
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | David Waisman | + 1513 word(s) | 1513 | 2021-04-23 06:13:34 | | | |
| 2 | David Waisman | Meta information modification | 1513 | 2021-04-27 20:08:43 | | | | |
| 3 | Lindsay Dong | Meta information modification | 1513 | 2021-04-28 05:38:58 | | |
Video Upload Options
Plasmin is an enzyme which is responsible for digesting several proteins that hold the tissues surrounding solid tumors together.
1. Introduction
A tumor is a heterogenous multicellular organ comprised of not only cancer cells but also supporting stromal cells. The environment surrounding the tumor mass is known as the tumor microenvironment (TME). TME is composed of stromal cells that are of diverse types, including fibroblasts, adipocytes, infiltrating lymphocytes and macrophages, lymphatic and vascular networks, and most importantly the extracellular matrix (ECM). Fibrinogen and fibrin are important components of the ECM. The enhanced conversion of plasminogen to plasmin is not only associated with increased invasive and metastatic potential of tumor cells but also contributes to tumor growth by activating growth factors and cytokines that are present in the ECM.
2. Plasmin–Plasminogen Activation System
The fibrinolytic system is involved not only in homeostasis but also in the progression of several pathological conditions, such as cardiovascular diseases; angiogenesis; and tumor invasion and metastasis [1]. The critical mediator of fibrinolysis is plasmin—a potent serine protease. Plasmin also plays a key role in ECM degradation directly and also indirectly by activating many of the proteases that function in matrix degradation. Plasminogen is synthesized and secreted into the blood by the liver as a zymogen which is converted to plasmin by the action of plasminogen activators [2]. In processes, such as wound healing and tissue remodeling that occurs in the TME, uPA is the predominant form of plasminogen activator, whereas tPA is the primary activator in the circulation [3]. The activities of both tPA and uPA are regulated by plasminogen activator inhibitor (PAI)-1 [4] and PAI-2 [5], whereas the activity of plasmin itself is inhibited by α2-antiplasmin [6][7] and α2-macroglobulin [8] (Figure 1). Plasminogen receptors localize the bound plasminogen near the vicinity of the tumor cell, allowing easy access by uPAR-bound uPA. Plasminogen receptors are also present on the cells of the TME, including endothelial cells, activated myofibroblasts, and macrophages.
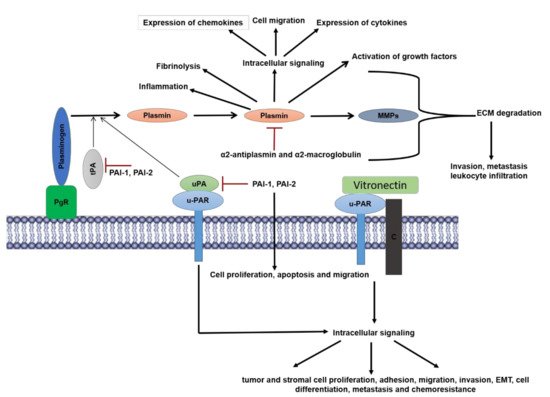
Figure 1. Overview of components and functions of plasminogen-activation system: Plasminogen is activated to the serine protease plasmin by a multicomponent system comprised of plasminogen receptors (PgR), urokinase-type-plasminogen activator (uPA), and tissue-plasminogen activator (tPA). uPA is bound to its receptor urokinase-plasminogen activator receptor (uPAR) whereas in most cases tPA is bound by the PgR. The activity of tPA and uPA are inhibited by the plasminogen-activator-inhibitor 1, 2 (PAI-1, PAI-2), of which PAI-1 is a more potent inhibitor. PAI-1 also promotes cellular proliferation, apoptosis, and migration. Plasmin activity is directly regulated by inhibitors α2 anti-plasmin and α2-macroglobulin. Plasmin mediates its physiological effects by virtue of its protease dependent and independent activities. Plasmin proteolytically activates other proteases such as matrix metalloproteinases (MMPs) that along with plasmin is involved in the degradation of extracellular matrix proteins (ECM) which promotes inflammation including leukocyte infiltration in tumors, cancer cell invasion and metastasis. Plasmin promotes tumor growth by proteolytically activating nascent growth factors in the ECM. Intracellular signaling by plasmin via several receptors (described above) mediates a plethora of effects that include expression of cytokines and chemokines, cell migration and inflammation. uPA and uPAR function both in plasminogen-dependent (protease activity) and plasminogen-independent mechanisms that promote multiple cellular responses. uPAR functions independently of uPA by binding to vitronectin and co-receptors such as integrins, to promote intracellular signaling, which result in tumor and stromal cell proliferation, adhesion, migration, invasion, epithelial-mesenchymal-transition (EMT), cell differentiation, metastasis and chemoresistance in cancer cells.
