
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Marcella Reale | + 3552 word(s) | 3552 | 2021-04-20 05:36:29 | | | |
| 2 | Karina Chen | Meta information modification | 3552 | 2021-04-28 05:09:28 | | |
Video Upload Options
Frequent diseases of the CNS, such as Alzheimer’s disease, Parkinson’s disease, multiple sclerosis, and psychiatric disorders (e.g., schizophrenia), elicit a neuroinflammatory response that contributes to the neurodegenerative disease process itself. The immune and nervous systems use the same mediators, receptors, and cells to regulate the immune and nervous systems as well as neuro-immune interactions. In various neurodegenerative diseases, peripheral inflammatory mediators and infiltrating immune cells from the periphery cause exacerbation to current injury in the brain. Acetylcholine (ACh) plays a crucial role in the peripheral and central nervous systems, in fact, other than cells of the CNS, the peripheral immune cells also possess a cholinergic system.
1. Cholinergic and Immune System Crosstalk in Widespread Neuro-Inflammatory Diseases
Neurodegenerative diseases are a group of chronic, progressive disorders characterized by the gradual loss of neurons, and specific portions of the brain, spinal cord, or peripheral nerves are affected, with consequent impairment of cognition, movement, strength, coordination, sensation, or autonomic control. In the pathogenesis of several neurodegenerative diseases such as Alzheimer’s disease (AD), Parkinson’s disease (PD), and multiple sclerosis (MS), although it may not typically represent an initiating factor, inflammation is associated with degeneration of the nervous system, and is characterized by the activation of microglia and astrocytes, secretion of pro-inflammatory cytokines and chemokines, and the recruitment of more immune cells from the periphery. Under physiological conditions, microglia exhibit a deactivated phenotype that, in the presence of pathogen or tissue damage, switch to an activated phenotype and thereby promote an inflammatory response. Classically activated microglia or “M1” release inflammatory mediators, including proinflammatory cytokines, free radicals, and complement, help microglia to communicate with astrocytes and peripheral immune cells. Chronic and excessive microglial activation leads to the pro-inflammatory and neurotoxic environment and contributes to neurodegeneration and cognitive dysfunction [1][2][3][4][5]. Microglia induced by Th2 type cytokines such as IL-4, IL-10, or IL-13, or alternatively induced M2, produced anti-inflammatory molecules and neurotrophic factors implicated in repairing/remodeling and neuroprotective effects on endangered neurons [6][7]. Also, astrocytes, a significant component of the blood-brain barrier, behave as effective immune cells in the CNS playing a dual role, in fact, they can both amplify the effects of inflammation and mediate cell damage and protect the CNS. Similar to microglia, astrocyte polarization into the pro-inflammatory A1 and anti-inflammatory A2 phenotype, which is similar to M1 and M2 polarization of microglia, has been described in vitro and in vivo. Activated astrocytes are endowed, as microglia, with the ability to secrete cytokines and chemokines, which exert an impact on both adaptive and innate immune responses. As a prominent source of cytokines, chemokines, and other inflammatory mediators, astrocytes are instrumental both in the propagation as well as in the down-regulation of inflammatory responses in the brain. Due to their strategic location at the blood–brain interface, they are important for blood–brain barrier function and in particular in controlling the spread of inflammatory cells from the perivascular space into the parenchyma [8]. Neurons, releasing neuropeptides and neurotransmitters, regulate inflammation and favor the differentiation of T-regulatory cells. On the other hand, Tregs infiltrate the brain and show an activated/memory phenotype, suggesting that T cells are activated peripherally and then reactivated in the CNS [9]. The involvement of adaptive cellular immune responses in neurodegenerative disorders has been deduced from detections of T-cell responses to specific CNS antigens, or shifts in CD4+ and CD8+ cell populations in the periphery as well as in the CNS [10]. T cells have been implicated in complex brain processes including spatial learning, memory, emotional behavior, and stress responsiveness. CD4+ T cells are recruited to the meninges and secrete interleukin-4 that skews macrophages and microglia to an M2 anti-inflammatory phenotype and induces the production of brain-derived neurotrophic factor by astrocytes, leading to the improvement of spatial learning and memory [11][12][13][14][15]. B cells and CD4+ and CD8+ T cells, can play a role in neurodegeneration which is also evidenced by the close association of T cells expressing TNF-related apoptosis-inducing ligand (TRAIL) with dying spinal motor neurons in MS, and alterations in peripheral levels of CD4+ and CD8+ T cells, as observed in AD [16][17].
Consequently, the activation of T cells in CNS, increased levels of the inflammatory cytokines TNF-α and IL-6, and the chemokine CXCL8 are detected in many neurodegenerative disorders. The same cytokines circulating in the blood can communicate with the brain or crossing the intact blood–brain barrier or spreading easily from the blood into the brain parenchyma [18]. In the brain, cytokines may activate endothelial cells, and in turn activate adjacent perivascular macrophages, which then communicate with microglia. The reported correlation between increased cytokine levels in cerebrospinal fluid (CSF) and plasma and neurodegeneration [19][20][21][22] indicates that the same mediators of immune responses are present in the brain and periphery, and a link between systemic inflammation and neurodegeneration was corroborated by migration from the periphery to the brain of inflammatory cells [23], confirming the communication between the brain and peripheral immune systems (Figure 1). Systemic inflammation with the secretion of pro-inflammatory cytokines in the periphery has been evidenced in MS and AD. Circulating monocytes can migrate in the brain, and together with resident microglial cells, respond to inflammatory stimuli and increase the immune response [24] playing a key role in neurodegenerative disease progression. Th1 and Th17 CD4+ T cells infiltrate both the white and gray matter of MS or AD patients, producing inflammatory mediators involved in neuronal loss, that positively correlate with the disease course [25].
