Choi, H.Y.; Yoo, H.J.; Seo, J.A.; Kim, S.G.; Kim, N.H.; Baik, S.H.; Choi, D.S.; Choi, K.M. Relationship between sarcopenia and nonalcoholic fatty liver disease: The Korean Sarcopenic Obesity Study. Hepatology 2014, 59, 1772–1778.
- Chung, G.E.; Kim, M.J.; Yim, J.Y.; Kim, J.S.; Yoon, J.W. Sarcopenia Is Significantly Associated with Presence and Severity of Nonalcoholic Fatty Liver Disease. J. Obes. Metab. Syndr. 2019, 28, 129–138.
- Lee, Y.H.; Kim, S.U.; Song, K.; Park, J.Y.; Kim, D.Y.; Ahn, S.H.; Lee, B.W.; Kang, E.S.; Cha, B.S.; Han, K.H. Sarcopenia is associated with significant liver fibrosis independently of obesity and insulin resistance in nonalcoholic fatty liver disease: Nationwide surveys (KNHANES 2008–2011). Hepatology 2016, 63, 776–786.
- Koo, B.K.; Kim, D.; Joo, S.K.; Kim, J.H.; Chang, M.S.; Kim, B.G.; Lee, K.L.; Kim, W. Sarcopenia is an independent risk factor for non-alcoholic steatohepatitis and significant fibrosis. J. Hepatol. 2017, 66, 123–131.
- Lee, M.J.; Kim, E.H.; Bae, S.J.; Kim, G.A.; Park, S.W.; Choe, J.; Jung, C.H.; Lee, W.J.; Kim, H.K. Age-Related Decrease in Skeletal Muscle Mass Is an Independent Risk Factor for Incident Nonalcoholic Fatty Liver Disease: A 10-Year Retrospective Cohort Study. Gut Liver 2019, 13, 67–76.
- Kim, G.; Kang, S.H.; Kim, M.Y.; Baik, S.K. Prognostic value of sarcopenia in patients with liver cirrhosis: A systematic review and meta-analysis. PLoS ONE 2017, 12, e0186990.
- Dasarathy, S.; Merli, M. Sarcopenia from mechanism to diagnosis and treatment in liver disease. J. Hepatol. 2016, 65, 1232–1244.
- Montano-Loza, A.J.; Meza-Junco, J.; Prado, C.M.; Lieffers, J.R.; Baracos, V.E.; Bain, V.G.; Sawyer, M.B. Muscle wasting is associated with mortality in patients with cirrhosis. Clin. Gastroenterol. Hepatol. 2012, 10, 166–173.e1.
- Tateyama, M.; Naoe, H.; Tanaka, M.; Tanaka, K.; Narahara, S.; Tokunaga, T.; Kawasaki, T.; Yoshimaru, Y.; Nagaoka, K.; Watanabe, T.; et al. Loss of skeletal muscle mass affects the incidence of minimal hepatic encephalopathy: A case control study. BMC Gastroenterol. 2020, 20, 371.
- Ooi, P.H.; Hager, A.; Mazurak, V.C.; Dajani, K.; Bhargava, R.; Gilmour, S.M.; Mager, D.R. Sarcopenia in Chronic Liver Disease: Impact on Outcomes. Liver Transpl. 2019, 25, 1422–1438.
- Van Vugt, J.L.; Levolger, S.; de Bruin, R.W.; van Rosmalen, J.; Metselaar, H.J.; IJzermans, J.N.M. Systematic Review and Meta-Analysis of the Impact of Computed Tomography-Assessed Skeletal Muscle Mass on Outcome in Patients Awaiting or Undergoing Liver Transplantation. Am. J. Transpl. 2016, 16, 2277–2292.
- Sinclair, M.; Poltavskiy, E.; Dodge, J.L.; Lai, J.C. Frailty is independently associated with increased hospitalisation days in patients on the liver transplant waitlist. World J. Gastroenterol. 2017, 23, 899–905.
- Van Vugt, J.L.A.; Buettner, S.; Alferink, L.J.M.; Bossche, N.; de Bruin, R.W.F.; Darwish Murad, S.; Polak, W.G.; Metselaar, H.J.; IJzermans, J.N.M. Low skeletal muscle mass is associated with increased hospital costs in patients with cirrhosis listed for liver transplantation-a retrospective study. Transpl. Int. 2018, 31, 165–174.