3. Structure and Function of Plasminogen and Plasmin
Plasminogen is a 92–94 kilodalton protein that circulates as a zymogen in the blood. Plasminogen is a 92–94 kilodalton protein that circulates as a zymogen in the blood. The variation in molecular weight is due to glycosylation differences, discussed further below. Plasminogen, like many hemostatic proteins, is produced in the liver and is found at a concentration of approximately 2 µM in the plasma [9]. It is a multidomain protein, comprised of an amino (N)-terminal peptide domain, five kringle domains, and the serine protease domain [10]. The classical catalytic triad is located within the serine protease domain: Histidine 603, Aspartate 646, and Serine 741.
Both, the rate, and location of plasmin formation, are tightly controlled. Plasmin generation is typically restricted to the sites of thrombosis and angiogenesis and the sites of ECM remodeling. Unrestrained plasmin generation is a factor in the depletion of pro-coagulant proteins in disseminated intravascular coagulation [11]. Plasmin generation on the surface of tumor cells leads to cell invasion and metastasis [12]. Local surfaces play a part in plasmin generation; both the fibrin surface and cell surface carry out a similar regulatory role [13].
Plasmin has proteolytic activity against a broad range of substrates, such as syndecans [14], laminins, fibronectin [15], vascular cell adhesion protein 1 [16], and vitronectin [17]. The broad range of substrates results in an extensive range of processes, both physiologic and pathologic, in which plasmin participates. These processes include fibrinolysis, signaling pathways, cell migration, wound healing, inflammation, and oncogenesis [18]. Stimulation of monocytes by plasmin results in enhanced expression of inflammatory cytokines (tumor necrosis factor-alpha (TNFα), interleukin (IL)-6, IL-1α/β, CD40) and chemokines (monocyte chemoattractant protein (MCP)-1/CCL2), tissue factors, and lipid mediators via activation of AP-1 and nuclear factor-kappa (NF-κB) transcription factors [19].
4. Plasminogen Activators and Plasminogen Activation Inhibitors
There are two physiologic activators of plasminogen—uPA and tPA—which are used clinically as thrombolytic therapeutics [20]. Both tPA and uPA are multidomain proteases. The active forms of each of these enzymes are inhibited by PAI-1 and PAI-2. PAI-1 is a 379-amino acid serine protease inhibitor that inhibits the activity of tPA and uPA [21][22][23] which is associated with inflammation and tumorigenesis. Interestingly although PAI-1 functions as an inhibitor of uPA and tPA, it serves a pro-tumorigenic role in various cancers such as lung and breast [24][25][26]. PAI-2, although originally identified as a less potent inhibitor of uPA, is a 47 kDa protein. It has been now shown to having a predominantly intracellular function and has been discovered to be a modulator of proteotoxic stress [27] unrelated to plasminogen activation/uPA inhibition.
