
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Annie Ladoux | + 3059 word(s) | 3059 | 2021-02-04 04:15:51 |
Video Upload Options
Adipose tissue resides in specific depots scattered in peripheral or deeper locations all over the body and it enwraps most of the organs. This tissue is always in a dynamic evolution as it must adapt to the metabolic demand and constraints. It exhibits also endocrine functions important to regulate energy homeostasis. This complex organ is composed of depots able to produce opposite functions to monitor energy: the so called white adipose tissue acts to store energy as triglycerides preventing ectopic fat deposition while the brown adipose depots dissipate it. It is composed of many cell types. Different types of adipocytes constitute the mature cells specialized to store or burn energy. Immature adipose progenitors (AP) presenting stem cells properties contribute not only to the maintenance but also to the expansion of this tissue as observed in overweight or obese individuals.
1. Introduction
Adipose tissue is the most expandable tissue of the organism as it plays a crucial role in controlling energy storage and release in the vertebrates. Besides its physiological function, it has a mechanical function as a cushion and insulates the body from cold and heat. This tissue extends when the energy intake is more important than the energy expenditure. An excessive fat storage leading to overweight and subsequent obesity participates to health disorders. Risk factors including abnormal plasma cholesterol and/or triglyceride levels, excess of body fat around the waist, insulin resistance, and hypertension promote the acquisition of the metabolic syndrome with an increased probability of heart disease, stroke, and type 2 diabetes [1] . In addition, a link between obesity and a high cancer incidence exists for many types of cancers [2]. As more than 1.9 billion individuals were considered overweight by the World Health Organization in 2016 (https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight), the obesity epidemics is a major Public Health concern. On the other hand, a group of genetic or therapeutically induced disorders called lipodystrophies [3], whereby the body is unable to produce healthy and functional fat mass, preventing then lipid storage, recapitulates also features leading to metabolic syndrome development.
Adipose tissue is widely distributed all over the human body where it enwraps most organs that instead have a precise location. Subcutaneous adipose tissue (SAT) is mainly located in the buttocks, the tights, and the abdomen. Inside the abdomen, adipose tissue is present in three prevalent locations i.e., attached to the stomach (omental), to the intestine (mesenteric) and behind the kidneys (retroperirenal) [4]. Other organs host adipose tissue such as the bone marrow and the breasts. It also resides in the face, around the heart (pericardial), or the vessels (perivascular and periarterial). These many locations suggest that all depots are not equivalent. Adipose tissue is composed of many cell types organized as structural units called lobules. Adipocytes, i.e., cells presenting lipid droplets where lipids are stored as triglycerides, represent the major cell type. In addition, adipose progenitors (AP), pericytes, blood vessels, nerves, and various immune cells constitute the stroma vascular fraction (SVF), which is crucial for adipose tissue development and adaptation to metabolic constraints [5]. These processes mainly rely on the adipose immature stromal cells that retain characteristics of mesenchymal stem cells. They are endowed with an extensive regeneration potential that has drawn a special consideration for cellular therapy and regenerative medicine.
2. Distinct Adipose Tissue Functions Are Supported by Different Types of Adipocytes
2.1. White Adipose Tissue and Adipocytes
For long, white adipose tissue (WAT) has been known as a dynamic lipid storage tissue able to remodel the size of the cells to comply with the metabolic demand and to control energy homeostasis. WAT represents the major adipose tissue mass and it is located in many intra-abdominal depots and subcutaneously. Adipocytes are involved in this process to avoid ectopic fat storage that results in the metabolic syndrome [6]. Because fatty acids and their metabolites can be toxic, lipids are stored as triglycerides in a single fat vacuole that occupies the whole cytoplasm of the adipocyte and is surrounded by specific proteins, perilipin 1 (PLIN1) being the most abundant [7]. They are released upon fasting. Storage of an excess of energy relies on two mechanisms: adipocyte hypertrophy (increase in size) and hyperplasia (increase in number). Then, the adipose tissue mass expands leading to overweight and obesity. Several processes are involved in this adaptation such as adipogenesis, lipolysis, and lipogenesis. In addition to this property, adipose tissue is endowed with endocrine function through secretion of adipocytokines, which control important physiological functions such as appetite, metabolism, immune response, or reproduction [8]. Leptin (LEP), which is involved in the hypothalamic regulation of food intake, was first identified in 1994 [9]. Adiponectin (ADIPOQ) plays a crucial role in controlling glucose levels and fatty acid oxidation [10][11] (see figure 1). Other cytokines such as apelin, visfatin, and cytokines related to the immune system, i.e., interleukin-1β (IL-1β), interleukin-6 (IL-6), tumor necrosis factor-alpha (TNF-α), and monocyte chemotactic protein-1 (MCP-1) are also produced by adipocytes under certain circumstances [12][13]. During obesity, a state of chronic inflammation is associated with a modification of the level of production of these adipokines resulting in the development of a state of insulin resistance associated with this syndrome.
