
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Zinnia Shah | + 2412 word(s) | 2412 | 2021-04-26 11:48:14 | | | |
| 2 | Vivi Li | Meta information modification | 2412 | 2021-04-27 04:14:17 | | |
Video Upload Options
Podophyllotoxin is an aryltetralin-type lignan isolated from species of Podophyllum. Two most common sources are the rhizomes of Podophyllum peltatum (American mayapple) and Sinopodophyllum hexandrum Royle (Barberry family). The podophyllotoxin extract has been documented for its use as a laxative, and as a remedy for various medical complications such as gonorrhea, tuberculosis, menstrual disorders, psoriasis, dropsy, cough, syphilis and venereal warts.
1. Introduction
Podophyllotoxin is an aryltetralin-type lignan isolated from species of Podophyllum [1][2]. Two most common sources are the rhizomes of Podophyllum peltatum (American mayapple) and Sinopodophyllum hexandrum Royle (Barberry family) [1][3]. These perennial herbs are distributed widely across the Himalayan region and Western China [4][5][6][7]. Plants with high podophyllotoxin or podophyllotoxin-analogues’ content have been extensively used in traditional medicine since a long time in different cultures [8][9][10][11]. This remarkable molecule was first isolated in 1880 by Podwyssotzki [12][13][14][15], while it had already been described as early as in 1753 by Linnaeus [16]. Due to its remarkable biological activity, podophyllotoxin has remained a subject of various investigations ever since. It is mainly obtained from the alcohol-soluble fraction of Podophyllum species, called podophyllin—a bitter tasting resin.
The podophyllotoxin extract has been documented for its use as a laxative, and as a remedy for various medical complications such as gonorrhea, tuberculosis, menstrual disorders, psoriasis, dropsy, cough, syphilis and venereal warts [8][9][10][11]. The podophyllotoxin-family is now confirmed to elicit various curative properties such as mitotoxic, neurotoxic, insecticidal, antimicrobial, anti-inflammatory, antispasmogenic, hypolipidemic, immunosuppressive, antioxidative, analgesic and cathartic activities [17]. Initial studies reporting its pronounced activity against cancer cells established greater interest in its antimitotic efficacy. The clinical practicality of podophyllotoxin was, however, largely tampered because of its undesirable secondary effects such as gastrointestinal toxicity, neurotoxicity, hair-loss, and bone marrow suppression, etc., which led to the finding of less-toxic derivatives or analogues. These molecules have since become a structural base for the development of new therapeutic agents.
Podophyllotoxin (C22H22O8) is a selective cyclolignan whose structure was first elucidated in 1930s. Extensive structural modifications of podophyllotoxin have culminated to eventually discover clinically viable anticancer drugs, namely; etiposide, teniposide [18][19][20][21][22][23][24][25] and etopophos [26], but it was not until twenty years after the discovery of these viable derivatives that their mechanism of action was understood. Podophyllotoxins were recognized to interact with the DNA and its replication process to carry out their antimitotic effects. Etiposide, for instance, inhibits DNA topoisomerase II (dnaTII) and causes cell cycle arrest in the S-phase. Podophyllotoxin is also known to prevent cell growth through inhibiting the polymerization of tubulin and thus subdue the configuration of mitotic spindles [26]. The therapeutic use of these drugs is however, in fact, hindered by myelosuppression, development of drug-resistance and their cytotoxic activity towards normal body cells. On these accounts, the synthesis of new potent podophyllotoxin-derivatives such as NK-611 [27], NPF [28], GL-311 [29], TOP-53 [30], F14512 [26], azatoxin, etc., is a continuing concern—structures of some of these derivatives are shown in Figure 1. These have thence opened a window for virtual designing of unlimited podophyllotoxin-derivatives geared at improving clinical efficacy. These studies necessitate reviewing all the recent advancement made on podophyllotoxin. Furthermore, the extinction of podophyllotoxin plant sources shifted the focus towards source alternatives, which includes the manipulation of microbes for the purpose. This review is aimed at providing an insight to podophyllotoxin research and hence helps delineate further investigations, which are needed in this field.
