
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ahmad Joshkon | + 4222 word(s) | 4222 | 2020-12-28 08:46:12 | | | |
| 2 | Dean Liu | -1692 word(s) | 2530 | 2021-04-27 04:28:18 | | | | |
| 3 | Dean Liu | -1692 word(s) | 2530 | 2021-04-27 04:29:20 | | |
Video Upload Options
CD146 is a cell adhesion molecule expressed on all the vascular tree and belongs to the immunoglobulin superfamily. Two isoforms of CD146 exists, a long isoform expressed at the cell junction and a short isoform located at the apical membrane of the cells. CD146 appears to be critical in regulating vascular permeability, cell-cell cohesion, leukocyte transmigration, and angiogenesis. As a consequence, CD146 is involved in the pathogenesis of various diseases including autoimmune diseases and cancers. Also, CD146 exists in a soluble form generated via the action of matrix metalloproteinases and referred to as soluble CD146 (sCD146). The concentration of sCD146 is quantifiable in the sera and cerebrospinal fluid of healthy individuals. Indeed, any variation in its physiological concentration is associated with certain diseases making it an excellent biomarker for diagnostic purposes.
1. Introduction
Unlike many other physiological processes that initiate and develop only during embryo implantation and fetal development [1], angiogenesis, which is characterized by the production of new blood vessels from pre-existing microvasculature, also occurs in the adulthood stage and is then referred to as neo-angiogenesis [2]. Indeed, besides embryonic development, angiogenesis is involved in diverse processes like reproduction, renewing of damaged vessels, nurturing of organs after ischemia or strokes, wound healing, or tissue repair. Given these fundamental roles, it is obvious that multiple proteins exist to modulate angiogenesis but also vascular system development. In fact, angiogenesis is finely regulated by soluble factors including proangiogenic growth factors (as VEGF, b-FGF, HGF, etc and their receptors) and anti-angiogenic factors (as thrombospondin 1, angiostatin, endostatin, PF4, etc) in addition to insoluble molecules present in the extracellular matrix (as collagen, fibronectin, etc) [3]. The homeostatic balance between these soluble factors contributes to the onset and maintenance of physiological vascularization. Notably, endothelial cells migrate, proliferate, and differentiate into capillaries in response to a concentration gradient of pro-angiogenic growth factors [4]. Many diseases have been attributed to the deregulation of both angiogenic stimuli and inhibitors [5][6][7]. Indeed, an increase in angiogenic stimuli and a decrease in the angiogenic inhibitors constitute the hallmark of many cancers [8], cardiovascular disorders [9], and chronic inflammatory diseases [10], leading to abnormal neovascularization. Along this line, the cell adhesion molecule of the mucin family, CD146, appears to be expressed at the endothelial junction but also at the apical membrane of endothelial cells where it was recently found to act as a co-receptor for the key angiogenic receptor Flk-1 (VEGFR-2) [11]. Indeed, CD146 is expressed on endothelial cells, smooth muscle cells, and pericytes, and thus on the entire vessel [12]. This membrane glycoprotein is also found as a circulating soluble form which displays multifaceted effects on endothelial and surrounding cells [13].
2. CD146: Generalities
CD146, also referred to as melanoma cell adhesion molecule (MCAM), hemopoietic cell adhesion molecule (HEMCAM), MUC18, S-Endo1, or A32 antigen, is a cell adhesion molecule essentially expressed on the entire vascular tree that belongs to the immunoglobulin superfamily [14]. It plays a significant role in regulating vascular permeability, cell-cell cohesion, leukocyte transmigration, and angiogenesis [15][16][17]. The extracellular domain of this single-pass membrane glycoprotein is composed of two variable regions (V) and three constant regions (C2) V-V-C2-C2-C2, while the intracellular domain is relatively short, containing a single tyrosine residue that may become phosphorylated [18][19]. Two membrane isoforms of CD146 exist, short and long, generated by alternative splicing of the transcript in exon 15, leading to a shift of the reading frame. Despite expressing identical extracellular and transmembrane domains, these two isoforms differ by their cytoplasmic tail. The short isoform (shCD146) displays a shorter cytoplasmic domain encompassing one phosphorylation site for protein kinase C (PKC) and an interaction site with proteins containing a PDZ domain. In contrast, the long isoform (lgCD146) displays two phosphorylation sites by PKC and a dileucine motif for protein targeting to the basolateral membrane [18][20]. Of interest, the expression of these isoforms is spatially selective. The long isoform is located at the cell junction and is involved in structural functions while the short isoform is essentially expressed at the apical membrane of the cell and contributes to angiogenesis. [18][21]. Additionally, shedding of membrane CD146 proteins, as induced by matrix metalloproteinases, generates a soluble form (sCD146) that is detected in the sera of healthy people at a concentration around 260 ± 60 ng/mL [22]. Of interest, CD146 is conserved among species, suggesting its evolutionary significance for physiological development.