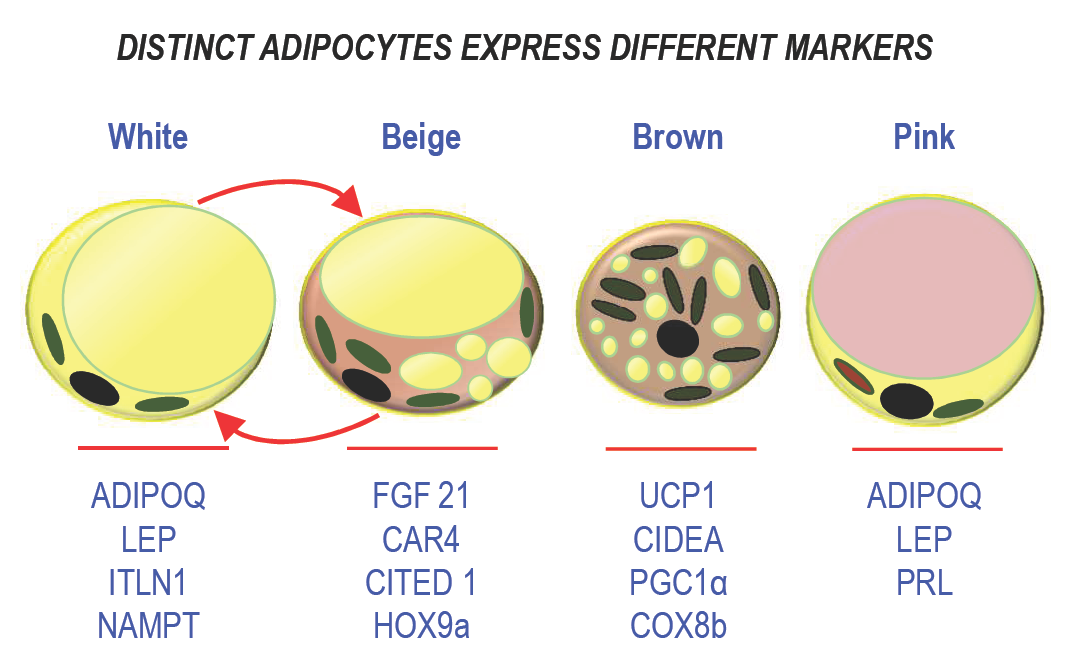
Figure 1: Schematic representation of the different types of adipocytes. The main markers are indicated below each type of adipocyte.
2.2. Brown Adipose Tissue and Adipocytes
Besides WAT, an adipose tissue with opposite metabolic function, i.e., the brown adipose tissue (BAT) exists. It dissipates energy in a heat-producing process called thermogenesis, the primary source of energy being fatty acids. This process is involved in a proper maintain of the body temperature. In humans, BAT depots are found in fetuses, and for long, it was considered to be limited to newborns in perirenal, periadrenal, axillary, and cervical regions [14]. After birth, it atrophies and its presence rapidly declines but depots remain preferentially in the upper part of the body, i.e., in the supraclavicular region, in the upper part of the body (neck and face [15][16]), and around deep organs (such as the kidneys [17]). It was traditionally considered insignificant in adults, except for subjects exposed to cold climates for a long time or those affected by pheochromocytoma. BAT depots are metabolically active as they can respond to cold or to catecholamines stimulation. Note that sexual dimorphisms were reported for BAT depots in humans, but without any significant impact on its activation [18].
Brown adipocytes display a different morphology as they own multiple fat vacuoles and abundant mitochondria in their cytoplasm. Dissipation of energy occurs through the uncoupling protein 1 (UCP1), which uses the mitochondrial proton gradient to produce heat instead of ATP [19]. Indeed, the mitochondria-enriched adipocytes are active as they are able to respond to cold exposure and to β-adrenergic stimulation through β3-adrenergic receptor activation. As WAT, BAT is a secretory organ. Its secretory profile is quite distinct from WAT, although BAT secretes classical adipokines such as leptin. Batokines, i.e., adipokines secreted by BAT, comprise fibroblast growth factor 21 (FGF21), IL6, bone morphogenic protein 8b (BMP-8b), and endothelin 1 [20].