5. Plasminogen Receptors
Plasminogen receptors serve as anchoring sites for plasminogen on the cell surface. The binding of plasminogen to these receptors involves the interaction between lysine residue(s) of the plasminogen receptor and the kringle domains of plasminogen. This interaction results in a conformational change in plasminogen which makes it a increases the rate of local plasmin generation. Many proteins have been identified as potential plasminogen receptors. Most of these proteins have C-terminal lysines which form binding sites for plasminogen. Included in this group are receptors such as α-enolase, histone H2B, S100A10 (p11) and Plg-RKT, However, other plasminogen receptors do not possess C-terminal lysines but still bind plasminogen in a lysine-dependent manner. Examples of plasminogen receptors that do not have the classic C-terminal lysine residue include high mobility group box-1 protein (HMGB-1) [28] (also known as amphoterin) and integrins of the β2 integrin family [29] and glucose-regulated protein 78 [30].
Interestingly, the majority of plasminogen receptors function in tumor-stromal cross-talk during cancer progression. The expression of plasminogen receptors, such as p11 and Plg-RKT, in macrophages, promote pro-tumorigenic phenotype in animal models. Importantly, these receptors have been shown to play a predominant role in macrophage recruitment to tumor sites and during inflammation.
6. Plasmin Functions in the Tumor Microenvironment—Regulation of Macrophage Function
The plasminogen-activation system plays a pivotal role in macrophage biology, specifically macrophage migration and phagocytosis. Aligned with the role of plasminogen in macrophage recruitment (summarized in Figure 3), depletion of several plasminogen receptors results in a phenotype resembling that in plasminogen-deficient mice. The role of plasminogen receptors on monocyte migration was mostly studied using an inflammation model.
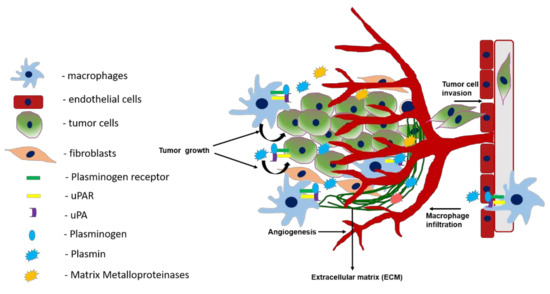
Figure 3. Role of the plasminogen activation system in tumor growth, angiogenesis, macrophage infiltration, and invasion. Activation of plasminogen bound to plasminogen receptors at the cell surface of macrophages and tumor cells is mediated by uPA bound to uPAR. This results in the production of plasmin which activates matrix metalloproteinases and together these proteases play a key role in the infiltration of macrophages to tumor sites. At the tumor site, macrophages promote tumor growth, angiogenesis, and metastasis. Plasmin produced by the tumor cells mediates tumor cell migration and invasion both by protease dependent and independent mechanisms.
7. The Role of the Plasmin and Plasminogen System as Biomarkers in Cancers
Cancer biomarkers are most often used as aid in diagnosis but can also be useful to determine tumor aggressiveness. Biomarkers also play a key role in identifying new personalized targets depending on the aggressiveness of the disease and toxicity to the therapeutic agents. In order for personalized therapies to be effective we require validated novel biomarkers for predicting aggressive vs. non-aggressive cancer, response to therapy and therapeutic resistance, and for predicting therapeutic side-effects and toxicity [31]. The best-established cancer biomarkers are uPA/uPAR and PAI-I [1][32]. Of the various cancers, the best-established case for the utilization of uPA/PAI-1 as biomarkers is for breast cancer.
8. Summary
Plasmin-mediated proteolysis contributes to the degradation of ECM, which can directly or indirectly affect tumor stroma formation, angiogenesis, and dissemination. Plasmin generation by stromal cells, such as macrophages is critical for tumor growth and progression in many forms of cancer including breast cancer. In this regard, the plasminogen receptors play a key role in regulating plasmin generation.
References
- Kwaan, H.C.; Lindholm, P.F. Fibrin and Fibrinolysis in Cancer. Semin. Thromb. Hemost. 2019, 45, 413–422.
- Raum, D.; Marcus, D.; Alper, C.A.; Levey, R.; Taylor, P.D.; Starzl, T.E. Synthesis of human plasminogen by the liver. Science 1980, 208, 1036–1037.
- Romer, J.; Bugge, T.H.; Pyke, C.; Lund, L.R.; Flick, M.J.; Degen, J.L.; Danø, K. Impaired wound healing in mice with a disrupted plasminogen gene. Nat. Med. 1996, 2, 287–292.