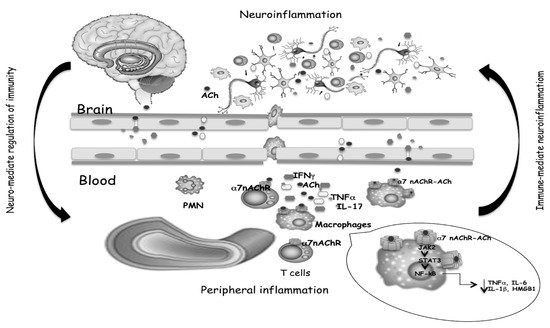
Figure 1. Cytokines and neurotransmitters mediate the bidirectional crosstalk between nervous and immune systems. The binding of ACh, produced by T cells, to α7 nAChR expressed on macrophages, triggers inhibitory signals that limit inflammatory cytokine release, as well as T cell activation and differentiation. Both the reduced production of peripheral cytokines and the reduced cell migration from the periphery to the brain, help to mitigate neuroinflammation.
On the other hand, it is not surprising that receptors for neurotransmitters expressed in the nervous system may be also expressed on the surface of immune system cells. Some subtypes of glutamate receptors (GluRs), ACh-receptors, serotonin receptors (5-HTRs), dopamine receptors (DARs), and adrenergic receptors are expressed on immune cells [26]. Neurotransmitter-mediated modulation may drive polarization of the peripheral T cell response toward Th1, Th17, or Tregs phenotype. Peripheral immune cells have their own cholinergic system able to release ACh which may act as a modulator of inflammation by binding to α7 nAChR on macrophages, and consequent inhibition of the nuclear translocation of NF-kB, phosphorylation of JAK2, STAT3 translocation into the nucleus and block of pro-inflammatory mediators production [27]. α7 nAChR-dependent cholinergic signaling, in addition to “early” inhibition of pro-inflammatory cytokines production, suppresses the HMGB1, a “late” mediator of systemic inflammation [28].
Peripheral decreases in ACh would also increase concentration and potentiate the effects of pro-inflammatory cytokines within the brain. AChE levels participate in the regulation of peripheral pro-inflammatory cytokine production, which can penetrate the brain and modify neuronal functioning. In fact, AChE hydrolyzes Ach, reducing its levels, and consequently activates α7 nAChR and the anti-inflammatory route. The ‘‘cholinergic hypothesis’’ assumes that impaired cholinergic function has a strategic relevance in neurodegenerative processes. In fact, previous studies have shown that cholinergic system dysfunction caused by dis-regulated ACh synthesis or hydrolysis and accompanied by an altered response of the cholinergic receptors, both of nicotinic and muscarinic types, plays a strategic role in neurodegenerative disease onset and progression.
In light of this, the CNS is no longer considered an “immune-privileged site” but a “special immune-controlled site” and cytokines, neurotransmitters, and trophic factors are mediators of the bidirectional neuro-immune interactions, crosstalk, and positive-feedback loops between immune cells and the central, peripheral, sympathetic, parasympathetic, and enteric nervous systems. The imbalance of this extensive communication may support the onset of immune disorders and may represent an important component of the pathogenic mechanisms involved in neurodegenerative diseases.
1.1. Multiple Sclerosis
Multiple sclerosis (MS) is one of the most common chronic inflammatory diseases of the CNS [29][30]. Traditionally, MS has been considered to be an autoimmune disease in which the interruption of the blood–brain barrier integrity, migration, and infiltration of activated lymphocytes, macrophages, and dendritic cells into the central nervous system (CNS) resulting in demyelination, neuro-axonal degeneration, and neurological deficits. Alternatively, it has been suggested that the formation of the oligodendrocyte–myelin complex that drives cyto-degeneration is the early event followed by the release of antigenic debris that induces an autoimmune and inflammatory response [31]. The impaired balance of Th17 cells and Tregs cells is involved in MS, in fact, Th17 cells produce pro-inflammatory cytokines while Treg mediated the production of cytokines that are involved in the maintenance of immune homeostasis and in the control of MS progression, and the Treg/Th17 ratio was negatively correlated with the severity of symptoms [32]. Th1 and Th17 cells specific for myelin oligodendrocyte glycoprotein (MOG) increase microglial activation and drive inflammation, increasing cytokine release [33], confirming that the interaction between peripheral immune cells and microglia was complex and often opposing. Several experimental models have strengthened that nicotine has an anti-inflammatory effect by preventing the polarization to Th1/Th17 [34], that nicotine upregulates the expression of cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) and Foxp3 and boosts the recruitment of Treg cells to the central nervous system CNS [35][36], and that α7 nAChR improves the differentiation of Treg cells.
The involvement of the cholinergic system in MS is not well-known, but studies on EAE mice have suggested how the treatment with cholinesterase inhibitors may recover motor and cognitive impairment and downgrade the neuro-inflammation. In addition, a role for the cholinergic system was strengthened by the observation that α7 nAChR activation can lead to the inhibition of lymphocyte proliferation [37][38] and to the inhibition of macrophage and microglia activation [39][40]. Stimulation of α7 nAChR on endothelial cells additionally controls the extravasation of leukocytes during inflammation [41], although it is not known if this may happen in the blood–brain barrier. Inflammatory cytokine production in peripheral blood cells was suppressed by cholinergic agonists, as reported by in vitro studies carried out to test the effects of cholinergic agonists on cytokine production. It has been reported that in stimulated human monocyte-derived macrophages Ach, choline, nicotine, and other agonists inhibited pro-inflammatory cytokine release through the α7 nAChR dependent mechanism [40][42][43][44]. Activation of α7 nAChR in PHA-induced PBMC from MS patients by nicotine reduced the production of both inflammatory cytokines IL-17 and IL-1β [45]. Stimulation of CD4+ cells by nicotine increased expression and activation of α7 nAChR and reduced the Th17 response [46].