2.1. CD146 Expression Pattern and Functions
CD146 is expressed all along the vascular tree regardless of the vessel size and anatomical location, including endothelial cells, smooth muscle cells, and pericytes [23]. This distribution pattern is important for maintaining vessel architecture through heterotypic interaction among these cells via CD146 and its binding partners. As mentioned earlier, the long and the short membrane isoforms have different localizations on endothelial cells. lgCD146 is mainly stored intracellularly when the cells are not confluent. However, at confluency, lgCD146 is redistributed to inter-cellular junctions, outside the tight or adherens junctions, and regulate cell–cell cohesion, paracellular permeability, and monocyte transmigration. shCD146 is involved in regulating endothelial cells adhesion, migration, proliferation, and consequently angiogenesis [21]. CD146 was first identified on melanoma cells as a poor prognostic marker correlating with disease progression, but was later found to be expressed on various cancer cell lines such as breast, kidney, gastric, ovarian, and prostate cancers [14][20][24]. The mechanisms underlying CD146 upregulation on cancer cells remain to be found. Elevated membrane CD146 expression and high soluble CD146 concentration in plasma are associated with increased cancer cell proliferation, motility, metastatic dissemination, and tumor angiogenesis, along with a decrease in patients’ overall survival [25]. Moreover, CD146 is also expressed on several immune cell subsets, in particular on T lymphocytes [26][27]. Along this line, it was demonstrated that soluble factors in the tumor microenvironment induce CD146 expression on tumor infiltrating T lymphocytes. Indeed, the density of CD146 expressed on tumor-infiltrating CD4+ T cells is higher than that on peripheral T cells [26][28]. Analysis of the RNA profile of CD4+ CD146+ T cells from peripheral blood shows high levels of genes associated with Th17 cells (IL-17A, ROR-γ, IL-22, IL-26, IL-23R, CXCL-13, IL1-β, GM-CSF) which exacerbate inflammatory reactions and indirectly promote tumor progression [27]. In addition, Th17 cells contribute to auto-immune diseases progression as in multiple sclerosis (MS) and systemic sclerosis (SS). In fact, CD146+Th17 cells constitute the principal T-cell subset in the cerebrospinal fluid of MS patients and is considered as a poor prognostic marker [29]. CD146 was also found to be expressed on the intermediate and extra-villous trophoblasts [30][31].
2.2. CD146 Ligands and Signaling
Initially considered as an orphan receptor, successive studies have identified new ligands for CD146 (Table 1). Most of the newly discovered ligands emphasize the role of CD146 in angiogenesis, as these ligands were found to promote angiogenic effects on endothelial cells. For example, in one study, authors showed that netrin-1, a neuronal guidance molecule, induces human umbilical vein endothelial cells (HUVEC) proliferation, migration, and tube formation by interacting with CD146 but not VEGFR2 [32]. They also showed that netrin-1 binds to the domain IV of CD146 to induce activation of P38 and Erk1/2, a characteristic signaling pathway implicated in VEGFR2 signaling, and relied this effect to the fact that CD146 also acts as a co-receptor for VEGFR2. Indeed, siRNA experiments targeting CD146 abolished the angiogenic effects of netrin-1 on HUVECs while the knock-down of VEGFR2 barely induced similar results. In the same study, the authors used a monoclonal anti-CD146 antibody, AA98, which blocks CD146. They showed that netrin-1 lost its angiogenic effects after treating HUVECs with AA98. Consistently, AA98 efficiently reduced the number of blood vessels in Matrigel plugs conditioned with netrin-1 and grafted in mice, when compared to the group treated with netrin-1 without the antibody. Likewise, in a zebrafish embryo model, antisense morpholino oligonucleotides (MO) targeting CD146 inhibited netrin-1-induced angiogenesis and blocked the development of parachordal vessels. These data underscore the relevance of CD146 as a potent angiogenic molecule both in vitro and in vivo and highlight the capability of CD146 to mediate angiogenic signals even in the absence of conventional pro-angiogenic molecules like VEGF or b-FGF.
