
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Sara Lopez Martinez | + 1793 word(s) | 1793 | 2021-04-20 13:23:49 | | | |
| 2 | Peter Tang | Meta information modification | 1793 | 2021-04-29 10:10:05 | | |
Video Upload Options
Adult stem cells (ASCs) were long suspected to exist in the endometrium. Indeed, several types of endometrial ASCs were identified in rodents and humans through diverse isolation and characterization techniques. Putative stromal and epithelial stem cell niches were identified in murine models using label-retention techniques. In humans, functional methods (clonogenicity, long-term culture, and multi-lineage differentiation assays) and stem cell markers (CD146, SUSD2/W5C5, LGR5, NTPDase2, SSEA-1, or N-cadherin) facilitated the identification of three main types of endogenous endometrial ASCs: stromal, epithelial progenitor, and endothelial stem cells. Further, exogenous populations of stem cells derived from bone marrow may act as key effectors of the endometrial ASC niche.
1. Introduction
Stem cells are undifferentiated cells capable of simultaneously self-renewing and differentiating into multiple tissue-specific cell types under appropriate stimuli [1][2]. Traditionally, stem cells can be classified according to their location and differentiation potency. The most potent stem cells are zygotes, classified as totipotent stem cells, which have the ability to generate a whole embryo and extra-embryonic structures. Then, pluripotent stem cells such as embryonic stem cells give rise to the three primary germ layers, namely endoderm, ectoderm, and mesoderm. Induced pluripotent stem cells, generated by the reprogramming of somatic cells, are included in this group. Unipotent stem cells have the narrowest differentiation capability and divide themselves repeatedly into a single cell type. Finally, multipotent cells give rise to specific lineages [3]. Adult stem cells (ASCs), also referred to as somatic stem cells, are a genre of multipotent stem cells located in specific differentiated organs and can differentiate into a limited type of mature cell to maintain tissue homeostasis [2]. The necessary conditions are provided by the specific anatomical location surrounding the ASCs. This microenvironment, called the stem cell niche, gives rise to autocrine, paracrine, and systemic signals that enable stem cell maintenance and differentiation into specific cell types that participate in tissue repair or regeneration [4][5]. Most of the described ASCs reside in the bone marrow, but they are also detected in several organs and play a crucial role in tissue homeostasis, renewal, and repair [6]. Consequently, this transdifferentiation capacity enables research into therapeutic approaches in tissues such as the blood [7], intestine [8], skin [9], muscle [10], brain [11], and endometrium [12].
The endometrium is the innermost lining of the uterus and its main function is preparing for implantation and attracting the blastocyst towards the uterus. The human endometrium is divided into two different layers with different properties. First, the functionalis, which is the upper layer, is formed by luminal epithelium and subjacent stroma as well as microvasculature. Second, the basalis is constituted by glands and stroma that are preserved throughout the female’s life [13]. During menstruation, the functionalis layer is removed from the body through the menstrual blood, while the basalis remains as an endometrial supply for regeneration of a new functional layer in the next cycle. The human endometrium has some features including menstruation that make it physiologically unique from the murine models. The human menstrual cycle consists of three stages: growth, differentiation, and shedding, which occur around 400 times until menopause [14]. In the mouse, the estrous cycle is divided into four stages (proestrus, estrus, metestrus, and diestrus) occurring every 4 to 5 days, but does not involve menstruation and regeneration of the functionalis layer [15]. Mice undergo up to 80 estrous cycles and can give rise to 8-10 litters during their reproductive life, so active repair and regeneration mechanisms in the endometrial mucosa are critically important [16]. This pronounced remodeling ability and the later proliferative changes seen in adult mammals have driven the hypothesis that there is a niche of ASCs in the endometrium that is activated in every cycle [17][18]. Accordingly, alterations in this endogenous niche could be responsible for endometrial pathology, causing fertility problems [19]. Endometrial pathologies such as Asherman syndrome (AS), caused by the presence of intrauterine adhesions [20], or endometrial atrophy (EA), characterized by an atrophic and usually thin endometrium [21], could originate from insufficient production of endogenous cells and/or a non-functional ASC niche. Other gynecological pathologies such as endometriosis could also be caused by variations in endogenous endometrial ASC activity [19].
Even though endometrial ASCs have been described in different mammals, such as cows [22], pigs [23], sheep [24], horses [25], and non-human primates [26], in this review we focus on the discovery of stem cells in mice and humans, pointing out how these ASCs were identified and describing the different techniques employed (Figure 1). In addition, we highlight the value of pre-clinical models of stem cell therapy to treat AS and EA.