2.3. Beige Adipose Tissue and Adipocytes
Recently, a new class of adipocytes, called beige (or brite) adipocytes was described. They display properties of brown adipocytes, but they are located within WAT depots. Note that in contrast to brown adipocytes, the expression of UCP1 is not constitutive in beige adipocytes, but inducible [21]. In addition to this unusual location for thermogenic adipocytes, they differ also from brown adipocytes in their developmental program as these adipocytes derive from white adipocytes. Upon stimulation, these cells are able to dissipate energy and to produce heat, like observed for brown adipocytes. WAT browning occurs almost exclusively in subcutaneous (SAT) depots in humans [22], which is another difference within the WAT depots. These adipocytes share common secreted batokines with BAT such as FGF21. Indeed, some adipokines are preferentially expressed in beige cells, for instance Meteorin-like, which contributes to an increase in beige thermogenesis in mice [23].
WAT browning represents a promising therapeutic strategy to combat obesity, owing that this mechanism occurs in obese patients upon stimulation with an efficient and well tolerated drug that remains to be identified [24].
2.4. Breast Adipose Tissue and Pink Adipocytes
Breast is composed of epithelial ducts associated with adipose lobules and the adipose tissue is the major contributor to the volume of the breast, and it plays a crucial role in the morphogenesis of mammary glands. Within the breast, special female-specific adipocytes were described and called “pink adipocytes.” During pregnancy, the size of mammary adipocytes increases to store lipids [25][26]. Lipogenesis stops when lactation starts. Then, mammary adipocytes trans-differentiate into secretory epithelial cells to promote lipid transfer during milk production. This process participates actively to breast remodeling as breast adipocytes display an extraordinary plasticity. Breast adipose tissue is a secretory organ like the other depots. It produces prolactin and steroid hormones like estrogens thanks to an aromatase. In addition, it participates in epithelial cell growth, angiogenesis, intercellular communication, and milk production [27], and it secretes many growth factors and enzymes involved in beast reshaping during development [28]. Distinct endocrine features can also be exemplified as follow. For instance, WAT in the breast and also in the buttocks is sensitive to estrogens, in contrast to WAT in the upper back which is more sensitive to glucocorticoids [27][29]. This unique association between adipose tissue and the mammary epithelium outlines functional differences with other body fat depots.
All together, these simple observations raise an important notion regarding adipose tissue biology. Depending on their location all over the human body, the distinct fat depots harbor different adipocyte populations with proper functional characteristics that are recapitulated in Figure 2.
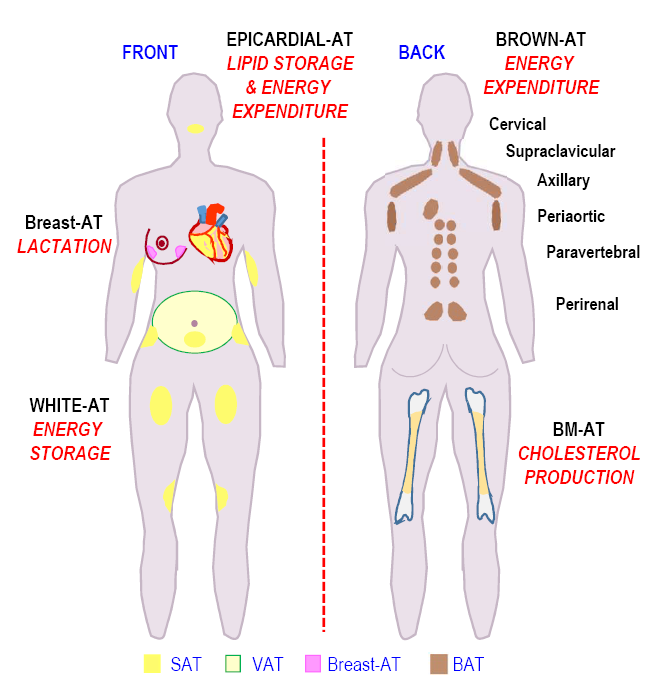 Figure 2. Locations of the adipose depots associated with their functions.
Figure 2. Locations of the adipose depots associated with their functions.
Schematic representation of the fat depots locations in the human body is shown in Figure 2. The main metabolic role of each adipose fat pad is mentioned. (Adipose tissue (AT), subcutaneous adipose tissue (SAT), visceral adipose tissue (VAT), Brown adipose tissue (BAT), Bone marrow adipose tissue (BM-AT)).
3. Distinctive features of some WAT depots.
Although the role in energy storage and release is well established for WAT, these many locations point out the existence of peculiar endocrine or metabolic features and one should consider that the intrinsic functions of the distinct depots are related to these specificities.