Table 1. CD146 binding partners and ligands. A summary of proteins validated to interact with membrane CD146 and the consequent biological effects.
|
|
Interaction |
Type of Interaction |
Biological Significance |
References |
|
|
VEGF-c |
Ligand |
Lymphatic system development |
[38] |
|
|
FGF-4 |
Ligand |
Regulate morphogenesis/ Mediate cellular polarity |
[39] |
|
|
Netrin-1 |
Ligand |
Enhance VEGF-a signaling/ Pro-angiogenic |
[32] |
|
|
Wnt-1 |
Ligand |
Induce fibroblasts activation and proliferation |
[40] |
|
|
Wnt-5a |
Ligand |
Cytoskeleton remodeling/ Increase cell migration |
[41] |
|
|
VEGFR-2 |
Co-receptor |
Enhance VEGFR2 signaling/ Pro-angiogenic |
[11] |
|
|
PDGFR-β |
Co-receptor |
Pericytes recruitment/ Vessel stabilization |
[36] |
|
|
Laminin-421 |
Ligand |
Cancer metastasis/ Retina development |
[42] |
|
|
Laminin-411 |
Ligand |
Lymphocytes extravasation in to CNS |
[43] |
|
|
Galectin-1 |
Ligand |
Endothelial cells survival/ Vascular development |
[33] |
|
|
Galectin-3 |
Ligand |
Cancer progression and metastasis |
[44] |
|
|
S100-A8/A9 |
Ligand |
Chemotactic effect on cancer cells/ Pro-metastatic |
[45] |
|
|
Matriptase |
Ligand |
Stimulate neuron differentiation/ Cancer invasion |
[46] |
Galectins are a family of soluble carbohydrate-binding lectins that mediate cell-to-cell and cell-to-ECM adhesions. As glycosylation accounts for nearly 35% of CD146 apparent molecular weight, it was tempting to speculate that CD146 could interact with sugar-binding proteins such as lectins. In particular, galectin-1, a protein produced by vascular, interstitial, epithelial, and immune cells, was found to interact readily with N-linked oligosaccharides of membrane CD146 and signal to protect endothelial cells from apoptosis as well as to regulate angiogenesis [33]. In this study, Jouve et al. evidenced that CD146 is mainly N-glycosylated and validated its interaction with galectin-1. They showed that galectin-1, by binding CD146, was protecting endothelial cells from apoptosis. In addition, in vivo experiments in zebrafish showed impaired vascular network formation and poorly developed intersomitic vessels upon knocking down galectin-1 [34] and CD146 [16], respectively. Moreover, in a galectin-1 knockout (KO) mouse model [34], tumor growth was markedly impaired and this effect was attributed to a weak tumor angiogenesis. In the same way, administration of anti-CD146 monoclonal antibody AA98 potently reduced tumor vessel formation in nude mice xenografted with human tumor cells (SMMC7721, SK-LMS-1, SW1990). Another study by Thijssen et al. [35] has shown that galectin-1 is significantly upregulated on activated endothelium and positively correlates with strong angiogenesis by augmenting VEGF receptors and transforming protein p21 (GTPase H-RAS) signaling and phosphorylation. Thus, galectin-1 effect on angiogenesis could be, at least in part, mediated by CD146, which is indeed a coreceptor for VEGFR2. Importantly, it was recently described that endothelial CD146 binds platelet-derived growth factor receptor-β (PDGFR-β) on pericyte and regulates the PDGF-induced activation of PDGFR-β signaling [36]. By this mechanism, CD146 appears to have a crucial role in recruiting adjacent pericytes in the endothelium, and hence, stabilize the developing vessels. All other ligands that are documented in the literature to interact directly with CD146 are summarized in Table 1 along with the consequent biological significance.