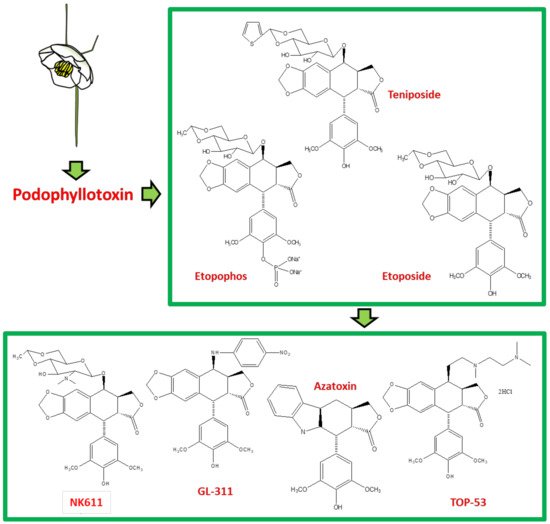
Figure 1. The sequential discovery of podophyllotoxin-group of drugs. FDA approved anticancer drugs etoposide, teniposide and etopophos were derived from the parent compound podophyllotoxin, which was originally extracted from mayapple plant as a curative for various diseases. As the side-effects for podophyllotoxin and its primary derivatives became evident, less toxic derivatives such as TOP-53, NK611, GL-331, azatoxin and various others were designed and synthesized.
2. Structural Characteristics of Podophyllotoxin
Podophyllotoxin has a five-ring system (ABCDE) having four chiral centers (C1-C4) in a row—Figure 2. There are five important structural characteristics of most podophyllotoxin species: (1) a tetracyclic group going from dioxole ring (A) to the lactone ring (D); (2) four oxygen atoms located at the functional groups lactone, methoxys, secondary alcohol and oxoles; (3) ring E with alpha-configuration located at position 1; (4) four asymmetrical adjacent centers and (5) the unique stereochemical properties of C4 define the molecules’ mechanism of action [31][32]. Studies have revealed, rational modification at C4 can improve the molecule’s topoisomerase II inhibitory activity, drug resistance profile, water solubility and antimitotic activities [33]. The lactone ring is known to exhibit transconfiguration, which easily epimerizes in low basic conditions [32].
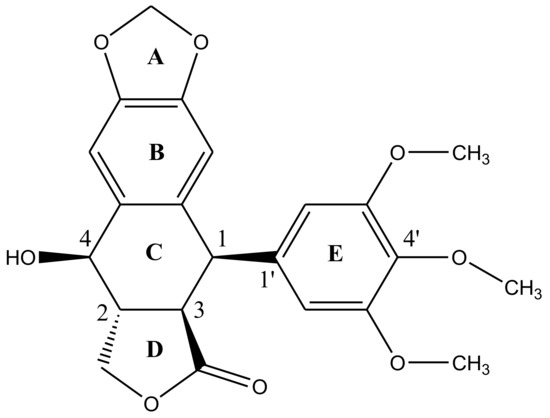
Figure 2. Structural representation of podophyllotoxin. The structure is constituted of 4 aromatic rings A, B, C and D arranged in an almost planar system whereas ring E is attached quasi-axially at C4 of ring C.
3. Derivatives, Analogues and Hybrids of Podophyllotoxin
Derivatives of this multifaceted molecule are synthesized as properties of its four rings. Ring A, for instance is not essential for the exhibition of antimitotic activity, and aromatization of ring C may cause displacement of axially positioned ring E—leading to a loss of cytotoxic property. Structural diversity hence brings many attractive medicinal properties and a comprehensive insight to the molecule’s mechanism of action. These derivatives were first introduced by Schreier through selective cleavage of ring A to produce a 6, 7-dihydroxy derivative (compound A), which was further methylated with diazomethane to produce yet another derivative (compound B) shown in Figure 3 [34]. Schreier’s methods were later on slightly modified to produce a large group of podophyllotoxin derivativescompound I–XXfrom compound C, Figure 3. However, the structural modifications are not alone to contribute towards the novelty of the derivatives, instead, changes in the chemical skeleton of podophyllotoxin cyclo-lignan add particularity to each individual derivative.