The involvement of the cholinergic system in MS was confirmed also by the detection of lower ACh levels in serum and CSF of MS patients compared with healthy subjects, and increased activity of the ACh hydrolyzing enzymes AChE and BChE and their expression levels in peripheral blood mononuclear cells. In MS patients, the expression of transcript for OCTN-1 and mediatophore, the two proteins typically expressed in immune cells and responsible for the non-vesicular ACh release, were also up-regulated, other than the ACh biosynthetic enzyme ChAT [47][48]. The dysregulated balance between ACh, AChE, and BChE could be responsible for higher pro-inflammatory cytokines production detected in MS patients [49].
The study of Jiang et al. observed that in MS patients the higher ACh content in NK cells was associated with an increased expanded disability status scale (EDSS) score other than with the higher number and volume of lesions, though ACh-producing NK cells might modulate infiltration of monocytes/macrophages and attenuate CNS inflammation [50]. In MS patients, it is likely that inflammation related to disease severity may induce NK cells to release lower ACh or express higher ChAT levels. Even NK cells produced ACh, which was degraded by enhanced production of AChE, contributing to disease severity. In fact, elevated serum levels of ACh in MS patients correlate with better disease outcomes.
Thus, taking into account that in immune cells of MS patients showed increased activity of the cholinergic hydrolyzing enzymes BChE and AChE, reduced levels of ACh, increased pro-inflammatory cytokines production, and a correlation between the genetic polymorphisms and activity of BChE and AChE hydrolyzing enzymes, understanding the role of cholinergic components in the aberrant immune response and severe neuro-inflammation may help the development of new treatments to ameliorate the clinical symptoms and delay or arrest the onset of the disabilities in MS.
1.2. Alzheimer’s Disease
Alzheimer’s disease (AD) is a progressive neurodegenerative disease and its neuropathological hallmarks are neuritic amyloid plaques derived from misfolded fragments of amyloid beta (Aβ) that aggregate to form oligomers, fibrils, and insoluble plaques, and neurofibrillary tangles originate from deposits of hyperphosphorylated degenerate filaments, which result from aggregations of the microtubular protein tau. The presence of neuronal plaques activates inflammatory responses mediated by astrocytes and microglia with resulting activation of the immune response and production of cytokines by macrophages and neutrophils, causing damage to neurons [51]. Aβ oligomers affected cholinergic synapses and the synaptic loss is correlated to cognitive impairment. ACh has an important role in cognitive processes, thus the cholinergic system is potentially an important factor in many forms of dementia, including AD, where ChAT transcription and activity is diminished in accordance with the progression of dementia and AChE interacts with the Aβ peptide and stimulates amyloid fibril formation [52]. In this context, AChE inhibitors (ChE-Is) are currently the most frequent treatment for AD patients. Autoradiography assay showed that, in the hippocampus of transgenic Tg2576 mice used as a model of AD, the choline uptake and the expression of muscarinic and nicotinic cholinergic receptors were reduced [53]. The inflammatory reaction to Aβ involves the release of a pro-inflammatory cytokine IL-1β, activated via cleavage by caspase-1 in the inflammasome. The activation of the anti-inflammatory cholinergic pathway through the α7 nAChR, inhibits the activation of the inflammasome [54].
As secondary components in senile plaques, cytokines, chemokines, complement components, and acute-phase proteins are over-produced or co-localized in AD brains [55]. Increased expression of IL-1β and TNFα in the olfactory bulb of the APP SW Tg2576 mouse was related to the accumulation of Aβ and to the increase in ChE and nAChR expression [56]. Increased levels of circulating pro-inflammatory cytokines, such as IL-1α, IL-1β, IL-6, and TNF-α, complement and the presence of activated microglia, have been described in patients with AD, and a direct correlation has been established between Aβ-induced neurotoxicity and cytokine production, and between AD progression, rapid cognitive decline and peripheral immunity and inflammation [57]. In fact, pro-inflammatory cytokines may induce the synthesis of Aβ that, by a positive feedback mechanism, may induce the expression of these cytokines in astrocytes and microglia, and the concept that AD, a disease affecting the central nervous system, can be considered a systemic disorder becomes more and more consistent [58].
A relationship between peripheral inflammatory mediators, Aβ levels, and ApoE genotype was evidenced in Alzheimer’s disease patients [59]. T cells may play a key role in AD-associated inflammation, and an higher percentage of T cells with a more marked response to β-amyloid were shown in AD patients, than healthy subjects. In the brain of AD mice, facilitated by increased BBB permeability, an increase of CD4+ and CD8+ cells linked to gliosis and amyloid pathology has been observed [60], and Th1 and Th17 cells induced microglial activation modulating phagocytic and secretory phenotype, while Th2 cells had no effect on microglial cytokine production [61].
AChE inhibitors, the most used drugs of AD treatment to slow neurodegeneration, induce a synaptic increase in ACh and a direct stimulation of ACh receptors, and may affect the inflammatory activity of cells participating in AD-associated inflammation, inducing a Th1 to Th2 switch [62] and decreasing inflammation by a dose-dependent reduction in inflammatory cytokine levels [63][64]. Since mAChRs can modulate cognition and ACh via mAChRs expressed on leukocytes may potentiate inflammatory reactions, these receptors could represent a significant therapeutic target to ameliorate the behavioral and cognitive deficits and to regulate immune cell activation in inflammatory conditions of several diseases.
The cholinergic anti-inflammatory pathway represents the efferent/motor arm of the inflammatory reflex, which is a neural circuit that controls the immune response against injuries. Thus, immunological reactions that take part in the immune response of AD patients may be influenced by the modulation of the non-neurological cholinergic system. Knowledge of the non-neuronal cholinergic pathways can prove invaluable in understanding how immunomodulation in AD can represent an important therapeutic target for the development of therapies capable of preventing or halting the development of neuroinflammation and cognitive decline.