3.1. Visceral (VAT) and subcutaneous WAT depots.
The physiological importance of WAT is specially underlined in two opposite situations: obesity characterized by an excess of adipose tissue and lipodystrophy (and/or lipoatrophy) which corresponds to generalized or partial adipose tissue deficiency that depends on the degree and the location of fat loss [30]. For instance, mutations in genes important for adipose differentiation such as peroxisome proliferator-activated receptor-γ (PPARγ), or protein kinase B (AKT2) as well as genes essential for lipid droplets structure and lipolysis such as perilipin 1 (PLIN1) cause fat loss mainly in the sub-cutaneous adipose tissue from the extremities [3][30]. This last observation reinforces the idea of fat depots disparity.
An excess of circulating fatty acids, encountered in both situations, contributes to ectopic fat accumulation and to insulin resistance which participates to the development of the metabolic syndrome. WAT belongs to the insulin-responsive tissues, together with muscles and the liver. Insulin displays two main functions in white adipocytes through binding and activation of a specific receptor. First, it increases glucose uptake and participates to regulation of glycaemia. Insulin resistance is accompanied by a reduction of the glucose transporter 4 translocation that attenuates glucose metabolism. Second, it inhibits lipolysis, a process which is exquisitely sensitive to insulin in adipocytes. This process is crucial to avoid inappropriate fat storage leading to lipid overload, ectopic fat deposition and aberrant high concentrations of plasmatic non esterified fatty acids (NEFA). These situations are mainly encountered in poorly controlled Type 2 diabetes with the risk of cardiovascular disease development. They are generally associated with an expansion of the visceral adipose tissue mass.
A higher rate of lipolysis was observed in the visceral (omental) adipose tissue as compared to the subcutaneous (femoral/gluteal) adipose depots more than 20 years ago [31] and this contributes to increase the plasma fatty acids levels [32]. Higher lipolysis may result from distinct sensitivities to beta adrenergic stimuli or to insulin which exerts a predominant anti-lipolytic effect in vivo. No clear role could be assigned to lipases and proteins associated to lipid droplets in this observation, except for an obese situation [33]. As SAT is less active than VAT in a metabolic context, this depot is endowed with a higher lipid storage capacity. This is relevant for two situations: supplying energy as FFAs in period of starvation or exercise and/or storing lipids to protect the tissues from lipotoxicity after rich-lipid dietary intake. In addition to differences in lipolysis, differences in free fatty acid uptake also exist between VAT and SAT: VAT being more efficient than SAT in humans [34]. Thus lipid turn over may be higher in VAT as compared to SAT as it is endowed with higher uptake capacity and higher lipolysis rate. Differential adipokines production has been observed for VAT and SAT. The intra-abdominal WAT display respectively an adipocytokine secretion profile related to inflammation and type-2 diabetes, whereas the subcutaneous WAT secretes less pro-inflammatory cytokines and more leptin [35]. Note that molecules involved in innate immunity and acute phase response are preferentially produced in VAT [36].
3.2. Gluteofemoral AT
The gluteofemoral fat is found on the buttocks, thighs and hips. Women preferentially accumulate fat in this region as compared to men. This depot is the main source of Long Chain Poly Unsaturated Fatty Acids (LCPUFAs) that are essential for development during pregnancy. It is able to release these fatty acids during lactation leading then to a reduction of this depot [37]. In addition, many studies confirm the protective properties of the gluteofemoral fat as compared to visceral fat for the development of Type 2 Diabetes (T2D) and its associated cardiometabolic risks. This is especially observed for patients affected with lipodytrophic syndromes, such as Dunnigan syndrome, that is accompanied by fat loss in the lower part of the body associated with insulin resistance, an abnormal lipid profile and T2D [38][39].
This tissue has reduced lipid turnover which is in lane with a slower fat redistribution [40][41] .
3.3. Bone Marrow AT
Human bone marrow AT (BM-AT) location is restricted to the bones and it represents more than 10% of the adipose mass. As its microenvironment mainly consists in hematopoietic and skeletal mature or progenitor cells it plays an active role in the regulation of hematopoiesis and bone formation. Indeed, bone marrow adipocytes (BM-adipocytes) appear as negative regulators of hematopoiesis by promoting quiescence of progenitor cells [42]. This tissue increases with age and people with prevalent vertebral fractures display higher mean BM-AT [43]. As for the other fat depots; human BM-adipocytes depots secrete adipokines [44]. Leptin has a positive effect on osteogenesis as it increases bone mineral density [45] while adiponectin acts as an anti-osteogenic agent [46].
BM-adipocytes are unilocular cells that do not express brown or beige markers even in animals or humans exposed to cold. Indeed, they exhibit a peculiar lipid metabolism as they displayed higher glucose uptake than other white fat pads. BM-AT is a major site of basal glucose uptake in humans, although BM-adipocytes resist to insulin stimulated-glucose uptake [47]. In contrast to sub-cutaneous adipocytes, these native adipocytes are devoid of lipolytic activity as they cannot release free fatty acids upon adrenergic stimulation. In consequence, they display a higher content in monoacylglycerol as well as in cholesterol [48].