- Ohashi, K.; Ishikawa, T.; Imai, M.; Suzuki, M.; Hoshii, A.; Abe, H.; Koyama, F.; Nakano, T.; Ueki, A.; Noguchi, H.; et al. Relationship between pre-sarcopenia and quality of life in patients with chronic liver disease: A cross-sectional study. Eur. J. Gastroenterol. Hepatol. 2019, 31, 1408–1413.
- Sinclair, M.; Grossmann, M.; Hoermann, R.; Angus, P.W.; Gow, P.J. Testosterone therapy increases muscle mass in men with cirrhosis and low testosterone: A randomised controlled trial. J. Hepatol. 2016, 65, 906–913.
- Sinha-Hikim, I.; Taylor, W.E.; Gonzalez-Cadavid, N.F.; Zheng, W.; Bhasin, S. Androgen receptor in human skeletal muscle and cultured muscle satellite cells: Up-regulation by androgen treatment. J. Clin. Endocrinol. Metab. 2004, 89, 5245–5255.
- Kovacheva, E.L.; Hikim, A.P.; Shen, R.; Sinha, I.; Sinha-Hikim, I. Testosterone supplementation reverses sarcopenia in aging through regulation of myostatin, c-Jun NH2-terminal kinase, Notch, and Akt signaling pathways. Endocrinology 2010, 151, 628–638.
- Wu, Y.; Zhao, W.; Zhao, J.; Pan, J.; Wu, Q.; Zhang, Y.; Bauman, W.A.; Cardozo, C.P. Identification of androgen response elements in the insulin-like growth factor I upstream promoter. Endocrinology 2007, 148, 2984–2993.
- Liguori, I.; Russo, G.; Aran, L.; Bulli, G.; Curcio, F.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Sarcopenia: Assessment of disease burden and strategies to improve outcomes. Clin. Interv. Aging 2018, 13, 913–927.
- Sinclair, M.; Grossmann, M.; Gow, P.J.; Angus, P.W. Testosterone in men with advanced liver disease: Abnormalities and implications. J. Gastroenterol. Hepatol. 2015, 30, 244–251.
- Donaghy, A.; Ross, R.; Wicks, C.; Hughes, S.C.; Holly, J.; Gimson, A.; Williams, R. Growth hormone therapy in patients with cirrhosis: A pilot study of efficacy and safety. Gastroenterology 1997, 113, 1617–1622.
- Bian, A.; Ma, Y.; Zhou, X.; Guo, Y.; Wang, W.; Zhang, Y.; Wang, X. Association between sarcopenia and levels of growth hormone and insulin-like growth factor-1 in the elderly. BMC Musculoskelet. Disord. 2020, 21, 214.
- Brioche, T.; Kireev, R.A.; Cuesta, S.; Gratas-Delamarche, A.; Tresguerres, J.A.; Gomez-Cabrera, M.C.; Vina, J. Growth hormone replacement therapy prevents sarcopenia by a dual mechanism: Improvement of protein balance and of antioxidant defenses. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, 1186–1198.
- Wallace, J.D.; Abbott-Johnson, W.J.; Crawford, D.H.; Barnard, R.; Potter, J.M.; Cuneo, R.C. GH treatment in adults with chronic liver disease: A randomized, double-blind, placebo-controlled, cross-over study. J. Clin. Endocrinol. Metab. 2002, 87, 2751–2759.
- Han, H.Q.; Zhou, X.; Mitch, W.E.; Goldberg, A.L. Myostatin/activin pathway antagonism: Molecular basis and therapeutic potential. Int. J. Biochem. Cell Biol. 2013, 45, 2333–2347.
- Lee, S.J.; Lehar, A.; Meir, J.U.; Koch, C.; Morgan, A.; Warren, L.E.; Rydzik, R.; Youngstrom, D.W.; Chandok, H.; George, J.; et al. Targeting myostatin/activin A protects against skeletal muscle and bone loss during spaceflight. Proc. Natl. Acad. Sci. USA 2020, 117, 23942–23951.
- Becker, C.; Lord, S.R.; Studenski, S.A.; Warden, S.J.; Fielding, R.A.; Recknor, C.P.; Hochberg, M.C.; Ferrari, S.L.; Blain, H.; Binder, E.F.; et al. Myostatin antibody (LY2495655) in older weak fallers: A proof-of-concept, randomised, phase 2 trial. Lancet Diabetes Endocrinol. 2015, 3, 948–957.