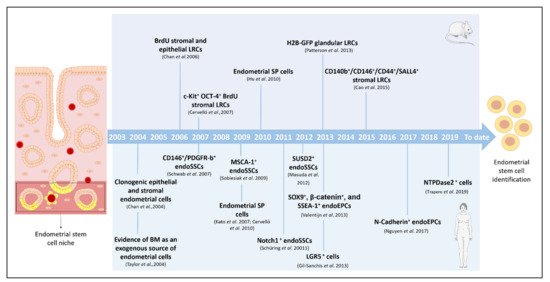
Figure 1. Milestones in the identification, isolation, and characterization of endometrial stem cells. Timeline of the principal findings concerning endometrial stem cells and specific isolation techniques in murine and human models. BM: bone marrow; BrdU: bromodeoxyuridine; CD: cluster of differentiation; endoSSCs: endometrial stromal stem cells; endoEPCs: endometrial epithelial progenitor cells; GFP: green fluorescent protein; H2B: histone 2B; LRCs: label-retaining cells; LGR: leucine-rich repeat containing G-protein-coupled receptor; MenSCs: menstrual blood stem cells; NTPDase2: ectonucleoside triphosphate diphosphohydrolase-2; SSEA-1: stage-specific embryonic antigen-1; SP: side population; SUSD2: sushi domain-containing-2.
2. Endometrial Stem Cells and Specific Niches
In 1978, Schofield proposed the concept of a stem cell niche for the first time, referring to an anatomically defined compartment in which stem cells reside and are able to renew and/or remain undifferentiated [27]. We know now how the niche is specialized and the dynamic microenvironment in which stem cells interact with differentiated cells, secreted factors, and/or components of the extracellular matrix (ECM) [28]. These stimuli determine the behavior (rate and pattern of division) of the stem cells in each specific tissue [29]. Prianishnikov was the first to propose the existence of ASCs in the endometrium [30] as an immature hormone-independent population, residing in the deepest basalis layer with the capability of differentiating towards hormone-responsible endometrial cells. The constant maintenance of this layer along the menstrual (or estrous) cycle makes it a reasonable candidate to be a reservoir of ASCs, even if some stem cells are also found in the functionalis [31]. In 1991, Padykula claimed that the population of stem-like cells migrates and produces different progenitor cells that differentiate specifically into vascular, epithelial, or stromal compartments, thanks to their specific niches [32]. Over the years, these endogenous endometrial ASCs have been proposed not only to be responsible for the cyclic endometrial growth and, consequently, for certain gynecological disorders, but also as useful in therapeutic approaches. According to the origin of the stem cells, endometrial stem cell niches include epithelial, stromal, and endothelial cells, and are likely to contribute to endometrial regeneration [33].
3. Stem Cell Therapy and the Endometrium: The Importance of Basic Research and Pre-Clinical Models
The knowledge acquired during the search for an endogenous endometrial stem cell population has contributed to stem cell therapy, widely used in other medical fields, for endometrial pathologies. Stem cell therapy is based on the capacity of the stem cells to arrive at the damaged site, self-renew, and differentiate into target tissue cells to empower the repopulation and regeneration of, in this case, the endometrium. However, with the growing implementation of stem cell therapies and low engraftment of the cells observed in some studies [34][35], other mechanisms of action have been proposed.
3.1. Paracrine Action of Stem Cells: Main Findings in the Endometrium
Currently, the paracrine hypothesis is probably the most documented and accepted one. Following this premise, stem cells secrete different biomolecules such as growth, angiogenic, or immunosuppressive factors, as well as chemokines and exosomes, which contribute to regeneration of the injured tissue [36]. In line with these secreted immunosuppressive factors, the stem cells are also thought to have an immunomodulatory potential to manage the inflammation status of the injured site and prepare the tissue for the incoming regenerative events [37]. Different studies in animal models corroborate this paracrine hypothesis by elucidating low stem cell engraftment when applied to a damaged endometrium [38]. In addition, this therapy has similar effectiveness whether it is administered through the tail vein or directly into the uterine horns [38][39]. These findings support the hypothesis that the regenerative potential of stem cells might not be due to proliferation of the administered stem cells themselves but to the paracrine factors they secrete or stimulate in the receptor organism. Interestingly, injected stem cells engraft into the spiral arterioles of the endometrium where the stem cell niche is thought to be located [40]. This finding reinforces the existence of this stem cell niche and the paracrine effect that the endogenous cells exert to attract the therapy (stem cells in this case) to directly act over the niche and promote tissue regeneration.