3.4. Epicardial and pericardial AT
Specific adipose tissue depots are located around the heart. They were poorly studied for an access restricted to small scraps obtained after open cardiac surgery.
Pericardial adipose tissue (PAT) is located between the two pericardial layers and owns a vascularization that originates from the internal mammary artery [49]. Epicardial adipose tissue (EAT) is a visceral thoracic fat depot in direct contact with the myocardium and coronary arteries [50]. Its vascularization is issued from coronary arteries. It is composed of small adipocytes, presenting brown adipose tissue features through expression of UCP1. This thermogenic profile has been suggested to regulate the temperature of the myocardium [51] and is intended to protect the myocardium from the occurrence of fatal ventricular arrhythmias in case of cold exposure. This tissue displays higher lipogenic and lipolytic rates as compared with other fat depots. Hence, EAT is more efficient to store or release lipids on demand [52]. As other fat depots, EAT releases polypeptides such as adiponectin [53] and adrenomedullin [54] which have cardioprotective properties as well as inflammatory cytokines [55] that may play a role in the development of heart diseases. As compared to SAT, EAT is characterized by a marked expression of transcription factors associated with cardiac development and function. At this point, it is unclear whether this signature corresponds to a memory of the tissue location of if it plays a role in the cardiac function. EAT displays also an overrepresentation of immune-related genes expression. However, there is an inverse correlation between a high UCP1 expression and expression of immune-related genes, especially those correlated with T cells responses and the adaptive immunity. The number of genes with a modified expression in obese versus lean patients is scant [52]. It secretes as well more matrix metalloproteases than SAT, i. e. enzymes that remodel the extracellular matrix.
In humans, an expansion of these tissues promotes the onset, the progression and the severity of coronary arterial disease and atrial fibrillations. Thus, beneficial effects of their reduction on the management of coronary arterial disease or cardiac rhythm disorders need to be further investigated with randomized controlled studies.
4. Conclusion
In conclusion, adipose tissue, which is scattered in the human body, is the cornerstone to control metabolism. This tissue, displays an extraordinary ability to adapt to metabolic constraints. This flexibility relies on several types of adipocytes, that all contain lipid droplets, exhibit some common markers such as perilipin, leptin, adiponectin… but are able either to store or to dissipate energy. Then, these two opposite functions provide an accurate balance to fit with physio-pathological conditions. Hence, distinct adipose depots are not equal as they have their own specificity. All the major differences concern lipid metabolism, the thermogenic ability and the interactions with other systems. For instance, BM-AT is specialized in cholesterol production and is less prone to lipolysis than SAT. EAT is characterized by a general activation of immune related pathways. However high UCP1 expression in this tissue was associated with down regulation of genes involved in the production of reactive oxygen species and immune responses. Better characterization of the distinct cell types of each depot may give crucial information to develop therapeutic opportunities to understand and treat metabolic diseases.
References
- Kopelman, P.G. Obesity as a medical problem. Nature 2000, 404, 635-643, doi:10.1038/35007508.
- Lauby-Secretan, B.; Scoccianti, C.; Loomis, D.; Grosse, Y.; Bianchini, F.; Straif, K.; International Agency for Research on Cancer Handbook Working, G. Body Fatness and Cancer--Viewpoint of the IARC Working Group. The New England journal of medicine 2016, 375, 794-798, doi:10.1056/NEJMsr1606602.
- Garg, A. Clinical review#: Lipodystrophies: genetic and acquired body fat disorders. The Journal of clinical endocrinology and metabolism 2011, 96, 3313-3325.
- Cinti, S. Adipocyte differentiation and transdifferentiation: plasticity of the adipose organ. Journal of endocrinological investigation 2002, 25, 823-835, doi:10.1007/BF03344046.
- Rodeheffer, M.S.; Birsoy, K.; Friedman, J.M. Identification of white adipocyte progenitor cells in vivo. Cell 2008, 135, 240-249, doi:10.1016/j.cell.2008.09.036.
- Ravussin, E.; Smith, S.R. Increased fat intake, impaired fat oxidation, and failure of fat cell proliferation result in ectopic fat storage, insulin resistance, and type 2 diabetes mellitus. Annals of the New York Academy of Sciences 2002, 967, 363-378, doi:10.1111/j.1749-6632.2002.tb04292.x.