- Woodhouse, L.; Gandhi, R.; Warden, S.J.; Poiraudeau, S.; Myers, S.L.; Benson, C.T.; Hu, L.; Ahmad, Q.I.; Linnemeier, P.; Gomez, E.V.; et al. A Phase 2 Randomized Study Investigating the Efficacy and Safety of Myostatin Antibody LY2495655 versus Placebo in Patients Undergoing Elective Total Hip Arthroplasty. J. Frailty Aging 2016, 5, 62–70.
- Saitoh, M.; Ishida, J.; Ebner, N.; Anker, S.D.; Springer, J.; von Haehling, S. Myostatin inhibitors as pharmacological treatment for muscle wasting and muscular dystrophy. J. Cachexia Sarcopenia Muscle Clin. Rep. 2017, 2, 1–10.
- Attie, K.M.; Borgstein, N.G.; Yang, Y.; Condon, C.H.; Wilson, D.M.; Pearsall, A.E.; Kumar, R.; Willins, D.A.; Seehra, J.S.; Sherman, M.L. A single ascending-dose study of muscle regulator ACE-031 in healthy volunteers. Muscle Nerve 2013, 47, 416–423.
- Campbell, C.; McMillan, H.J.; Mah, J.K.; Tarnopolsky, M.; Selby, K.; McClure, T.; Wilson, D.M.; Sherman, M.L.; Escolar, D.; Attie, K.M. Myostatin inhibitor ACE-031 treatment of ambulatory boys with Duchenne muscular dystrophy: Results of a randomized, placebo-controlled clinical trial. Muscle Nerve 2017, 55, 458–464.
- Pearsall, R.S.; Davies, M.V.; Cannell, M.; Li, J.; Widrick, J.; Mulivor, A.W.; Wallner, S.; Troy, M.E.; Spaits, M.; Liharska, K.; et al. Follistatin-based ligand trap ACE-083 induces localized hypertrophy of skeletal muscle with functional improvement in models of neuromuscular disease. Sci. Rep. 2019, 9, 11392.
- Zhu, J.; Li, Y.; Lu, A.; Gharaibeh, B.; Ma, J.; Kobayashi, T.; Quintero, A.J.; Huard, J. Follistatin improves skeletal muscle healing after injury and disease through an interaction with muscle regeneration, angiogenesis, and fibrosis. Am. J. Pathol. 2011, 179, 915–930.
- Glasser, C.E.; Gartner, M.R.; Wilson, D.; Miller, B.; Sherman, M.L.; Attie, K.M. Locally acting ACE-083 increases muscle volume in healthy volunteers. Muscle Nerve 2018, 57, 921–926.
- Lach-Trifilieff, E.; Minetti, G.C.; Sheppard, K.; Ibebunjo, C.; Feige, J.N.; Hartmann, S.; Brachat, S.; Rivet, H.; Koelbing, C.; Morvan, F.; et al. An antibody blocking activin type II receptors induces strong skeletal muscle hypertrophy and protects from atrophy. Mol. Cell. Biol. 2014, 34, 606–618.
- Rooks, D.; Praestgaard, J.; Hariry, S.; Laurent, D.; Petricoul, O.; Perry, R.G.; Lach-Trifilieff, E.; Roubenoff, R. Treatment of Sarcopenia with Bimagrumab: Results from a Phase II, Randomized, Controlled, Proof-of-Concept Study. J. Am. Geriatr. Soc. 2017, 65, 1988–1995.
- Polkey, M.I.; Praestgaard, J.; Berwick, A.; Franssen, F.M.E.; Singh, D.; Steiner, M.C.; Casaburi, R.; Tillmann, H.C.; Lach-Trifilieff, E.; Roubenoff, R.; et al. Activin Type II Receptor Blockade for Treatment of Muscle Depletion in Chronic Obstructive Pulmonary Disease. A Randomized Trial. Am. J. Respir. Crit. Care Med. 2019, 199, 313–320.