The immunomodulatory action of these stem cells in the target tissue was also elucidated in the endometrial milieu. Downregulation of some genes such as chemokine CXCL8 is hypothesized to reduce the inflammation status and induce the expression of other factors such as SERPINE1 or proto-oncogene c-JUN, preparing the tissue for the incoming regeneration processes [40].
3.2. Stem Cell Therapy for Treating Endometrial Pathologies
To treat AS and EA, different sources of stem cells have been used. The most explored sources for endometrial regeneration are the umbilical cord [41][42], amniotic membrane [43][44], bone marrow and adipose tissue [45], in addition to menstrual blood [46], or even autologous endometrial biopsies [47][48]. These last four types of stem cells usually have an autologous origin. Thus, complications such as graft-versus-host disease, associated with allogenic stem cell transplant, do not apply; however, they can involve other disadvantages such as an insufficient number of cells [49]. This could explain why most published works in the field, most of them in the form of pilot studies or case reports, directly try the therapy in humans [50]. However, using pre-clinical models, usually rodents (mice and rats), mimicking either the adhesions and fibrotic tissue characteristic of AS patients or the thin and/or atrophic endometrium of patients with EA, is important before translating medical approaches to women [51], which is always the final goal. The importance of these animal models resides in their capacity to predict the outcome of future clinical trials.
3.2.1. Pre-Clinical Models of Endometrial Injury
Bone marrow is an excellent source for obtaining stem cells to treat endometrial pathologies. The contribution of these stem cells to the endometrial stem cell niche has probably encouraged their use. As mentioned before, the bone marrow contains a heterologous cell population, [52], thus, it is important to distinguish which subpopulation of stem cells is administered into murine models of AS or injured endometrium. Some studies use the non-hematopoietic or stromal BMDSCs that are positive for CD29, CD44, CD73, and/or CD90, but negative for CD45 or CD34 [39][53][54][55]. Other research groups inject hematopoietic BMDSCs cells using different markers such as CD133 antigen [38]. Furthermore, other studies report the use of the whole bone marrow stem cell fractions [56][57].
Studies using other stem cell sources [41][42][43][44][45][46][47][48] to treat animal models of endometrial injury (umbilical cord, adipose tissue, amniotic membrane, menstrual blood, and endometrial biopsies) mainly explored the mesenchymal stem cell type using markers such as CD29, CD44, CD73, CD90, and CD105.
References
- Dulak, J.; Szade, K.; Szade, A.; Nowak, W.; Józkowicz, A. Adult stem cells: Hopes and hypes of regenerative medicine. Acta Biochim. Pol. 2015, 62, 329–337.
- Clevers, H. What is an adult stem cell? Science 2015, 350, 1319–1320.
- Zakrzewski, W.; Dobrzyński, M.; Szymonowicz, M.; Rybak, Z. Stem cells: Past, present, and future. Stem Cell Res. Ther. 2019, 10, 1–22.
- Cervelló, I.; Santamaria, X.; Miyazaki, K.; Maruyama, T.; Simon, C. Cell Therapy and Tissue Engineering from and toward the Uterus. Semin. Reprod. Med. 2015, 33, 366–372.
- Chacón-Martínez, C.A.; Koester, J.; Wickström, S.A. Signaling in the stem cell niche: Regulating cell fate, function and plasticity. Development 2018, 145, 165399.
- Gurusamy, N.; Alsayari, A.; Rajasingh, S.; Rajasingh, J. Adult Stem Cells for Regenerative Therapy. Prog. Mol. Biol. Transl. Sci. 2018, 160, 1–22.
- Amouzegar, A.; Dey, B.R.; Spitzer, T.R. Peripheral Blood or Bone Marrow Stem Cells? Practical Considerations in Hematopoietic Stem Cell Transplantation. Transfus. Med. Rev. 2019, 33, 43–50.
- Ayyaz, A.; Kumar, S.; Chan, K.; Wrana, J.L.; Gregorieff, A.; Sangiorgi, B.; Ghoshal, B.; Gosio, J.; Ouladan, S.; Fink, M.; et al. Single-cell transcriptomes of the regenerating intestine reveal a revival stem cell. Nature 2019, 569, 121–125.