- Brasaemle, D.L. Thematic review series: adipocyte biology. The perilipin family of structural lipid droplet proteins: stabilization of lipid droplets and control of lipolysis. J Lipid Res 2007, 48, 2547-2559, doi:10.1194/jlr.R700014-JLR200.
- Trayhurn, P.; Wood, I.S. Adipokines: inflammation and the pleiotropic role of white adipose tissue. The British journal of nutrition 2004, 92, 347-355, doi:10.1079/bjn20041213.
- Zhang, Y.; Proenca, R.; Maffei, M.; Barone, M.; Leopold, L.; Friedman, J.M. Positional cloning of the mouse obese gene and its human homologue. Nature 1994, 372, 425-432, doi:10.1038/372425a0.
- Wang, Z.V.; Scherer, P.E. Adiponectin, the past two decades. J Mol Cell Biol 2016, 8, 93-100, doi:10.1093/jmcb/mjw011.
- Yanai, H.; Yoshida, H. Beneficial Effects of Adiponectin on Glucose and Lipid Metabolism and Atherosclerotic Progression: Mechanisms and Perspectives. International journal of molecular sciences 2019, 20, doi:10.3390/ijms20051190.
- Cao, H. Adipocytokines in obesity and metabolic disease. J Endocrinol 2014, 220, T47-59, doi:10.1530/JOE-13-0339.
- Ouchi, N.; Parker, J.L.; Lugus, J.J.; Walsh, K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol 2011, 11, 85-97, doi:10.1038/nri2921.
- Lidell, M.E. Brown Adipose Tissue in Human Infants. Handbook of experimental pharmacology 2019, 251, 107-123, doi:10.1007/164_2018_118.
- Cypess, A.M.; White, A.P.; Vernochet, C.; Schulz, T.J.; Xue, R.; Sass, C.A.; Huang, T.L.; Roberts-Toler, C.; Weiner, L.S.; Sze, C., et al. Anatomical localization, gene expression profiling and functional characterization of adult human neck brown fat. Nature medicine 2013, 19, 635-639, doi:10.1038/nm.3112.
- Kouidhi, M.; Villageois, P.; Mounier, C.M.; Menigot, C.; Rival, Y.; Piwnica, D.; Aubert, J.; Chignon-Sicard, B.; Dani, C. Characterization of human knee and chin adipose-derived stromal cells. Stem cells international 2015, 2015, 592090, doi:10.1155/2015/592090.
- Svensson, P.A.; Lindberg, K.; Hoffmann, J.M.; Taube, M.; Pereira, M.J.; Mohsen-Kanson, T.; Hafner, A.L.; Rizell, M.; Palming, J.; Dani, C., et al. Characterization of brown adipose tissue in the human perirenal depot. Obesity 2014, 22, 1830-1837, doi:10.1002/oby.20765.
- Fletcher, L.A.; Kim, K.; Leitner, B.P.; Cassimatis, T.M.; O'Mara, A.E.; Johnson, J.W.; Halprin, M.S.; McGehee, S.M.; Brychta, R.J.; Cypess, A.M., et al. Sexual Dimorphisms in Adult Human Brown Adipose Tissue. Obesity 2020, 28, 241-246, doi:10.1002/oby.22698.
- Klingenberg, M. Uncoupling protein--a useful energy dissipator. Journal of bioenergetics and biomembranes 1999, 31, 419-430.
- Villarroya, F.; Cereijo, R.; Villarroya, J.; Giralt, M. Brown adipose tissue as a secretory organ. Nature reviews. Endocrinology 2017, 13, 26-35, doi:10.1038/nrendo.2016.136.
- Jash, S.; Banerjee, S.; Lee, M.J.; Farmer, S.R.; Puri, V. CIDEA Transcriptionally Regulates UCP1 for Britening and Thermogenesis in Human Fat Cells. iScience 2019, 20, 73-89, doi:10.1016/j.isci.2019.09.011.
- Sidossis, L.; Kajimura, S. Brown and beige fat in humans: thermogenic adipocytes that control energy and glucose homeostasis. J Clin Invest 2015, 125, 478-486, doi:10.1172/JCI78362.
- Rao, R.R.; Long, J.Z.; White, J.P.; Svensson, K.J.; Lou, J.; Lokurkar, I.; Jedrychowski, M.P.; Ruas, J.L.; Wrann, C.D.; Lo, J.C., et al. Meteorin-like is a hormone that regulates immune-adipose interactions to increase beige fat thermogenesis. Cell 2014, 157, 1279-1291, doi:10.1016/j.cell.2014.03.065.
- Kwok, K.H.; Lam, K.S.; Xu, A. Heterogeneity of white adipose tissue: molecular basis and clinical implications. Experimental & molecular medicine 2016, 48, e215, doi:10.1038/emm.2016.5.