1.3. Parkinson’s Disease
Although Parkinson’s disease (PD) is a complex and multisystem disorder, neuroinflammation contributes to disease progression through loss of dopaminergic (DA) neurons in the substantia nigra (SN) of the brain. Loss of cholinergic neurons, reductions of ChAT, evidenced cholinergic deficits in PD with potential relevance for symptoms including cognitive and attentional impairments. Microglia activated by α-synuclein, have the potential to produce several neurotrophic factors, apoptosis-related proteins, and many pro-inflammatory cytokines, via the transcription factor NF-κB, exacerbating the progression of PD [65]. Notably, microglia are enriched in SN compared to other regions of the brain. These findings, coupled to reduced antioxidant capacity and enhanced sensitivity of neurons to pro-inflammatory molecules, support a role for microglia-mediated DA degeneration in PD [66]. Other than microglia activation, the neuroinflammation in PD is characterized by astrocyte activation and blood–brain barrier damage. The study of Brochard et al. showed that CD4 and CD8 T lymphocytes are infiltrated in the substantia nigra, indicating that in addition to central glial activation, peripheral T lymphocytes are involved in the neuroinflammation during the pathogenesis of PD [67]. These central/peripheral immune cells drive the production of many pro-inflammatory cytokines to amplify inflammatory signals, and neurotoxins that act on dopamine neurons inducing their death.
In summary, during the course of PD the development of immune inflammation, which is characterized by glial cell activation, peripheral immune cell infiltration, immune complex deposition, and the production and release of many pro-inflammatory cytokines and chemokines, was observed [68]. These findings suggest that Parkinson’s had an important inflammatory contribution, and this inflammation could be related to the cholinergic system [69].
Increasing evidence connect mitochondrial perturbation with neuronal diseases, such as AD and PD [70][71]. In addition to the expression on the cell surface, α7 nAchR is expressed also in the mitochondrial outer membrane in non-neuronal cells, including monocytes and B lymphocytes, and α7 nAchR signaling attenuates ATP-induced mitochondrial perturbation, and ACh inhibits ATP-induced NLRP3 inflammasome activation by preventing mitochondrial DNA release into the cytoplasm, representing the new anti-inflammatory targets. Additionally, the pro-inflammatory responses may be modulated by activation of α7 in macrophages via activation of the Jak2/STAT3 signaling cascade [72], and the upregulation of heme oxygenase-1 (HO-1) may be an alternative mechanism for the cholinergic regulation of pro-inflammatory responses [73]. Thus, activation of this non-neuronal cholinergic system may underlie neuroprotection through the effect of α7 nAchR agonists or AChE inhibitors that improve ACh levels [74].
2. CholinomiRs: A Novel Approach for Neuroinflammatory Diseases
MicroRNAs (miRNAs) are non-coding transcripts of 18–25 nucleotides and usually target mRNAs to influence post-transcriptional gene expression affecting the mRNA stability and/or translational repression of their target mRNAs. MiRNAs are involved in pathophysiological processes such as differentiation, proliferation, apoptosis, metabolism, the fate of neuronal and immune cells, and the regulation of the cholinergic system [75][76][77]. Over 200 miRNAs, which modulate both neuronal and immune processes, are identified to target different cholinesterase transcripts and have been designated by Nadorp and Soreq “CholinomiRs” [78]. Simulating the activation of α7 nAChR or influencing cholinesterase activity, miRNA could be helpful for inflammatory and neurodegenerative diseases and represent attractive targets for designing therapies aimed to restore the cholinergic anti-inflammatory pathway also in neuroinflammatory conditions [79].
A large number of miRNAs, targeting ChAT, VAChT, AChE-S, AChE-R, and BChE, can regulate several biological pathways, by the increase of AChE synthesis and decrease of ChAT production that modulate ACh levels.
Nardop and Soreq suggested the overlapping miRNA regulation as a new surveillance mechanism that can balance cholinergic neurotransmission and may be of value for both basic and translational aspects of neuroinflammation-related disorders [78] and showed that few overlapping miRs for BChE and AChE were detected, suggesting that these enzymes are subject to distinct modes of miR regulation.
In the central nervous system miR-124 acts as a controller of microglia quiescence, but also as a modulator of monocyte and macrophage activation in the periphery. In experimental autoimmune encephalomyelitis (EAE), miR-124 was down-regulated in activated microglia, and the conversion of activated macrophages to an inactive phenotype such as resting microglia was linked to the overexpression of miR-124. Peripheral administration of miR-124 reduced CNS inflammation and suppressed EAE by inhibiting macrophages, myelin-specific T cells, and migration of encephalitogenic T cells from the periphery to the site of inflammation [80]. miR-124 driving macrophage polarization from an M1 toward an M2 phenotype may modulate the inflammatory diseases associated with macrophage activation in EAE. Activation of α7 nAChR up-regulates miR-124 expression in LPS-stimulated macrophages reducing pro-inflammatory cytokine production, and in turn, miR-124 targeting STAT3, decreases STAT3 and its phosphorylation and reduces IL-6 transcription, or miR-124 targeting TACE modulates TNF-α release [81]. The activation of α7 nAchR in macrophages increased also the expression of miR 2055b that mediates the cholinergic anti-inflammatory activity by targeting HMGB1, suggesting that it is also a potential therapeutic target for the treatment of inflammatory diseases [82].
miR-132 is the miRNA that targets AChE in both the brain and the periphery and attenuates the inflammation. In alveolar macrophages, miR-132 inhibits LPS-induced inflammation by enhancing the cholinergic anti-inflammatory pathway and reducing the AChE level. In EAE, miR-132 is downregulated and associated with the severity of EAE, and the activation of miR-132 has an anti-inflammatory effect and mediates attenuation of EAE [83]. miRNA-199a and miR-186 suppress cholinesterases to increase cholinergic signaling, resulting in decreased expression of pro-inflammatory cytokines [78].