- Gonzales, K.A.U.; Fuchs, E. Skin and Its Regenerative Powers: An Alliance between Stem Cells and Their Niche. Dev. Cell 2017, 43, 387–401.
- Mashinchian, O.; Pisconti, A.; Le Moal, E.; Bentzinger, C.F. The Muscle Stem Cell Niche in Health and Disease. In Current Topics in Developmental Biology, 1st ed.; David Sasson: Bombay, India, 2018; Volume 126, pp. 23–65.
- Kelava, I.; Lancaster, M.A. Stem Cell Models of Human Brain Development. Cell Stem Cell 2016, 18, 736–748.
- Cervelló, I.; Mas, A.; Gil-Sanchis, C.; Simon, C. Somatic Stem Cells in the Human Endometrium. Semin. Reprod. Med. 2013, 31, 69–76.
- Simón, C.; Horcajadas, J.A.; García-Velasco, J.; Pellicer, A. El Endometrio Humano: Desde la Investigación a La Clínica, 1st ed.; Editorial Médica Panamericana: Buenos Aires, Argentina, 2009; pp. 2–42.
- Jabbour, H.N.; Kelly, R.W.; Fraser, H.M.; Critchley, H.O.D. Endocrine Regulation of Menstruation. Endocr. Rev. 2006, 27, 17–46.
- Byers, S.L.; Wiles, M.V.; Dunn, S.L.; Taft, R.A. Mouse estrous cycle identification tool and images. PLoS ONE 2012, 7, e35538.
- Gargett, C.E.; Nguyen, H.P.T.; Ye, L. Endometrial regeneration and endometrial stem/progenitor cells. Rev. Endocr. Metab. Disord. 2012, 13, 235–251.
- Teixeira, J.; Rueda, B.R.; Pru, J.K. Uterine Stem cells. In StemBook, 1st ed.; The Stem Cell Research Community: Cambridge, MA, USA; Harvard Stem Cell Institute: Cambridge, MA, USA, 2008; Available online: (accessed on 2 January 2021).
- Prianishnikov, V.A. On the concept of stem cell and a model of functional-morphological structure of the endometrium. Contraception 1978, 18, 213–223.
- Deane, J.A.; Gualano, R.C.; Gargett, C.E. Regenerating endometrium from stem/progenitor cells: Is it abnormal in endometriosis, Asherman’s syndrome and infertility? Curr. Opin. Obstet. Gynecol. 2013, 25, 193–200.
- Dreisler, E.; Kjer, J.J. Asherman’s syndrome: Current perspectives on diagnosis and management. Int. J. Women Health 2019, 11, 191–198.
- Lebovitz, O.; Orvieto, R. Treating patients with “thin” endometrium-an ongoing challenge. Gynecol. Endocrinol. 2014, 30, 409–414.
- Cabezas, J.; Lara, E.; Pacha, P.; Rojas, D.; Veraguas, D.; Saravia, F.; Rodríguez-Alvarez, L.; Castro, F.O. The Endometrium of Cycling Cows Contains Populations of Putative Mesenchymal Progenitor Cells. Reprod. Domest. Anim. 2014, 49, 550–559.
- Miernik, K.; Karasinski, J. Porcine uterus contains a population of mesenchymal stem cells. Reproduction 2012, 143, 203–209.
- Letouzey, V.; Tan, K.S.; Deane, J.A.; Ulrich, D.; Gurung, S.; Ong, Y.R.; Gargett, C.E. Isolation and Characterisation of Mesenchymal Stem/Stromal Cells in the Ovine Endometrium. PLoS ONE 2015, 10, e0127531.
- Cabezas, J.; Rojas, D.; Navarrete, F.; Ortiz, R.; Rivera, G.; Saravia, F.; Rodriguez-Alvarez, L.; Castro, F. Equine mesenchymal stem cells derived from endometrial or adipose tissue share significant biological properties, but have distinctive pattern of surface markers and migration. Theriogenology 2018, 106, 93–102.
- Padykula, H.A.; Coles, L.G.; Okulicz, W.C.; Rapaport, S.I.; McCracken, J.A.; King, N.W., Jr.; Longcope, C.; Kaiserman-Abramof, I.R. The Basalis of the Primate Endometrium: A Bifunctional Germinal Compartment. Biol. Reprod. 1989, 40, 681–690.