- Cinti, S. Pink Adipocytes. Trends Endocrinol Metab 2018, 29, 651-666, doi:10.1016/j.tem.2018.05.007.
- Wang, Q.A.; Song, A.; Chen, W.; Schwalie, P.C.; Zhang, F.; Vishvanath, L.; Jiang, L.; Ye, R.; Shao, M.; Tao, C., et al. Reversible De-differentiation of Mature White Adipocytes into Preadipocyte-like Precursors during Lactation. Cell metabolism 2018, 28, 282-288 e283, doi:10.1016/j.cmet.2018.05.022.
- Hovey, R.C.; Aimo, L. Diverse and active roles for adipocytes during mammary gland growth and function. J Mammary Gland Biol Neoplasia 2010, 15, 279-290, doi:10.1007/s10911-010-9187-8.
- Kothari, C.; Diorio, C.; Durocher, F. The Importance of Breast Adipose Tissue in Breast Cancer. International journal of molecular sciences 2020, 21, doi:10.3390/ijms21165760.
- Tchkonia, T.; Giorgadze, N.; Pirtskhalava, T.; Tchoukalova, Y.; Karagiannides, I.; Forse, R.A.; DePonte, M.; Stevenson, M.; Guo, W.; Han, J., et al. Fat depot origin affects adipogenesis in primary cultured and cloned human preadipocytes. Am J Physiol Regul Integr Comp Physiol 2002, 282, R1286-1296, doi:10.1152/ajpregu.00653.2001.
- Nolis, T. Exploring the pathophysiology behind the more common genetic and acquired lipodystrophies. Journal of human genetics 2014, 59, 16-23, doi:10.1038/jhg.2013.107.
- Arner, P. Differences in lipolysis between human subcutaneous and omental adipose tissues. Annals of medicine 1995, 27, 435-438, doi:10.3109/07853899709002451.
- Hajer, G.R.; van Haeften, T.W.; Visseren, F.L. Adipose tissue dysfunction in obesity, diabetes, and vascular diseases. European heart journal 2008, 29, 2959-2971, doi:10.1093/eurheartj/ehn387.
- Wang, Y.; Sullivan, S.; Trujillo, M.; Lee, M.J.; Schneider, S.H.; Brolin, R.E.; Kang, Y.H.; Werber, Y.; Greenberg, A.S.; Fried, S.K. Perilipin expression in human adipose tissues: effects of severe obesity, gender, and depot. Obesity research 2003, 11, 930-936, doi:10.1038/oby.2003.128.
- Jensen, M.D.; Sarr, M.G.; Dumesic, D.A.; Southorn, P.A.; Levine, J.A. Regional uptake of meal fatty acids in humans. Am J Physiol Endocrinol Metab 2003, 285, E1282-1288, doi:10.1152/ajpendo.00220.2003.
- Lee, M.J.; Wu, Y.; Fried, S.K. Adipose tissue remodeling in pathophysiology of obesity. Curr Opin Clin Nutr Metab Care 2010, 13, 371-376, doi:10.1097/MCO.0b013e32833aabef.
- Lee, M.J.; Wu, Y.; Fried, S.K. Adipose tissue heterogeneity: implication of depot differences in adipose tissue for obesity complications. Molecular aspects of medicine 2013, 34, 1-11, doi:10.1016/j.mam.2012.10.001.
- Kramer, K., F. M.; Stunkard, A.J.; Marshall, K.A.; McKinney, S.; Liebschutz, J. Breast-feeding reduces maternal lower-body fat. Journal of the American Dietetic Association 1993, 93, 429-433, doi:10.1016/0002-8223(93)92289-a.
- Garg, A. Acquired and inherited lipodystrophies. The New England journal of medicine 2004, 350, 1220-1234, doi:10.1056/NEJMra025261.
- Garg, A.; Peshock, R.M.; Fleckenstein, J.L. Adipose tissue distribution pattern in patients with familial partial lipodystrophy (Dunnigan variety). The Journal of clinical endocrinology and metabolism 1999, 84, 170-174, doi:10.1210/jcem.84.1.5383.
- Piche, M.E.; Vasan, S.K.; Hodson, L.; Karpe, F. Relevance of human fat distribution on lipid and lipoprotein metabolism and cardiovascular disease risk. Current opinion in lipidology 2018, 29, 285-292, doi:10.1097/MOL.0000000000000522.
- Piche, M.E.; Parry, S.A.; Karpe, F.; Hodson, L. Chylomicron-Derived Fatty Acid Spillover in Adipose Tissue: A Signature of Metabolic Health? The Journal of clinical endocrinology and metabolism 2018, 103, 25-34, doi:10.1210/jc.2017-01517.