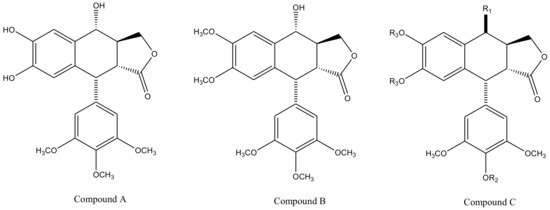
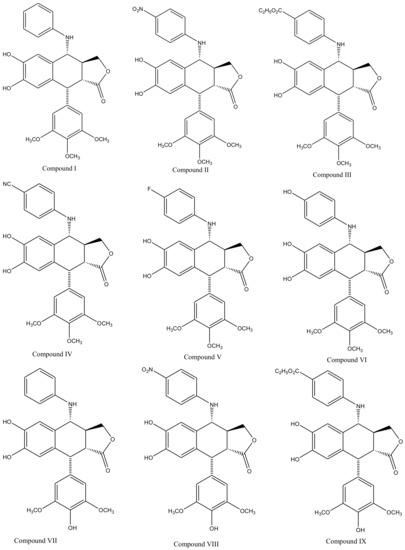
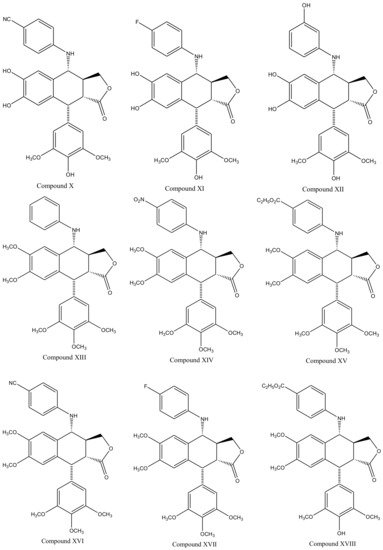
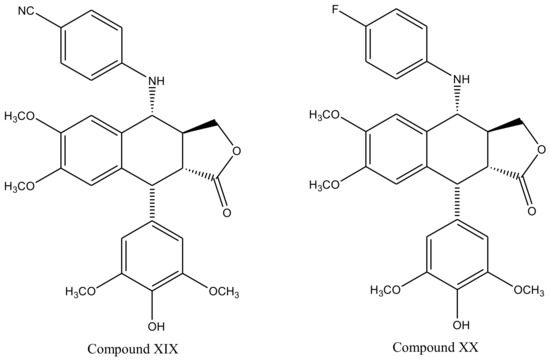
Figure 3. Podophyllotoxin derivatives produced by selective cleavage of ring A through Schreier’s method and the modified-Schreier method. These ring-A-open compounds, however, appeared biologically less active than podophyllotoxin itself.
With slight modifications in the podophyllotoxin skeleton, the ring-A-open compounds appeared biologically less active than podophyllotoxin itself [35]; functional studies indicate that an intact A-ring system is important for the compounds’ DNA topoisomerase II (dtopII) inhibiting activity. Based on these findings, three crucially significant domains in the parent pharmacophore model were reported [36]. Modifications at these domains further revealed the unexplored potential of novel clinically applicable derivatives (shown in Figure 4)with a mechanism of action distinct to that of the previously well-established derivative; etiposide [37]. The paucity of studies on B-ring modifications in podophyllotoxin led the investigation of α-peltatin and β-peltatin. These two compounds (Figure 5) exhibited significant antiviral and antitumor activities. In order to investigate the influence of B-ring substitutions and modifications, a series of ester- and ether-derivatives of both structures were prepared by various researchers but were reported to be biologically less active than their parent compounds [38][39][40][41][42][43].