- Declerck, P.J.; Gils, A. Three decades of research on plasminogen activator inhibitor-1: A multifaceted serpin. Semin. Thromb. Hemost. 2013, 39, 356–364.
- Dear, A.E.; Medcalf, R.L. The cellular and molecular biology of plasminogen activator inhibitor type-2. Fibrinolysis 1995, 9, 321–330.
- Aoki, N. Discovery of alpha2-plasmin inhibitor and its congenital deficiency. J. Thromb. Haemost. 2005, 3, 623–631.
- Gallimore, M.J. More on: Discovery of alpha2-plasmin inhibitor and its congenital deficiency. J. Thromb. Haemost. 2006, 4, 284–285.
- De Boer, J.P.; Creasey, A.A.; Chang, A.; Abbink, J.J.; Roem, D.; Eerenberg, A.J. Alpha-2-macroglobulin functions as an inhibitor of fibrinolytic, clotting, and neutrophilic proteinases in sepsis: Studies using a baboon model. Infect. Immun. 1993, 61, 5035–5043.
- Ranson, M.; Andronicos, N.M. Plasminogen binding and cancer: Promises and pitfalls. Front. Biosci. 2003, 8, s294–s304.
- Forsgren, M.; Råden, B.; Israelsson, M.; Larsson, K.; Hedén, L.O. Molecular cloning and characterization of a full-length cDNA clone for human plasminogen. FEBS Lett. 1987, 213, 254–260.
- The cell biology of the plasminogen system. Faseb, J. 1995. Available online: (accessed on 6 August 2020).
- Poddar, N.K.; Maurya, S.K.; Saxena, V. Role of Serine Proteases and Inhibitors in Cancer. In Proteases in Physiology and Pathology; Chakraborti, S., Dhalla, N.S., Eds.; Springer: Singapore, 2017; pp. 257–287.
- Fleury, V.; Anglés-Cano, E. Characterization of the binding of plasminogen to fibrin surfaces: The role of carboxy-terminal lysines. Biochemistry 1991, 30, 7630–7638.
- Schmidt, A.; Echtermeyer, F.; Alozie, A.; Brands, K.; Buddecke, E. Plasmin- and thrombin-accelerated shedding of syndecan-4 ectodomain generates cleavage sites at Lys(114)-Arg(115) and Lys(129)-Val(130) bonds. J. Biol. Chem. 2005, 280, 34441–34446.
- Liotta, L.A.; Goldfarb, R.H.; Brundage, R.; Siegal, G.P.; Terranova, V.; Garbisa, S. Effect of plasminogen activator (urokinase), plasmin, and thrombin on glycoprotein and collagenous components of basement membrane. Cancer Res. 1981, 41, 4629–4636.
- Tjwa, M.; Moura, R.; Moons, L.; Plaisance, S.; De Mol, M.; Jansen, S.; Dewerchin, M.; Verfaillie, C.; Carmeliet, P. Fibrinolysis-independent role of plasmin and its activators in the haematopoietic recovery after myeloablation. J. Cell Mol. Med. 2009, 13, 4587–4595.
- Gechtman, Z.; Sharma, R.; Kreizman, T.; Fridkin, M.; Shaltiel, S. Synthetic peptides derived from the sequence around the plasmin cleavage site In Vitronectin: Use in mapping the PAI-1 binding site. FEBS Lett. 1993, 315, 293–297.
- Andreasen, P.A.; Egelund, R.; Petersen, H.H. The plasminogen activation system in tumor growth, invasion, and metastasis. CMLS Cell Mol. Life Sci. 2000, 57, 25–40.
- Carmo, A.A.F.; Costa, B.R.C.; Vago, J.P.; De Oliveira, L.C.; Tavares, L.P.; Nogueira, C.R.C.; Ribeiro, A.L.C.; Garcia, C.C.; Barbosa, A.S.; Brasil, B.S.A.F.; et al. Plasmin Induces In Vivo Monocyte Recruitment through Protease-Activated Receptor-1-, MEK/ERK-, and CCR2-Mediated Signaling. J. Immunol. 2014, 193, 3654–3663.