Concerning sCD146, its binding partners are still largely unknown. In fact, angiomotin was described to be among the first binding partners of sCD146 on endothelial cells. Stalin et al. [22] showed that the interaction between sCD146 and angiomotin on endothelial cells induces cell proliferation, migration, and pseudo-capillary formation in Matrigel. This interaction activates angiogenesis and exerts a competitive inhibitory effect on angiostatin. Recently, integrin αvβ1 has been reported to interact with sCD146, an interaction that appears to be important in regulating blood brain barrier (BBB) permeability [37].
2.3. CD146 Mechanism of Action
CD146 is mainly a monomeric protein. However, it has been shown to dimerize and multimerize in response to physiological stimuli [38]. Indeed, the stimulation of endothelial cells with VEGF or by means of anti-CD146 AA98 antibody results in CD146 dimerization as revealed by fluorescence resonance energy transfer (FRET) technology. This dimerization leads to conformational changes in CD146 structure and induces changes in ligand binding. However, whether this dimerization causes receptor auto-activation and -phosphorylation is not elucidated yet.
CD146 can interact with diverse ligands that mediate and alter endothelial functions (Figure 1). In fact, CD146 activation induces the phosphorylation of p125FAK and paxillin along with p59fyn recruitment in cultured endothelial cells, leading to actin cytoskeleton reorganization and activation of transcription factors that modulate cell migration and survival [39]. Moreover, the intracellular domain of CD146 interacts physically with actin linked proteins of the ezrin-radixin-moesin (ERM) family, bringing them to the level of membrane protrusions. This CD146-mediated formation of microvilli like extensions will then allow the activation of RhoA by sequestering Rho guanine nucleotide dissociation inhibitory factor 1 (RhoGDI1), leading to an increase in cell motility. In addition, the stimulation of melanoma cells with Wnt5a induces CD146 redistribution within polarized structures known as W-RAMP (Wnt5a-mediated receptor-actin-myosin polarity), leading to membrane retraction and cell migration in a RhoA-mediated mechanism [40]. Finally, a defect in CD146 expression is associated with an upregulation of the canonical Wnt pathway and a downregulation of the non-canonical pathway [41].
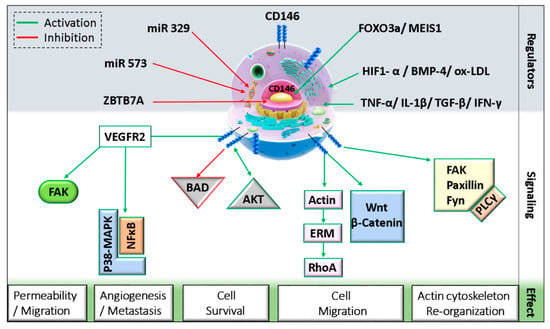
Figure 1. Schematic representation of CD146 cellular localization and regulation.(MEIS1: myeloid ecotropic viral integration site 1; HIF1-α: hypoxia-inducible factor 1 alpha; TGF-β: transforming growth factor-beta; IFN-γ: interferon-gamma; BMP-4: bone morphogenetic protein 4; ox-LDL: oxidized low density lipoprotein; ZBTB7A: zinc finger and BTB domain-containing protein 7A; FAK: focal adhesion kinase; BAD: Bcl-2 associated agonist of cell death; ERM: ezrin, radixin, and moesin; PLC-γ: phospholipase C-gamma).