- Schofield, R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells 1978, 4, 7–25.
- Lane, S.W.; Williams, D.A.; Watt, F.M. Modulating the stem cell niche for tissue regeneration. Nat. Biotechnol. 2014, 32, 795–803.
- Ferraro, F.; Lo Celso, C.; Scadden, D. Adult stem cells and their niches. Adv. Exp. Med. Biol. 2010, 695, 155–168.
- Prianishnikov, V.A. A functional model of the structure of the epithelium of normal, hyperplastic, and malignant human endometrium: A review. Gynecol. Oncol. 1978, 6, 420–428.
- Cousins, F.L.; Dorien, F.O.; Gargett, C.E. Endometrial stem/progenitor cells and their role in the pathogenesis of endometriosis. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 50, 27–38.
- Padykula, H.A. Regeneration in the Primate Uterus: The Role of Stem Cells. Ann. N. Y. Acad. Sci. 1991, 622, 47–56.
- Santamaria, X.; Mas, A.; Cervelló, I.; Taylor, H.; Simon, C. Uterine stem cells: From basic research to advanced cell therapies. Hum. Reprod. Update 2018, 24, 673–693.
- Terrovitis, J.V.; Smith, R.R.; Marbán, E. Assessment and optimization of cell engraftment after transplantation into the heart. Circ. Res. 2010, 106, 479–494.
- Von Bahr, L.; Batsis, I.; Moll, G.; Hägg, M.; Szakos, A.; Sundberg, B.; Uzunel, M.; Ringden, O.; Le Blanc, K. Analysis of Tissues Following Mesenchymal Stromal Cell Therapy in Humans Indicates Limited Long-Term Engraftment and No Ectopic Tissue Formation. Stem Cells 2012, 30, 1575–1578.
- Ratajczak, M.Z.; Kucia, M.; Jadczyk, T.; Greco, N.J.; Wojakowski, W.; Tendera, M.; Ratajczak, J. Pivotal role of paracrine effects in stem cell therapies in regenerative medicine: Can we translate stem cell-secreted paracrine factors and microvesicles into better therapeutic strategies? Leukemia 2012, 26, 1166–1173.
- Molina, E.R.; Smith, B.T.; Shah, S.R.; Shin, H.; Mikos, A.G. Immunomodulatory properties of stem cells and bioactive molecules for tissue engineering. J. Control. Release 2015, 219, 107–118.
- Cervelló, I.; Gil-Sanchis, C.; Santamaría, X.; Cabanillas, S.; Díaz, A.; Faus, A.; Pellicer, A.; Simón, C. Human CD133+ bone marrow-derived stem cells promote endometrial proliferation in a murine model of Asherman syndrome. Fertil. Steril. 2015, 104, 1552–1560.e3.
- Wang, J.; Ju, B.; Pan, C.; Gu, Y.; Zhang, Y.; Sun, L.; Zhang, B.; Zhang, Y. Application of Bone Marrow-Derived Mesenchymal Stem Cells in the Treatment of Intrauterine Adhesions in Rats. Cell. Physiol. Biochem. 2016, 39, 1553–1560.
- De Miguel–Gómez, L.; Ferrero, H.; López-Martínez, S.; Campo, H.; López-Pérez, N.; Faus, A.; Hervás, D.; Santamaría, X.; Pellicer, A.; Cervelló, I. Stem cell paracrine actions in tissue regeneration and potential therapeutic effect in human endometrium: A retrospective study. BJOG Int. J. Obstet. Gynecol. 2019, 127, 551–560.
- Xu, L.; Ding, L.; Wang, L.; Cao, Y.; Zhu, H.; Lu, J.; Li, X.; Song, T.; Hu, Y.; Dai, J. Umbilical cord-derived mesenchymal stem cells on scaffolds facilitate collagen degradation via upregulation of MMP-9 in rat uterine scars. Stem Cell Res. Ther. 2017, 8, 84.
- Zhang, L.; Li, Y.; Guan, C.-Y.; Tian, S.; Lv, X.-D.; Li, J.-H.; Ma, X.; Xia, H.-F. Therapeutic effect of human umbilical cord-derived mesenchymal stem cells on injured rat endometrium during its chronic phase. Stem Cell Res. Ther. 2018, 9, 1–15.
- Gan, L.; Duan, H.; Xu, Q.; Tang, Y.-Q.; Li, J.-J.; Sun, F.-Q.; Wang, S. Human amniotic mesenchymal stromal cell transplantation improves endometrial regeneration in rodent models of intrauterine adhesions. Cytotherapy 2017, 19, 603–616.