References
- Qin, L.; Wu, X.; Block, M.L.; Liu, Y.; Breese, G.R.; Hong, J.S.; Knapp, D.J.; Crews, F.-T. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia 2007, 555, 453–462.
- Bodea, L.G.; Wang, Y.; Linnartz-Gerlach, B.; Kopatz, J.; Sinkkonen, L.; Musgrove, R.; Kaoma, T.; Muller, A.; Vallar, L.; Di Monte, D.A.; et al. Neurodegeneration by activation of the microglial complement-phagosome pathway. J. Neurosci. 2014, 3425, 8546–8556.
- Süβ, P.; Lana, A.J.; Schlachetzki, J.C.M. Chronic peripheral inflammation: A possible contributor to neurodegenerative diseases. Neural. Regen. Res. 2021, 169, 1711–1714.
- Sandhu, J.K.; Kulka, M. Decoding Mast Cell-Microglia Communication in Neurodegenerative Diseases. Int. J. Mol. Sci. 2021, 223, 1093.
- Guzman-Martinez, L.; Maccioni, R.B.; Andrade, V.; Navarrete, L.P.; Pastor, M.G.; Ramos-Escobar, N. Neuroinflammation as a Common Feature of Neurodegenerative Disorders. Front. Pharmacol. 2019, 10, 1008.
- Block, M.L.; Hong, J.S. Chronic microglial activation and progressive dopaminergic neurotoxicity. Biochem. Soc. Trans. 2007, 35, 1127–1132.
- Bachiller, S.; Jiménez-Ferrer, I.; Paulus, A.; Yang, Y.; Swanberg, M.; Deierborg, T.; Boza-Serrano, A. Microglia in Neurological Diseases: A Road Map to Brain-Disease Dependent-Inflammatory Response. Front. Cell Neurosci. 2018, 12, 488.
- Brambilla, R. The contribution of astrocytes to the neuroinflammatory response in multiple sclerosis and experimental autoimmune encephalomyelitis. Acta Neuropathol. 2019, 137, 757–783.
- Xie, L.; Choudhury, G.R.; Winters, A.; Yang, S.-H.; Jin, K. Cerebral regulatory T cells restrain microglia/macrophage-mediated inflammatory responses via IL-10. Eur. J. Immunol. 2015, 45, 180–191.
- Korin, B.; Ben-Shaanan, T.L.; Schiller, M.; Dubovik, T.; Azulay-Debby, H.; Boshnak, N.T.; Koren, T.; Rolls, A. High-dimensional, single-cell characterization of the brain’s immune compartment. Nat. Neurosci. 2017, 20, 1300–1309.
- Kipnis, J. Multifaceted interactions between adaptive immunity and the central nervous system. Science 2016, 353, 766–771.
- Walsh, J.T.; Kipnis, J. Regulatory T cells in CNS injury: The simple, the complex and the confused. Trends Mol. Med. 2011, 17, 541–547.
- Ziv, Y.; Ron, N.; Butovsky, O.; Landa, G.; Sudai, E.; Greenberg, N.; Cohen, H.; Kipnis, J.; Schwartz, M. Immune cells contribute to the maintenance of neurogenesis and spatial learning abilities in adulthood. Nat. Neurosci. 2006, 9, 268–275.
- Radjavi, A.; Smirnov, I.; Kipnis, J. Brain antigen-reactive CD4+ T cells are sufficient to support learning behavior in mice with limited T cell repertoire. Brain Behav. Immun. 2014, 35, 58–63.
- Morimoto, K.; Nakajima., K. Role of the Immune System in the Development of the Central Nervous System. Front. Neurosci. 2019, 13, 916.
- Neumann, H.; Medana, I.M.; Bauer, J.; Lassmann, H. Cytotoxic T lymphocytes in autoimmune and degenerative CNS diseases. Trends Neurosci. 2002, 25, 313–319.
- Larbi, A.; Pawelec, G.; Witkowski, J.M.; Schipper, H.M.; Derhovanessian, E.; Goldeck, D.; Fulop, T. Dramatic shifts in circulating CD4 but not CD8 T cell subsets in mild Alzheimer’s disease. J. Alzheimers Dis. 2009, 17, 91–103.
- Quan, N. Immune-to-brain signaling: How important are the blood-brain barrier-independent pathways? Mol. Neurobiol. 2008, 372, 142–152.
- Perry, V.H. The influence of systemic inflammation on inflammation in the brain: Implications for chronic neurodegenerative disease. Brain Behav. Immun. 2004, 18, 407–413.
- Perry, V.H.; Cunningham, C.; Holmes, C. Systemic infections and inflammation affect chronic neurodegeneration. Nat. Rev. Immunol. 2007, 7, 161–167.
- Yang, Q.; Wang, G.; Zhang, F. Role of Peripheral Immune Cells-Mediated Inflammation on the Process of Neurodegenerative Diseases. Front. Immunol. 2020, 11, 582825.
- Cunningham, C.; Campion, S.; Lunnon, K.; Murray, C.L.; Woods, J.F.; Deacon, R.M.; Rawlins, J.N.P.; Perry, V.H. Systemic inflammation induces acute behavioral and cognitive changes and accelerates neurodegenerative disease. Biol. Psychiatry 2009, 65, 304–312.
- Prinz, M.; Priller, J. The role of peripheral immune cells in the CNS in steady state and disease. Nat. Neurosci. 2017, 20, 136–144.
- Kierdorf, K.; Masuda, T.; Jordao, M.J.C.; Prinz, M. Macrophages at CNS interfaces: Ontogeny and function in health and disease. Nat. Rev. Neurosci. 2019, 20, 547–562.