On endothelial cells, both VEGFR2 and CD146 extracellular domains were found to interact physically even without VEGF binding [11]. A study showed that CD146 dimerizes upon VEGF-A stimulation in a mechanism involving RAC1, Nox4, and ROS production [42]. The inhibition of CD146 using siRNAs, miRNAs, or inhibitory antibodies as AA98 decreased VEGF-A-induced VEGFR2, p38/MAPK, and AKT phosphorylation and reduced NFκB activation in HUVECs [11]. Similarly, in lung vascular endothelial cells from CD146KO mice, VEGF-A stimulation resulted in decreased VEGFR2 and FAK phosphorylation [43]. Additionally, by regulating further junctional proteins such as VE-cadherin and PECAM, CD146 mediates VEGF-induced permeability in endothelial cells.
As mentioned earlier, CD146 can interact with netrin-1 and mediate angiogenesis [32]. It has been shown in zebrafish that CD146 silencing inhibits netrin-1-induced vascularization. However, netrin-1 does not always stimulate angiogenesis. The controversy about its angiogenic role is attributed to the binding partner, as netrin-1 preferentially binds CD146 at low concentrations but UNC5B at high concentrations. Indeed, netrin-1 has higher affinity for CD146 as compared to its cognate ligand UNC5B. By binding UNC5B, signals will be triggered to counteract those of CD146 and thus inhibit angiogenesis [32]. On the other hand, netrin-1 can induce CD146 dimerization and VEGFR2 phosphorylation, and the inhibition of CD146 decreases netrin-1 effect on HUVEC migration, proliferation, and tube formation in vitro by reducing VEGFR2, ERK1/2, and p38 activation.
Soluble CD146 is also involved in activating several signaling pathways. Stalin et al. showed that soluble CD146 binds angiomotin P80 on endothelial progenitor cells and HUVECs and regulates vessel formation and cell migration [22]. Mechanistically, soluble CD146 triggers a signalosome constituted of angiomotin, short CD146, VEGFR1, VEGFR2, and presenilin-1 in lipid rafts and phosphorylates VEGF receptors 1 and 2 on endothelial cells [44]. Within this signalosome, soluble CD146 promotes the proteolytic cleavage of short CD146 extracellular domain through matrix metalloproteinases/a disintegrin and metalloproteinase (MMP/ADAM), followed by intracellular cleavage of the cytoplasmic domain by presenilin-1. The generated so-called short CD146 intracellular fragment (shCD146 IC) will be then translocated to the nucleus where it associates with the transcription factor CSL to regulate the transcription of FADD, Bcl-xl, and eNOS genes that are involved in cell survival and angiogenesis [44].
Soluble CD146 also significantly contributes to tumor progression in different manners. This form, by associating with its cognate binding partners on cancer cells, activates the oncogene c-myc, which in turn activates signaling pathways leading to increased cell proliferation and migration [13].
References
- Bergh, P.A.; Navot, D. The impact of embryonic development and endometrial maturity on the timing of implantation. Fertil. Steril. 1992, 58, 537–542.
- Risau, W. Mechanisms of angiogenesis. Nature 1997, 386, 671–674.
- Neve, A.; Cantatore, F.P.; Maruotti, N.; Corrado, A.; Ribatti, D. Extracellular Matrix Modulates Angiogenesis in Physiological and Pathological Conditions. BioMed Res. Int. 2014, 2014.
- Lamalice, L.; Le Boeuf, F.; Huot, J. Endothelial Cell Migration During Angiogenesis. Circ. Res. 2007, 100, 782–794.
- Usui, Y.; Westenskow, P.D.; Murinello, S.; Dorrell, M.I.; Scheppke, L.; Bucher, F.; Sakimoto, S.; Paris, L.P.; Aguilar, E.; Friedlander, M. Angiogenesis and Eye Disease. Annu. Rev. Vis. Sci. 2015, 1, 155–184.
- Fernández, M.; Semela, D.; Bruix, J.; Colle, I.; Pinzani, M.; Bosch, J. Angiogenesis in liver disease. J. Hepatol. 2009, 50, 604–620.
- Farouk, H.M.; Hamza, S.H.; El Bakry, S.A.; Youssef, S.S.; Aly, I.M.; Moustafa, A.A.; Assaf, N.Y.; El Dakrony, A.H.M. Dysregulation of angiogenic homeostasis in systemic sclerosis. Int. J. Rheum. Dis. 2013, 16, 448–454.