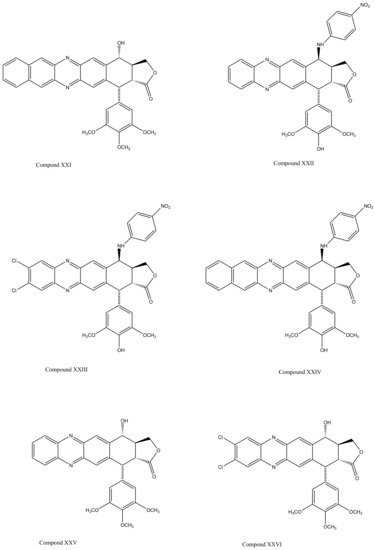
Figure 4. The significance of ring A for biological functionality of podophyllotoxin and its derivatives introduced three crucially significant domains in the parent pharmacophore model. Modifying these domains provided a number of new derivatives as shown (Compound XX1–XXVI). These compounds show distinct mechanism of actions in comparison to the previously discovered podophyllotoxin derivatives.
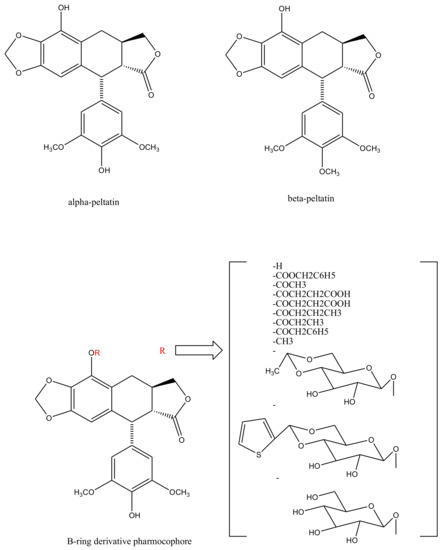
Figure 5. Structures of alpha-peltatin and beta-peltatin. These two B-ring modified podophyllotoxin derivatives are known to exhibit significant antiviral and antitumor activities. The B-ring modified pharmacophore was identified from these compounds and was taken further to synthesize more derivatives—as shown bracketed—none of which elicited any clinical efficacy.
Several C-ring modified podophyllotoxin analogues have also been subjected to extensive research. Unlike the two natural C-aromatized lignans; justicidin A and diphyllin; the many synthetic C-ring aromatized compounds were prepared but reported to have no cytotoxic activities. This observation proposed that the axial E-ring conformation was lost during synthetic preparations. The loss of the axial E-ring conformation is now known to be directly linked to the loss of molecule’s cytotoxic and dtopII inhibiting activity [44]. Notably, however, delactonized analogues of podophyllotoxin—apopicropodophyllotoxin; and its beta-isomer—β-apopicropodophyllotoxin give strong antimitotic activities, otherwise indicating the untrammelled antimitotic potential of podophyllotoxin-like compounds in the absence of the lactone ring [45][46][47]. The antitumor activity of podophyllotoxin and its derivatives is mainly due to the ability to inhibit tubulin polymerization [48], which is a property of the axially placed E-ring. The cis-fused lactone rings, however, modulating its selectivity from tubulin to IGF-1 (insulin- like growth factor I) receptor to trigger cell death [49][50][51].
Transfused γ-lactone D ring is also strict requirement for podophyllotoxin’s antitumor activity. Since it is susceptible to isomerization, the opening of this lactone ring is undesirable for it limits the physiological lifetime of these compounds and their biological effectiveness as well. A series of delactonized D-ring derivatives were prepared [52][53][54][55][56][57] and studied by various researchers, most of which were found to be less cytotoxic than the parent compound itself. However, an ethyl hydrazide derivative (Figure 6) was found to retain its clinical efficacy but was discontinued due to severe adverse aftereffects [58]. Studies that later discovered immunosuppressive ability of these derivatives were attempted to revive D-ring modified podophyllotoxin—antitumor drug preparations [59][60]. 4′-d-β-DMEP (4′-d-β-Demethylepipodophyllotoxin) is a natural lignin, which is used in chemotherapy as an aglycon of etoposide [61][62]. 4′-d-α-DMEP is a novel biological derivative of 4′-d-β-DMEP reported by Jia et al. (2020), which has a higher antitumor activity [63].