- Hanna, M.G.; Badrising, U.A.; Benveniste, O.; Lloyd, T.E.; Needham, M.; Chinoy, H.; Aoki, M.; Machado, P.M.; Liang, C.; Reardon, K.A.; et al. Safety and efficacy of intravenous bimagrumab in inclusion body myositis (RESILIENT): A randomised, double-blind, placebo-controlled phase 2b trial. Lancet Neurol. 2019, 18, 834–844.
- Rooks, D.; Swan, T.; Goswami, B.; Filosa, L.A.; Bunte, O.; Panchaud, N.; Coleman, L.A.; Miller, R.R.; Garcia Garayoa, E.; Praestgaard, J.; et al. Bimagrumab vs Optimized Standard of Care for Treatment of Sarcopenia in Community-Dwelling Older Adults: A Randomized Clinical Trial. JAMA Netw. Open 2020, 3, e2020836.
- Kumar, A.; Davuluri, G.; Silva, R.N.E.; Engelen, M.; Ten Have, G.A.M.; Prayson, R.; Deutz, N.E.P.; Dasarathy, S. Ammonia lowering reverses sarcopenia of cirrhosis by restoring skeletal muscle proteostasis. Hepatology 2017, 65, 2045–2058.
- Butterworth, R.F. L-Ornithine L-Aspartate for the Treatment of Sarcopenia in Chronic Liver Disease: The Taming of a Vicious Cycle. Can. J. Gastroenterol. Hepatol. 2019, 2019, 8182195.
- Hey, P.; Gow, P.; Testro, A.G.; Apostolov, R.; Chapman, B.; Sinclair, M. Nutraceuticals for the treatment of sarcopenia in chronic liver disease. Clin. Nutr. ESPEN 2021, 41, 13–22.
- Owen, O.E.; Reichle, F.A.; Mozzoli, M.A.; Kreulen, T.; Patel, M.S.; Elfenbein, I.B.; Golsorkhi, M.; Chang, K.H.; Rao, N.S.; Sue, H.S.; et al. Hepatic, gut, and renal substrate flux rates in patients with hepatic cirrhosis. J. Clin. Investig. 1981, 68, 240–252.
- Kabadi, U.M. The association of hepatic glycogen depletion with hyperammonemia in cirrhosis. Hepatology 1987, 7, 821–824.
- Anand, A.C. Nutrition and Muscle in Cirrhosis. J. Clin. Exp. Hepatol. 2017, 7, 340–357.
- Cabre, E.; Abad-Lacruz, A.; Nunez, M.C.; Gonzalez-Huix, F.; Fernandez-Banares, F.; Gil, A.; Esteve-Comas, M.; Moreno, J.; Planas, R.; Guilera, M.; et al. The relationship of plasma polyunsaturated fatty acid deficiency with survival in advanced liver cirrhosis: Multivariate analysis. Am. J. Gastroenterol. 1993, 88, 718–722.
- Enguita, M.; Razquin, N.; Pamplona, R.; Quiroga, J.; Prieto, J.; Fortes, P. The cirrhotic liver is depleted of docosahexaenoic acid (DHA), a key modulator of NF-kappaB and TGFbeta pathways in hepatic stellate cells. Cell Death Dis. 2019, 10, 14.
- Bischoff, S.C.; Bernal, W.; Dasarathy, S.; Merli, M.; Plank, L.D.; Schutz, T.; Plauth, M. ESPEN practical guideline: Clinical nutrition in liver disease. Clin. Nutr. 2020, 39, 3533–3562.
- Tsien, C.D.; McCullough, A.J.; Dasarathy, S. Late evening snack: Exploiting a period of anabolic opportunity in cirrhosis. J. Gastroenterol. Hepatol. 2012, 27, 430–441.
- Plank, L.D.; Gane, E.J.; Peng, S.; Muthu, C.; Mathur, S.; Gillanders, L.; McIlroy, K.; Donaghy, A.J.; McCall, J.L. Nocturnal nutritional supplementation improves total body protein status of patients with liver cirrhosis: A randomized 12-month trial. Hepatology 2008, 48, 557–566.
- Norman, K.; Kirchner, H.; Freudenreich, M.; Ockenga, J.; Lochs, H.; Pirlich, M. Three month intervention with protein and energy rich supplements improve muscle function and quality of life in malnourished patients with non-neoplastic gastrointestinal disease—A randomized controlled trial. Clin. Nutr. 2008, 27, 48–56.