- Longstaff, C.; Kolev, K. Basic mechanisms and regulation of fibrinolysis. J. Thromb. Haemost. 2015, 13, S98–S105.
- Dosne, A.M.; Dupuy, E.; Bodevin, E. Production of a fibrinolytic inhibitor by cultured endothelial cells derived from human umbilical vein. Thromb. Res. 1978, 12, 377–387.
- Loskutoff, D.J.; van Mourik, J.A.; Erickson, L.A.; Lawrence, D. Detection of an unusually stable fibrinolytic inhibitor produced by bovine endothelial cells. Proc. Natl. Acad. Sci. USA 1983, 80, 2956–2960.
- Ginsburg, D.; Zeheb, R.; Yang, A.Y.; Rafferty, U.M.; A Andreasen, P.; Nielsen, L.; Dano, K.; Lebo, R.V.; Gelehrter, T.D. cDNA cloning of human plasminogen activator-inhibitor from endothelial cells. J. Clin. Investig. 1986, 78, 1673–1680.
- Bajou, K.; Noel, A.; Gerard, R.D.; Masson, V.; Brunner, N.; Holsthansen, C.; Skobe, M.; E Fusenig, N.; Carmeliet, P.; Collen, D.; et al. Absence of host plasminogen activator inhibitor 1 prevents cancer invasion and vascularization. Nat. Med. 1998, 4, 923–928.
- Bajou, K.; Maillard, C.; Jost, M.; Lijnen, R.H.; Gils, A.; Declerck, P.; Carmeliet, P.; Foidart, J.-M.; Noël, A. Host-derived plasminogen activator inhibitor-1 (PAI-1) concentration is critical for In Vivo tumoral angiogenesis and growth. Oncogene 2004, 23, 6986–6990.
- Bajou, K.; Peng, H.; Laug, W.E.; Maillard, C.; Noel, A.; Foidart, J.M. Plasminogen Activator Inhibitor-1 Protects Endothelial Cells from FasL-Mediated Apoptosis. Cancer Cell. 2008, 14, 324–334.
- Lee, J.A.; Yerbury, J.J.; Farrawell, N.; Shearer, R.F.; Constantinescu, P.; Hatters, D.M.; Schroder, W.A.; Suhrbier, A.; Wilson, M.R.; Saunders, D.N.; et al. SerpinB2 (PAI-2) Modulates Proteostasis via Binding Misfolded Proteins and Promotion of Cytoprotective Inclusion Formation. PLoS ONE 2015, 10, e0130136.
- Miles, L.A.; Ny, L.; Wilczynska, M.; Shen, Y.; Ny, T.; Parmer, R.J. Plasminogen Receptors and Fibrinolysis. Int. J. Mol. Sci. 2021, 22, 1712.
- Godier, A.; Hunt, B.J. Plasminogen receptors and their role in the pathogenesis of inflammatory, autoimmune and malignant disease. J. Thromb. Haemost. 2013, 11, 26–34.
- Gonzalez-Gronow, M.; Gomez, C.F.; de Ridder, G.G.; Ray, R.; Pizzo, S.V. Binding of tissue-type plasminogen activator to the glucose-regulated protein 78 (GRP78) modulates plasminogen activation and promotes human neuroblastoma cell proliferation In Vitro. J. Biol. Chem. 2014, 289, 25166–25176.
- Duffy, M.J.; Crown, J. A Personalized Approach to Cancer Treatment: How Biomarkers Can Help. Clin. Chem. 2008, 54, 1770–1779.
- McMahon, B.J.; Kwaan, H.C. Components of the Plasminogen-Plasmin System as Biologic Markers for Cancer. In Advances in Cancer Biomarkers: From Biochemistry to Clinic for a Critical Revision Advances in Experimental Medicine and Biology; Scatena, R., Ed.; Springer: Dordrecht, The Netherlands, 2015; pp. 145–156.