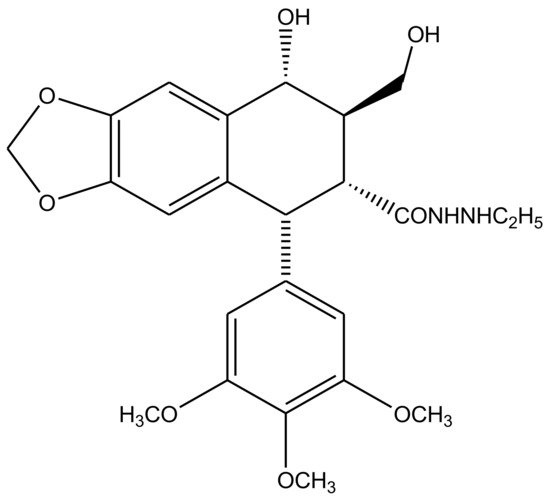
Figure 6. A series of delactonized D-ring derivatives have remained the focus of many studies. An ethyl hydrazide derivative, drawn here, was reportedly the only D-ring modified compound, which showed clinical efficacy, but was discontinued after occurrence of various cases of severe side-effects related to its use.
E-ring modifications have only actually focused on enhancing E-ring’s involvement in the cytotoxic mechanism of the compound. With an increased access to the compounds’ physicochemical behavior, a variety of exciting derivatives with successful clinical application have been developed, such as those shown in Figure 7 [64][65].
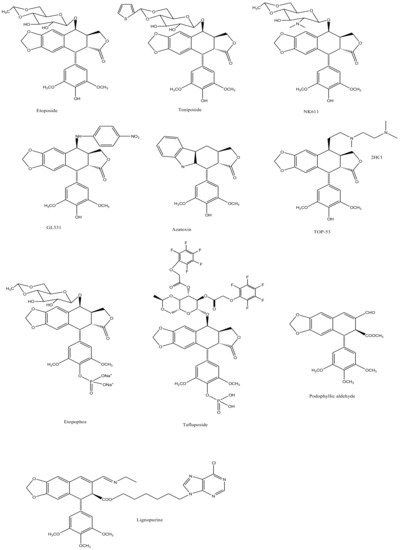
Figure 7. The figure shows structural illustrations of E-ring modified derivatives much of which have proved to be clinically significant.
4. Pharmacological Significance of Podophyllotoxin and Its Derivatives
Podophyllotoxin and its derivatives possess a wide-spectrum of pharmacological potential. The achievability of broad chemical modifications brings the podophyllotoxin pharmacophore its diverse applicability as a medicinal compound—tabulated in Table 1. Antineoplastic activity is the most outstanding of all its clinical properties. Various studies validate podophyllotoxin derivatives including etopophos, teniposide, etoposide, etoposide phosphate, GL331, NK-611, TOP53 and NPF as anticancer drugs [64][66][67][68][69][70]. Many clinical studies have reported the efficacy of these compounds against various forms of cancer including lung cancer, Wilmstumours, diverse types of genital tumors such as carcinoma verrucosus and for non-Hodgkin lymphoma, multiform glioblastoma lymphoma and nonlymphocytic leukemia [17]. Podophyllotoxin has also shown activity against various (multi) drug resistant tumor cells. As an example, Hu and coworkers presented a 4-β-anilino-podophyllotoxin derivative as a potential anticancer drug against the KB/VCR cells in both conditions; in vivo and in vitro [71]. For the development of new anticancer drugs, podophyllotoxin containing analogues are of prime focus in recent studies. Ming et al. [72] explained that an endophytic fungus named as Dysosma versipellis has both anticancer and antimicrobial properties. The aqueous extract of Podophyllum peltatum [41] fractionated by reverse-phase chromatography was observingly the most active component that was found to cause the inhibition in the replication process of herpes simplex type 1 virus and measles.