- Kebir, H.; Kreymborg, K.; Ifergan, I.; Dodelet-Devillers, A.; Cayrol, R.; Bernard, M.; Giuliani, F.; Arbour, N.; Becher, B.; Part, A. Human TH17 lymphocytes promote blood-brain barrier disruption and central nervous system inflammation. Nat. Med. 2007, 13, 1173–1175.
- Pacheco, R.; Riquelme, E.; Kalergis, A.M. Emerging evidence for the role of neurotransmitters in the modulation of T cell responses to cognate ligands. Cent. Nerv. Syst. Agents Med. Chem. 2010, 10, 65–83.
- Gallowitsch-Puerta, M.; Pavlov, V.A. Neuro-immune interactions via the cholinergic anti-inflammatory pathway. Life Sci. 2007, 80, 2325–2329.
- Wang, H.; Liao, H.; Ochani, M.; Justiniani, M.; Lin, X.; Yang, L.; Al-Abed, Y.; Wang, H.; Metz, C.; Miller, E.J.; et al. Cholinergic agonists inhibit HMGB1 release and improve survival in experimental sepsis. Nat. Med. 2004, 10, 1216–1221.
- Lassmann, H.; Brück, W.; Lucchinetti, C.F. The immunopathology of multiple sclerosis: An overview. Brain Pathol. 2007, 17, 210–218.
- Stys, P.K.; Zamponi, G.W.; Van Minnen, J.; Geurts, J.J. Will the real multiple sclerosis please stand up? Nat. Rev. Neurosci. 2012, 13, 507–514.
- Hemmer, B.; Nessler, S.; Zhou, D.; Kieseier, B.; Hartung, H.P. Immunopathogenesis and immunotherapy of multiple sclerosis. Nat. Clin. Pract. Neurol. 2006, 2, 201–211.
- Jamshidian, A.; Shaygannejad, V.; Pourazar, A.; Zarkesh-Esfahani, S.H.; Gharagozloo, M. Biased Treg/Th17 balance away from regulatory toward inflammatory phenotype in relapsed multiple sclerosis and its correlation with severity of symptoms. J. Neuroimmunol. 2013; 2621, 106–112.
- Murphy, A.C.; Lalor, S.J.; Lynch, M.A.; Mills, K.H.G. Infiltration of Th1 and Th17 cells and activation of microglia in the CNS during the course of experimental autoimmune encephalomyelitis. Brain Behav. Immun. 2010, 24, 641–651.
- Lu, J.; Wu, W. Cholinergic modulation of the immune syste -A novel therapeutic target for myocardial inflammation. Int. Immunopharmacol. 2021, 93, 107391.
- Hao, J.; Simard, A.R.; Turner, G.H.; Wu, J.; Whiteaker, P.; Lukas, R.J.; Shi, F.D. Attenuation of CNS inflammatory responses by nicotine involves α7 and non-α7 nicotinic receptors. Exp. Neurol. 2011, 227, 110–119.
- Wang, D.; Zhou, R.B.; Yao, Y.M.; Zhu, X.M.; Yin, Y.M.; Zhao, G.J.; Dong, N.; Sheng, Z.Y. Stimulation of α7 nicotinic acetylcholine receptor by nicotine increases suppressive capacity of naturally occurring CD4+CD25+ regulatory T cells in mice in vitro. J. Pharmacol. Exp. Ther. 2010, 335, 553–561.
- De Rosa, M.J.; Dionisio, L.; Agriello, E.; Bouzat, C.; Del Carmen Esandi, M. Alpha 7nicotinic acetylcholine receptor modulates lymphocyte activation. Life Sci. 2009, 85, 444–449.
- Nizri, E.; Irony-Tur-Sinai, M.; Lory, O.; Orr-Urtreger, A.; Lavi, E.; Brenner, T. Activation of the cholinergic anti-inflammatory system by nicotine attenuates neuroinflammation via suppression of Th1 and Th17 responses. J. Immunol. 2009, 183, 6681–6688.
- Wang, H.; Yu, M.; Ochani, M.; Amella, C.A.; Tanovic, M.; Susarla, S.; Li, J.H.; Wang, H.; Yang, H.; Ulloa, L.; et al. Nicotinic acetylcholine receptor alpha7 subunit is anessential regulator of inflammation. Nature 2003, 421, 384–388.
- Parrish, W.R.; Rosas-Ballina, M.; Gallowitsch-Puerta, M.; Ochani, M.; Ochani, K.; Yang, L.H.; Hudson, L.; Lin, X.; Patel, N.; Johnson, S.M.; et al. Modulation of TNF release by choline requires alpha7 subunit nicotinic acetylcholine receptormediated signaling. Mol. Med. 2008, 14, 567–574.
- Saeed, R.W.; Varma, S.; Peng-Nemeroff, T.; Sherry, B.; Balakhaneh, D.; Huston, J.; Tracey, K.J.; Al-Abed, Y.; Metz, C.N. Cholinergic stimulation blocks endothelial cell activation and leukocyte recruitment during inflammation. J. Exp. Med. 2005, 201, 1113–1123.
- Czura, C.J.; Friedman, S.G.; Tracey, K.J. Neural inhibition of inflammation: The cholinergic anti-inflammatory pathway. J. Endotoxin Res. 2003, 9, 409–413.
- Yoshikawa, H.; Kurokawa, M.; Ozaki, N.; Nara, K.; Atou, K.; Takada, E.; Suzuki, N. Nicotine inhibits the production of proinflammatory mediators in human monocytes by suppression of I-kappaB phosphorylation and nuclear factor-kappaB transcriptional activity through nicotinic acetylcholine receptor alpha7. Clin. Exp. Immunol. 2006, 146, 116–123.