- Ouyang, X.; You, S.; Zhang, Y.; Zhang, C.; Zhang, G.; Shao, X.; He, F.; Hu, L. Transplantation of Human Amnion Epithelial Cells Improves Endometrial Regeneration in Rat Model of Intrauterine Adhesions. Stem Cells Dev. 2020, 29, 1346–1362.
- Kilic, S.Ş.; Yuksel, B.; Pinarli, F.A.; Albayrak, A.; Boztok, B.; Delibasi, T. Effect of stem cell application on Asherman syndrome, an experimental rat model. J. Assist. Reprod. Genet. 2014, 31, 975–982.
- Hu, J.; Song, K.; Zhang, J.; Zhang, Y.; Tan, B. Effects of menstrual blood-derived stem cells on endometrial injury repair. Mol. Med. Rep. 2019, 19, 813–820.
- Young, Y.; Park, K.; Jin, Y.; Suk, M.; Ching, H.; Rosenwaks, Z.; Ku, S. Acta Biomaterialia Synergistic regenerative effects of functionalized endometrial stromal cells with hyaluronic acid hydrogel in a murine model of uterine damage. Acta Biomater. 2019, 89, 139–151.
- Domnina, A.; Novikova, P.; Obidina, J.; Fridlyanskaya, I.; Alekseenko, L.; Kozhukharova, I.; Lyublinskaya, O.; Zenin, V.; Nikolsky, N. Human mesenchymal stem cells in spheroids improve fertility in model animals with damaged endometrium. Stem Cell Res. Ther. 2018, 9, 1–12.
- Champlin, R. Selection of Autologous or Allogeneic Transplantation. In Cancer Medicine; Bast, R.C., Kufe, D.W., Pollock, R.E., Weichselbaum, R.R., Hollan, J.F., Frei, E., Eds.; BC Decker: Hamilton, Canada, 2003.
- Queckbörner, S.; Davies, L.C.; Von Grothusen, C.; Santamaria, X.; Simon, C.; Gemzell-Danielsson, K. Cellular therapies for the endometrium: An update. Acta Obstet. Gynecol. Scand. 2019, 98, 672–677.
- Andersen, M.D.; Alstrup, A.K.O.; Duvald, C.S.; Mikkelsen, E.F.R.; Vendelbo, M.H.; Ovesen, P.G.; Pedersen, M. Animal Models of Fetal Medicine and Obstetrics. In Experimental Animal Models of Human Diseases—An Effective Therapeutic Strategy, 1st ed.; Bartholomew, I., Ed.; IntechOpen: London, UK, 2018; pp. 343–374.
- Kucia, M.; Reca, R.; Jala, V.R.; Dawn, B.; Ratajczak, J.; Ratajczak, M.Z. Bone marrow as a home of heterogenous populations of nonhematopoietic stem cells. Leukemia 2005, 19, 1118–1127.
- Jing, Z.; Qiong, Z.; Yonggang, W.; Yanping, L. Rat bone marrow mesenchymal stem cells improve regeneration of thin endometrium in rat. Fertil. Steril. 2014, 101, 587–594.e3.
- Gao, L.; Huang, Z.; Lin, H.; Tian, Y.; Li, P.; Lin, S. Bone marrow mesenchymal stem cells (BMSCs) restore functional endometrium in the rat model for severe sherman syndrome. Reprod. Sci. 2019, 26, 436–444.
- Yang, H.; Wu, S.; Feng, R.; Huang, J.; Liu, L.; Liu, F.; Chen, Y. Vitamin C plus hydrogel facilitates bone marrow stromal cell-mediated endometrium regeneration in rats. Stem Cell Res. Ther. 2017, 8, 267.
- Alawadhi, F.; Du, H.; Cakmak, H.; Taylor, H.S. Bone Marrow-Derived Stem Cell (BMDSC) Transplantation Improves Fertility in a Murine Model of Asherman’s Syndrome. PLoS ONE 2014, 9, e96662.
- Yi, K.W.; Mamillapalli, R.; Sahin, C.; Song, J.; Tal, R.; Taylor, H.S. Bone marrow-derived cells or C-X-C motif chemokine 12 (CXCL12) treatment improve thin endometrium in a mouse model. Biol. Reprod. 2019, 100, 61–70.