Moreover, picropodophyllotoxin, deoxypodophyllotoxin and peltatins are also determined as useful antiviral compounds [73][74][75]. Activity against Sindbis and cytomegalovirus has also been recorded [76]. They either decrease the capacity of the infected cell to release virus or restrain these viruses in the replication cycle at an essential early stage, following the virus absorption into cells. Not only this, podophyllotoxin can also be used for treating Condyloma acuminatum that is usually caused by HPV (papilloma virus) [77] and for treating other perianal and venereal warts [78]. With a goal to achieve better therapeutic effectiveness, cocktail therapies are currently in use with other registered chemotherapeutic agents, combined with additional techniques that are beneficial in fighting against cancer and other viral infections. Podophyllotoxins with interferon therapy has shown greater effectivity against genital human infections, combination with cisplatin, on the other hand, is useful for treating neuroblastomas. Recently a study reports the study progression of etoposide in phase II clinical trials (ClinicalTrials NTC04356690) as a redeveloped drug to treat COVID-19 patients suffering with cytokine storm complication [79]. Similarly, in recent years podophyllotoxin derivatives have shown some interesting insecticidal activities against the larvae of Brontispa longissima and Mythimna separata [80]. Furthermore, derivatives are also being synthesized and used for the treatment of malaria and psoriasis [81]. Podophyllotoxins also hold dermatological significance and prove to be potential therapeutic agents for psoriasis vulgaris. Podophyllotoxin exhibited considerable ichthyotoxic activity and phyto growth inhibitory activities, even though the effects were weaker as compared to deoxy podophyllotoxin, in all cases observed [82][83].
Besides the major antiviral and anticancer activities, podophyllotoxin and its derived compounds have also exhibited some further interesting activities such as anti-trypanosomal activity, antimelanocortin-4 receptor (MC4R) activity, antioxidative activity, immunosuppressive, antioxidative, antispasmogenic, hypolipidemic, emetic, laxative and anti-inflammatory activity. Moreover, during the last century, many trials were carried out in an attempt to cure diseases such as syphilis, cough, gonorrhea, dropsy, gout, psoriasis, tuberculosis, tumor, menstrual disorders and venereal warts by using podophyllotoxins. According to Hartwell et al. (1951), podophyllotoxin is particularly abundant in the genus Podophyllum that has been utilized since ancient times as bothanthelminthic and cathartic agents, for medicinal purposes [3]. Podophyllotoxin is demonstrated as a promising and relatively safe drug, for treating genital warts and is also an active ingredient of registered wartec ointments and condylox liquids.
Table 1. Pharmacological activities of podophyllotoxin and its derivatives.
| Activity | Podophyllotoxin Derivative | Mechanism | Conclusion | Ref. |
|---|---|---|---|---|
| Cytotoxic activity | Cleistantoxin | Activity was checked against MCF-7, MCF-7R, KBand HT29 cancer cell lines | Cleistantoxin showed strong cytotoxic activity | [84] |
| Antibacterial activity | New precursors of podophyllotoxin were synthesized, and screened to check their antibacterial activity | Activity was checked against Klebsiella pneumoniae, Streptococcus faecalis, Citrobacter sp., Pseudomonas aeruginosa, Escherchia coli, Salmonella typhi and Shigella sonnei | Ethyl-2-(3′-methyl-4′-methoxybenzoyl)-3-(4″ methoxyphenol)-cyclopropane-1-carboxylic acid and Ethyl-2-(3′-methyl-4′-methoxybenzyol-3-1 3″, 4″-dimethoxyphenyl)-cyclopropane-carboxylic acid, both of them showed significant antibacterial activity | [85] |
| Antitumour activity | VP 16-213 (NSC-141540) | Activity was checked against L1210 ascites tumour in N/D mice | In 24 hours, divided treatment after every 3 hours, resulted in significant cure, hence, VP 16-213 is a cell cycle specific drug | [86] |
| Insecticidal activity | 20 podophyllotoxin analogues were tested | Activity was checked against fifth-instar larvae of Brontispa longissima in vivo | Among 20 analogues Deoxypodophyllotoxin showed more protential for insecticidal activity than a commercial insecticide (toosendanin) | [87] |
| Antineoplastic activity | New hybrids of podophyllotoxin and indirubin | Activity was checked against human leukemia cancer cells as a multifunctional anti-MDR agent | Podophyllotoxin-indirubin hybrid (Da-1) showed potential to overcome drug resistance. It is a novel hybrid havingpotent antiproliferative activity | [88] |
| Cyclolignans, derived from podophyllotoxin | Activity was checked againstA-549 human lung carcinoma, P-388 murine leukemia and HT-29 colon carcinoma | A number of substances were active in assay at concentrations below 1 pM; deoxypodophyllotoxin being the most potent compound in all cases | [89] |
References
- Cane, C.; Moracs, R.M. Podophyllotoxin. Phytochemistry 2000, 54, 115–120.