- Bender, R.J.; Mac Gabhann, F. Dysregulation of the vascular endothelial growth factor and semaphorin ligand-receptor families in prostate cancer metastasis. BMC Syst. Biol. 2015, 9, 55.
- Lähteenvuo, J.; Rosenzweig, A. Invited Review: The Role of Angiogenesis in Cardiovascular Aging. Circ. Res. 2012, 110, 1252–1264.
- Boyle, M.; Chun, C.; Strojny, C.; Narayanan, R.; Bartholomew, A.; Sundivakkam, P.; Alapati, S. Chronic Inflammation and Angiogenic Signaling Axis Impairs Differentiation of Dental-Pulp Stem Cells. PLoS ONE 2014, 9.
- Jiang, T.; Zhuang, J.; Duan, H.; Luo, Y.; Zeng, Q.; Fan, K.; Yan, H.; Lu, D.; Ye, Z.; Hao, J.; et al. CD146 is a coreceptor for VEGFR-2 in tumor angiogenesis. Blood 2012, 120, 2330–2339.
- Bardin, N.; Anfosso, F.; Massé, J.-M.; Cramer, E.; Sabatier, F.; Bivic, A.L.; Sampol, J.; Dignat-George, F. Identification of CD146 as a component of the endothelial junction involved in the control of cell-cell cohesion. Blood 2001, 98, 3677–3684.
- Stalin, J.; Nollet, M.; Garigue, P.; Fernandez, S.; Vivancos, L.; Essaadi, A.; Muller, A.; Bachelier, R.; Foucault-Bertaud, A.; Fugazza, L.; et al. Targeting soluble CD146 with a neutralizing antibody inhibits vascularization, growth and survival of CD146-positive tumors. Oncogene 2016, 35, 5489–5500.
- Lehmann, J.M.; Riethmüller, G.; Johnson, J.P. MUC18, a marker of tumor progression in human melanoma, shows sequence similarity to the neural cell adhesion molecules of the immunoglobulin superfamily. Proc. Natl. Acad. Sci. USA 1989, 86, 9891–9895.
- Chen, J.; Luo, Y.; Hui, H.; Cai, T.; Huang, H.; Yang, F.; Feng, J.; Zhang, J.; Yan, X. CD146 coordinates brain endothelial cell-pericyte communication for blood-brain barrier development. Proc. Natl. Acad. Sci. USA 2017, 114, E7622–E7631.
- Bardin, N.; Blot-Chabaud, M.; Despoix, N.; Kebir, A.; Harhouri, K.; Arsanto, J.-P.; Espinosa, L.; Perrin, P.; Robert, S.; Vely, F.; et al. CD146 and its Soluble Form Regulate Monocyte Transendothelial Migration. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 746–753.
- Chan, B.; Sinha, S.; Cho, D.; Ramchandran, R.; Sukhatme, V.P. Critical roles of CD146 in zebrafish vascular development. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2005, 232, 232–244.
- Guezguez, B.; Vigneron, P.; Alais, S.; Jaffredo, T.; Gavard, J.; Mège, R.-M.; Dunon, D. A dileucine motif targets MCAM-l cell adhesion molecule to the basolateral membrane in MDCK cells. FEBS Lett. 2006, 580, 3649–3656.
- Xu, W.; Hua, H.; Chiu, Y.-H.; Li, G.; Zhi, H.; Yu, Z.; Ren, F.; Luo, Y.; Cui, W. CD146 Regulates Growth Factor-Induced mTORC2 Activity Independent of the PI3K and mTORC1 Pathways. Cell Rep. 2019, 29, 1311–1322.e5.
- Nollet, M.; Stalin, J.; Moyon, A.; Traboulsi, W.; Essaadi, A.; Robert, S.; Malissen, N.; Bachelier, R.; Daniel, L.; Foucault-Bertaud, A.; et al. A novel anti-CD146 antibody specifically targets cancer cells by internalizing the molecule. Oncotarget 2017, 8, 112283–112296.