- Rosas-Ballina, M.; Goldstein, R.S.; Gallowitsch-Puerta, M.; Yang, L.; Valdés-Ferrer, S.I.; Patel, N.B.; Chavan, S.; Al-Abed, Y.; Yang, H.; Tracey, K.J. The selective alpha7 agonist GTS-21 attenuates cytokine production in human whole blood and human monocytes activated by ligands for TLR2, TLR3, TLR4, TLR9, and RAGE. Mol. Med. 2009, 15, 195–202.
- Reale, M.; De Angelis, F.; Di Nicola, M.; Capello, E.; Di Ioia, M.; De Luca, G.; Lugaresi, A.; Tata, A.M. Relation between pro-inflammatory cytokines and acetylcholine levels in relapsing-remitting multiple sclerosis patients. Int. J. Mol. Sci. 2012, 1310, 12656–12664.
- Liu, Z.; Han, B.; Li, P.; Wang, Z.; Fan, Q. Activation of α7nAChR by nicotine reduced the Th17 response in (CD4+)T lymphocytes. Immunol. Investig. 2014, 437, 667–674.
- Di Bari, M.; Reale, M.; Di Nicola, M.; Orlando, V.; Galizia, S.; Porfilio, I.; Costantini, E.; D’Angelo, C.; Ruggieri, S.; Biagioni, S.; et al. Dysregulated homeostasis of acetylcholine levels in immune cells of RR-multiple sclerosis patients. Int. J. Mol. Sci. 2016, 17, 2009.
- Di Bari, M.; Di Pinto, G.; Reale, M.; Mengod, G.; Tata, A.M. Cholinergic system and neuroinflammation: Implication in multiple sclerosis. CNS Agents Med. Chem. 2017, 17, 109–115.
- Gatta, V.; Mengod, G.; Reale, M.; Tata, A.M. Possible Correlation between Cholinergic System Alterations and Neuro/Inflammation in Multiple Sclerosis. Biomedicines 2020, 86, 153.
- Jiang, W.; Li, D.; Han, R.; Zhang, C.; Jin, W.N.; Wood, K.; Liu, Q.; Shi, F.D.; Hao, J. Acetylcholine-producing NK cells attenuate CNS inflammation via modulation of infiltrating monocytes/ macrophages. Proc. Natl. Acad Sci. USA 2017, 114, E6202–E6211.
- Castellani, R.J.; Perry, G. The complexities of the pathology–pathogenesis relationship in Alzheimer disease. Biochemical. Pharmacol. 2014, 88, 671–676.
- De Ferrari, G.V.; Canales, M.A.; Shin, I.; Weiner, L.M.; Silman, I.; Inestrosa, N.C. A structural motif of acetylcholinesterase that promotes amyloid beta-peptide fibril formation. Biochemistry 2001, 4035, 10447–10457.
- Apelt, J.; Kumar, A.; Schliebs, R. Impairment of cholinergic neurotransmission in adult and aged transgenic Tg2576 mouse brain expressing the Swedish mutation of human beta-amyloid precursor protein. Brain Res. 2002; 953, 17–30.
- Lu, B.; Kwan, K.; Levine, Y.A.; Olofsson, P.S.; Yang, H.; Li, J.; Joshi, S.; Wang, H.; Andersson, U.; Chavan, S.S.; et al. α7 nicotinic acetylcholine receptor signaling inhibits inflammasome activation by preventing mitochondrial DNA release. Mol. Med. 2014, 20, 350–358.
- Wyss-Coray, T.; Rogers, J. Inflammation in Alzheimer disease-a brief review of the basic science and clinical literature. Cold Spring Harb. Perspect. Med. 2012, 21, a006346.
- Reale, M.; D’Angelo, C.; Costantini, E.; Di Nicola, M.; Yarla, N.S.; Kamal, M.A.; Salvador, N.; Perry, G. Expression Profiling of Cytokine, Cholinergic Markers, and Amyloid-β Deposition in the APPSWE/PS1dE9 Mouse Model of Alzheimer’s Disease Pathology. J. Alzheimers Dis. 2018, 621, 467–476.
- Holmes, C.; Cunningham, C.; Zotova, E.; Woolford, J.; Dean, C.; Kerr, S.U.; Culliford, D.; Perry, V.H. Systemic inflammation and disease progression in Alzheimer disease. Neurology 2009, 73, 768–774.
- Dursun, E.; Gezen-Ak, D.; Hanağası, H.; Bilgiç, B.; Lohmann, E.; Ertan, S.; Atasoy, İ.L.; Alaylıoğlu, M.; Araz, Ö.S.; Önal, B.; et al. The interleukin 1 alpha, interleukin 1 beta, interleukin 6 and alpha-2-macroglobulin serum levels in patients with early or late onset Alzheimer’s disease, mild cognitive impairment or Parkinson’s disease. J. Neuroimmunol. 2015, 283, 50–57.
- Reale, M.; Kamal, M.A.; Velluto, L.; Gambi, D.; Di Nicola, M.; Greig, N.H. Relationship between Inflammatory Mediators, Aβ Levels and ApoE Genotype in Alzheimer Disease. Curr. Alzheimer Res. 2012, 9, 447–457.
- Ferretti, M.T.; Merlini, M.; Spani, C.; Gericke, C.; Schweizer, N.; Enzmann, G.; Engelhardt, B.; Kulic, L.; Suter, T.; Nitsch, R.M. T-cell brain infiltration and immature antigen-presenting cells in transgenic models of Alzheimer’s disease-like cerebral amyloidosis. Brain Behav. Immun. 2016, 54, 211–225.
- McQuillan, K.; Lynch, M.A.; Mills, K.H. Activation of mixed glia by Abeta-specific Th1 and Th17 cells and its regulation by Th2 cells. Brain Behav. Immun. 2010, 24, 598–607.
- Reale, M.; Iarlori, C.; Gambi, F.; Feliciani, C.; Isabella, L.; Gambi, D. The acetylcholinesterase inhibitor, Donepezil, regulates a Th2 bias in Alzheimer’s diseasepatients. Neuropharmacology 2006, 505, 606–613.