- Naveiras, O.; Nardi, V.; Wenzel, P.L.; Hauschka, P.V.; Fahey, F.; Daley, G.Q. Bone-marrow adipocytes as negative regulators of the haematopoietic microenvironment. Nature 2009, 460, 259-263, doi:10.1038/nature08099.
- Aparisi Gomez, M.P.; Ayuso Benavent, C.; Simoni, P.; Aparisi, F.; Guglielmi, G.; Bazzocchi, A. Fat and bone: the multiperspective analysis of a close relationship. Quantitative imaging in medicine and surgery 2020, 10, 1614-1635, doi:10.21037/qims.2020.01.11.
- Sulston, R.J.; Cawthorn, W.P. Bone marrow adipose tissue as an endocrine organ: close to the bone? Hormone molecular biology and clinical investigation 2016, 28, 21-38, doi:10.1515/hmbci-2016-0012.
- Lecka-Czernik, B. Marrow fat metabolism is linked to the systemic energy metabolism. Bone 2012, 50, 534-539, doi:10.1016/j.bone.2011.06.032.
- Ambrosi, T.H.; Schulz, T.J. The emerging role of bone marrow adipose tissue in bone health and dysfunction. Journal of molecular medicine 2017, 95, 1291-1301, doi:10.1007/s00109-017-1604-7.
- Suchacki, K.J.; Tavares, A.A.S.; Mattiucci, D.; Scheller, E.L.; Papanastasiou, G.; Gray, C.; Sinton, M.C.; Ramage, L.E.; McDougald, W.A.; Lovdel, A., et al. Bone marrow adipose tissue is a unique adipose subtype with distinct roles in glucose homeostasis. Nature communications 2020, 11, 3097, doi:10.1038/s41467-020-16878-2.
- Attane, C.; Esteve, D.; Chaoui, K.; Iacovoni, J.S.; Corre, J.; Moutahir, M.; Valet, P.; Schiltz, O.; Reina, N.; Muller, C. Human Bone Marrow Is Comprised of Adipocytes with Specific Lipid Metabolism. Cell reports 2020, 30, 949-958 e946, doi:10.1016/j.celrep.2019.12.089.
- Iacobellis, G.; Corradi, D.; Sharma, A.M. Epicardial adipose tissue: anatomic, biomolecular and clinical relationships with the heart. Nature clinical practice. Cardiovascular medicine 2005, 2, 536-543, doi:10.1038/ncpcardio0319.
- Gaborit, B.; Sengenes, C.; Ancel, P.; Jacquier, A.; Dutour, A. Role of Epicardial Adipose Tissue in Health and Disease: A Matter of Fat? Comprehensive Physiology 2017, 7, 1051-1082, doi:10.1002/cphy.c160034.
- Sacks, H.S.; Fain, J.N.; Holman, B.; Cheema, P.; Chary, A.; Parks, F.; Karas, J.; Optican, R.; Bahouth, S.W.; Garrett, E., et al. Uncoupling protein-1 and related messenger ribonucleic acids in human epicardial and other adipose tissues: epicardial fat functioning as brown fat. The Journal of clinical endocrinology and metabolism 2009, 94, 3611-3615, doi:10.1210/jc.2009-0571.
- Chechi, K.; Vijay, J.; Voisine, P.; Mathieu, P.; Bosse, Y.; Tchernof, A.; Grundberg, E.; Richard, D. UCP1 expression-associated gene signatures of human epicardial adipose tissue. JCI insight 2019, 4, doi:10.1172/jci.insight.123618.
- Iacobellis, G.; Pistilli, D.; Gucciardo, M.; Leonetti, F.; Miraldi, F.; Brancaccio, G.; Gallo, P.; di Gioia, C.R. Adiponectin expression in human epicardial adipose tissue in vivo is lower in patients with coronary artery disease. Cytokine 2005, 29, 251-255, doi:10.1016/j.cyto.2004.11.002.
- Silaghi, A.; Achard, V.; Paulmyer-Lacroix, O.; Scridon, T.; Tassistro, V.; Duncea, I.; Clement, K.; Dutour, A.; Grino, M. Expression of adrenomedullin in human epicardial adipose tissue: role of coronary status. Am J Physiol Endocrinol Metab 2007, 293, E1443-1450, doi:10.1152/ajpendo.00273.2007.
- Mazurek, T.; Zhang, L.; Zalewski, A.; Mannion, J.D.; Diehl, J.T.; Arafat, H.; Sarov-Blat, L.; O'Brien, S.; Keiper, E.A.; Johnson, A.G., et al. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation 2003, 108, 2460-2466, doi:10.1161/01.CIR.0000099542.57313.C5.