- Kebir, A.; Harhouri, K.; Guillet, B.; Liu, J.W.; Foucault-Bertaud, A.; Lamy, E.; Kaspi, E.; Elganfoud, N.; Vely, F.; Sabatier, F.; et al. CD146 short isoform increases the proangiogenic potential of endothelial progenitor cells in vitro and in vivo. Circ. Res. 2010, 107, 66–75.
- Stalin, J.; Harhouri, K.; Hubert, L.; Subrini, C.; Lafitte, D.; Lissitzky, J.-C.; Elganfoud, N.; Robert, S.; Foucault-Bertaud, A.; Kaspi, E.; et al. Soluble Melanoma Cell Adhesion Molecule (sMCAM/sCD146) Promotes Angiogenic Effects on Endothelial Progenitor Cells through Angiomotin. J. Biol. Chem. 2013, 288, 8991–9000.
- Wang, Z.; Yan, X. CD146, a multi-functional molecule beyond adhesion. Cancer Lett. 2013, 330, 150–162.
- Zeng, P.; Li, H.; Lu, P.-H.; Zhou, L.-N.; Tang, M.; Liu, C.-Y.; Chen, M.-B. Prognostic value of CD146 in solid tumor: A Systematic Review and Meta-analysis. Sci. Rep. 2017, 7, 4223.
- Stalin, J.; Traboulsi, W.; Vivancos-Stalin, L.; Nollet, M.; Joshkon, A.; Bachelier, R.; Guillet, B.; Lacroix, R.; Foucault-Bertaud, A.; Leroyer, A.S.; et al. Therapeutic targeting of soluble CD146/MCAM with the M2J-1 monoclonal antibody prevents metastasis development and procoagulant activity in CD146-positive invasive tumors. Int. J. Cancer 2020, 147, 1666–1679.
- Elshal, M.F.; Khan, S.S.; Takahashi, Y.; Solomon, M.A.; McCoy, J.P. CD146 (Mel-CAM), an adhesion marker of endothelial cells, is a novel marker of lymphocyte subset activation in normal peripheral blood. Blood 2005, 106, 2923–2924.
- Gabsi, A.; Heim, X.; Dlala, A.; Gati, A.; Sakhri, H.; Abidi, A.; Amri, S.; Neili, B.; Leroyer, A.S.; Bertaud, A.; et al. TH17 cells expressing CD146 are significantly increased in patients with Systemic sclerosis. Sci. Rep. 2019, 9.
- Dagur, P.K.; Tatlici, G.; Gourley, M.; Samsel, L.; Raghavachari, N.; Liu, P.; Liu, D.; McCoy, J.P. CD146+ T Lymphocytes are Increased in Both the Peripheral Circulation and in the Synovial Effusions of Patients with Various Musculoskeletal Diseases and Display Pro-inflammatory Gene Profiles. Cytometry B Clin. Cytom. 2010, 78, 88–95.
- Brucklacher-Waldert, V.; Stuerner, K.; Kolster, M.; Wolthausen, J.; Tolosa, E. Phenotypical and functional characterization of T helper 17 cells in multiple sclerosis. Brain J. Neurol. 2009, 132, 3329–3341.
- Liu, Q.; Zhang, B.; Zhao, X.; Zhang, Y.; Liu, Y.; Yan, X. Blockade of adhesion molecule CD146 causes pregnancy failure in mice. J. Cell. Physiol. 2008, 215, 621–626.
- Liu, Q.; Yan, X.; Li, Y.; Zhang, Y.; Zhao, X.; Shen, Y. Pre-eclampsia is associated with the failure of melanoma cell adhesion molecule (MCAM/CD146) expression by intermediate trophoblast. Lab. Investig. J. Tech. Methods Pathol. 2004, 84, 221–228.
- Tu, T.; Zhang, C.; Yan, H.; Luo, Y.; Kong, R.; Wen, P.; Ye, Z.; Chen, J.; Feng, J.; Liu, F.; et al. CD146 acts as a novel receptor for netrin-1 in promoting angiogenesis and vascular development. Cell Res. 2015, 25, 275–287.