- Reale, M.; Di Nicola, M.; Velluto, L.; D’Angelo, C.; Costantini, E.; Lahiri, D.K.; Kamal, M.A.; Yu, Q.-S.; Greig, N.H. Selective Acetyl- and Butyrylcholinesterase Inhibitors Reduce Amyloid-b Ex Vivo Activation of Peripheral Chemo-cytokines From Alzheimer’s Disease Subjects: Exploring the Cholinergic Anti-inflammatory Pathway. Curr. Alzheimer Res. 2014, 11, 608–622.
- Heneka, M.T.; Kummer, M.P.; Latz, E. Innate immune activation in neurodegenerative disease. Nat. Rev. Immunol. 2014, 14, 463–477.
- Bellucci, A.; Bubacco, L.; Longhena, F.; Parrella, E.; Faustini, G.; Porrini, V.; Bono, F.; Missale, C.; Pizzi, M. Nuclear Factor-κB Dysregulation and α-Synuclein Pathology: Critical Interplay in the Pathogenesis of Parkinson’s Disease. Front. Aging Neurosci. 2020, 12, 68.
- Alvarez-Erviti, L.; Couch, Y.; Richardson, J.; Cooper, J.M.; Wood, M.J. Alpha-synuclein release by neurons activates the inflammatory response in a microglial cell line. Neurosci. Res. 2011, 694, 337–342.
- Brochard, V.; Combadière, B.; Prigent, A.; Laouar, Y.; Perrin, A.; Beray-Berthat, V.; Bonduelle, O.; Alvarez-Fischer, D.; Callebert, J.; Launay, J.M.; et al. Infiltration of CD4+ lymphocytes into the brain contributes to neurodegeneration in a mouse model of Parkinson disease. J. Clin. Investig. 2009, 1191, 182–192.
- Reale, M.; Iarlori, C.; Thomas, A.; Gambi, D.; Perfetti, B.; Di Nicola, M.; Onofrj, M. Peripheral cytokines profile in Parkinson’s disease. Brain Behav. Immun. 2009, 231, 55–63.
- Harms, A.S.; Ferreira, S.A.; Romero-Ramos, M. Periphery and brain, innate and adaptive immunity in Parkinson’s disease. Acta Neuropathol. 2021.
- Lin, M.T.; Beal, M.F. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006, 443, 787.
- Tejera, D.; Mercan, D.; Sanchez-Caro, J.M.; Hanan, M.; Greenberg, D.; Soreq, H.; Latz, E.; Golenbock, D.; Heneka, M.T. Systemic inflammation impairs microglial Aβ clearance through NLRP3 inflammasome. EMBO J. 2019, 38, e101064.
- Peña, G.; Cai, B.; Liu, J.; Van der Zanden, E.P.; Deitch, E.A.; De Jonge, W.J.; Ulloa, L. Unphosphorylated STAT3 modulates alpha 7 nicotinic receptor signaling and cytokine production in sepsis. Eur. J. Immunol. 2010, 40, 2580–2589.
- Tsoyi, K.; Jang, H.J.; Kim, J.W.; Chang, H.K.; Lee, Y.S.; Pae, H.O.; Kim, H.J.; Seo, H.G.; Lee, J.H.; Chung, H.T.; et al. Stimulation of alpha7 nicotinic acetylcholine receptor by nicotine attenuates inflammatory response in macrophages and improves survival in experimental model of sepsis through heme oxygenase-1 induction. Antioxid Redox Signal. 2011, 14, 2057–2070.
- Maurer, S.V.; Williams, C.L. The Cholinergic System Modulates Memory and Hippocampal Plasticity via Its Interactions with Non-Neuronal Cells. Front. Immunol. 2017, 8, 1489.
- O’Connell, R.M.; Rao, D.S.; Baltimore, D. microRNA regulation of inflammatory responses. Annu. Rev. Immunol. 2012, 30, 295–312.
- Liu, G.; Abraham, E. MicroRNAs in immune response and macrophage polarization. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 170–177.
- Priyadarshini, M.; Arivarasu, N.A.; Shah, A.; Tabrez, S.; Priyamvada, S.; Aatif, M. MicroRNA: Novel modulators of the cholinergic anti-inflammatory pathway. Antiinflamm. Antiallergy Agents Med. Chem. 2013, 12, 136–140.
- Nadorp, B.; Soreq, H. Predicted overlapping microRNA regulators of acetylcholine packaging and degradation in neuroinflammation-related disorders. Front. Mol. Neurosci. 2014, 7, 9.
- Ofek, K.; Soreq, H. Cholinergic involvement and manipulation approaches in multiple system disorders. Chem. Biol. Interact. 2013, 203, 113–119.
- Ponomarev, E.D.; Veremeyko, T.; Barteneva, N.; Krichevsky, A.M.; Weiner, H.L. MicroRNA-124 promotes microglia quiescence and suppresses EAE by deactivating macrophages via the C/EBP-α-PU.1 pathway. Nat. Med. 2011, 17, 64–70.
- Sun, Y.; Li, Q.; Gui, H.; Xu, D.P.; Yang, Y.L.; Su, D.F.; Liu, X. MicroRNA-124 mediates the cholinergic anti-inflammatory action through inhibiting the production of pro-inflammatory cytokines. Cell Res. 2013, 23, 1270–1283.
- Zhou, W.; Wang, J.; Li, Z.; Li, J.; Sang, M. MicroRNA-2055b inhibits HMGB1 expression in LPS-induced sepsis. Int. J. Mol. Med. 2016, 38, 312–318.
- Hanieh, H.; Alzahrani, A. MicroRNA-132 suppresses autoimmune encephalomyelitis by inducing cholinergic anti-inflammation: A new Ahr-based exploration. Eur. J. Immunol. 2013, 43, 2771–2782.