- Manguso, F.; D’Ambra, G.; Menchise, A.; Sollazzo, R.; D’Agostino, L. Effects of an appropriate oral diet on the nutritional status of patients with HCV-related liver cirrhosis: A prospective study. Clin. Nutr. 2005, 24, 751–759.
- Les, I.; Doval, E.; Garcia-Martinez, R.; Planas, M.; Cardenas, G.; Gomez, P.; Flavia, M.; Jacas, C.; Minguez, B.; Vergara, M.; et al. Effects of branched-chain amino acids supplementation in patients with cirrhosis and a previous episode of hepatic encephalopathy: A randomized study. Am. J. Gastroenterol. 2011, 106, 1081–1088.
- Marchesini, G.; Bianchi, G.; Merli, M.; Amodio, P.; Panella, C.; Loguercio, C.; Rossi Fanelli, F.; Abbiati, R.; Italian, B.S.G. Nutritional supplementation with branched-chain amino acids in advanced cirrhosis: A double-blind, randomized trial. Gastroenterology 2003, 124, 1792–1801.
- Muto, Y.; Sato, S.; Watanabe, A.; Moriwaki, H.; Suzuki, K.; Kato, A.; Kato, M.; Nakamura, T.; Higuchi, K.; Nishiguchi, S.; et al. Effects of oral branched-chain amino acid granules on event-free survival in patients with liver cirrhosis. Clin. Gastroenterol. Hepatol. 2005, 3, 705–713.
- Plauth, M.; Egberts, E.H.; Hamster, W.; Torok, M.; Muller, P.H.; Brand, O.; Furst, P.; Dolle, W. Long-term treatment of latent portosystemic encephalopathy with branched-chain amino acids. A double-blind placebo-controlled crossover study. J. Hepatol. 1993, 17, 308–314.
- Lo, J.H.T.; Pong, U.K.; Yiu, T.; Ong, M.T.Y.; Lee, W.Y.W. Sarcopenia: Current treatments and new regenerative therapeutic approaches. J. Orthop. Transl. 2020, 23, 38–52.
- Vahidinia, Z.; Azami Tameh, A.; Nejati, M.; Beyer, C.; Talaei, S.A.; Etehadi Moghadam, S.; Atlasi, M.A. The protective effect of bone marrow mesenchymal stem cells in a rat model of ischemic stroke via reducing the C-Jun N-terminal kinase expression. Pathol. Res. Pract. 2019, 215, 152519.
- Dabrowska, S.; Andrzejewska, A.; Lukomska, B.; Janowski, M. Neuroinflammation as a target for treatment of stroke using mesenchymal stem cells and extracellular vesicles. J. Neuroinflamm. 2019, 16, 178.
- Reza-Zaldivar, E.E.; Hernandez-Sapiens, M.A.; Gutierrez-Mercado, Y.K.; Sandoval-Avila, S.; Gomez-Pinedo, U.; Marquez-Aguirre, A.L.; Vazquez-Mendez, E.; Padilla-Camberos, E.; Canales-Aguirre, A.A. Mesenchymal stem cell-derived exosomes promote neurogenesis and cognitive function recovery in a mouse model of Alzheimer’s disease. Neural Regen. Res. 2019, 14, 1626–1634.
- Cunningham, C.J.; Redondo-Castro, E.; Allan, S.M. The therapeutic potential of the mesenchymal stem cell secretome in ischaemic stroke. J. Cereb. Blood Flow Metab. 2018, 38, 1276–1292.
- Park, C.W.; Kim, K.S.; Bae, S.; Son, H.K.; Myung, P.K.; Hong, H.J.; Kim, H. Cytokine secretion profiling of human mesenchymal stem cells by antibody array. Int. J. Stem Cells 2009, 2, 59–68.
- Pourmohammadi-Bejarpasi, Z.; Roushandeh, A.M.; Saberi, A.; Rostami, M.K.; Toosi, S.M.R.; Jahanian-Najafabadi, A.; Tomita, K.; Kuwahara, Y.; Sato, T.; Roudkenar, M.H. Mesenchymal stem cells-derived mitochondria transplantation mitigates I/R-induced injury, abolishes I/R-induced apoptosis, and restores motor function in acute ischemia stroke rat model. Brain Res. Bull. 2020, 165, 70–80.