- Jouve, N.; Despoix, N.; Espeli, M.; Gauthier, L.; Cypowyj, S.; Fallague, K.; Schiff, C.; Dignat-George, F.; Vély, F.; Leroyer, A.S. The Involvement of CD146 and Its Novel Ligand Galectin-1 in Apoptotic Regulation of Endothelial Cells. J. Biol. Chem. 2013, 288, 2571–2579.
- Thijssen, V.L.J.L.; Postel, R.; Brandwijk, R.J.M.G.E.; Dings, R.P.M.; Nesmelova, I.; Satijn, S.; Verhofstad, N.; Nakabeppu, Y.; Baum, L.G.; Bakkers, J.; et al. Galectin-1 is essential in tumor angiogenesis and is a target for antiangiogenesis therapy. Proc. Natl. Acad. Sci. USA 2006, 103, 15975–15980.
- Thijssen, V.L.; Barkan, B.; Shoji, H.; Aries, I.M.; Mathieu, V.; Deltour, L.; Hackeng, T.M.; Kiss, R.; Kloog, Y.; Poirier, F.; et al. Tumor Cells Secrete Galectin-1 to Enhance Endothelial Cell Activity. Cancer Res. 2010, 70, 6216–6224.
- Chen, J.; Luo, Y.; Huang, H.; Wu, S.; Feng, J.; Zhang, J.; Yan, X. CD146 is essential for PDGFRβ-induced pericyte recruitment. Protein Cell 2018, 9, 743–747.
- Wang, D.; Duan, H.; Feng, J.; Xiang, J.; Feng, L.; Liu, D.; Chen, X.; Jing, L.; Liu, Z.; Zhang, D.; et al. Soluble CD146, a cerebrospinal fluid marker for neuroinflammation, promotes blood-brain barrier dysfunction. Theranostics 2020, 10, 231–246.
- Bu, P.; Zhuang, J.; Feng, J.; Yang, D.; Shen, X.; Yan, X. Visualization of CD146 dimerization and its regulation in living cells. Biochim. Biophys. Acta 2007, 1773, 513–520.
- Anfosso, F.; Bardin, N.; Francès, V.; Vivier, E.; Camoin-Jau, L.; Sampol, J.; Dignat-George, F. Activation of Human Endothelial Cells via S-Endo-1 Antigen (CD146) Stimulates the Tyrosine Phosphorylation of Focal Adhesion Kinase p125FAK. J. Biol. Chem. 1998, 273, 26852–26856.
- Luo, Y.; Zheng, C.; Zhang, J.; Lu, D.; Zhuang, J.; Xing, S.; Feng, J.; Yang, D.; Yan, X. Recognition of CD146 as an ERM-binding protein offers novel mechanisms for melanoma cell migration. Oncogene 2012, 31, 306–321.
- Liu, D.; Du, L.; Chen, D.; Ye, Z.; Duan, H.; Tu, T.; Feng, J.; Yang, Y.; Chen, Q.; Yan, X. Reduced CD146 expression promotes tumorigenesis and cancer stemness in colorectal cancer through activating Wnt/β-catenin signaling. Oncotarget 2016, 7, 40704–40718.
- Zhuang, J.; Jiang, T.; Lu, D.; Luo, Y.; Zheng, C.; Feng, J.; Yang, D.; Chen, C.; Yan, X. NADPH oxidase 4 mediates reactive oxygen species induction of CD146 dimerization in VEGF signal transduction. Free Radic. Biol. Med. 2010, 49, 227–236.
- Zeng, Q.; Wu, Z.; Duan, H.; Jiang, X.; Tu, T.; Lu, D.; Luo, Y.; Wang, P.; Song, L.; Feng, J.; et al. Impaired tumor angiogenesis and VEGF-induced pathway in endothelial CD146 knockout mice. Protein Cell 2014, 5, 445–456.
- Stalin, J.; Harhouri, K.; Hubert, L.; Garrigue, P.; Nollet, M.; Essaadi, A.; Muller, A.; Foucault-Bertaud, A.; Bachelier, R.; Sabatier, F.; et al. Soluble CD146 boosts therapeutic effect of endothelial progenitors through proteolytic processing of short CD146 isoform. Cardiovasc. Res. 2016, 111, 240–251.