- Gordaliza, M.; Garcia, P.A.; Miguel del Corral, J.M. Podophyllotoxin: Distribution, sources, applications, and new cytotoxic derivatives. Toxicon 2004, 44, 441–459.
- Hartwell, J.L.; Schrecker, A.W. Components of Podophyllin. V. The Constitution of Podophyllotoxin. J. Am. Chem. Soc. 1951, 73, 2909–2916.
- Ayres, D.C.; Loike, J.D. Lignans, Chemical, Biological and Clinical Properties; Cambridge University Press: Cambridge, UK, 1990; Chapters 3 and 4.
- King, J. Discovery of Podophyllin. Coll. J. M. Sci. 1875, 2, 557–559.
- Liu, C.J.; Hou, S.S. Current Research Status of Podophyllotoxin Lignans. Nat. Prod. Res. Dev. 1997, 9, 81–89.
- Chen, Y.H. A Study of the Resources of Chinese Podophyllin Plants. Acta Pharm. Sin. 1979, 14, 101–107.
- Yang, X.Z.; Shao, H.; Zhang, L.Q.; Zhou, C.; Xuan, Q.; Yang, C.Y. Present Situation of Studies on Resources of Podophyllotoxin. Chin. Tradit. Herb. Drugs 2001, 32, 1042–1044.
- Liu, H.J.; Xu, Y.; Su, G.Q.; Li, C.Y.; Wang, L.; Liu, Y.J. Research Progress in Sinopodophyllum emodi. Chin. Tradit. Herb. Drugs 2004, 35, 98–100.
- Macrae, D.W.; Towers, N.G.H. Biological Activity of Lignans. Phytochemistry 1984, 23, 1207–1220.
- Bohlin, L.; Rosen, B. Podophyllotoxin derivatives: Drug discovery and development. Drug Discov. Today 1996, 1, 343–351.
- Cragg, G.M.; Kingston, D.G. Anticancer Agents from Natural Products, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2011; p. 97.
- Podwyssotzki, V. The active constituents of podophyllotoxin. Pharm. J. Trans. 1881, 12, 217–218.
- Podwyssotzki, V. On the active constituents of podophyllin. Am. J. Pharm. 1882, 12, 102–115.
- Podwyssotzki, V. Pharmakologische Studien uber podophyllum peltatum. NaunynSchmied Arch. Exp. Path. Phar. 1884, 13, 29–52.
- Species Plantrum; Salvius: Stockholm, Sweden, 1753; p. 505.
- Yu, X.; Che, Z.P.; Xu, H. Recent Advances in the Chemistry and Biology of Podophyllotoxins. Chem. Eur. J. 2016, 10, 1002–1006.
- Stoll, A.; Renz, J.; Wartburg, A.V. The isolation of podophyllotoxin glucoside. J. Am. Chem. Soc. 1954, 76, 3103–3104.
- Stoll, A.; von Wartburg, A.; Angliker, E.; Renz, J. The isolation of 4′- Demethylpodophyllotoxin glucoside from rhizomes podophyllum emodi wall. J. Am. Chem. Soc. 1954, 76, 5004–5005.
- Emmenggger, H.; Stahelin, H.; Rutschmann, J.; von Wartburg, A. Chemistry and Pharmacology of podophyllum glucosides and derivatives. I. Arzneim. 1961, 11, 327–333.
- Kuhn, M.; von Wartburg, A. Mitosis-inhibiting substances. XXI. Synthesis of epipodophyllotoxin b-D-glucopyranoside. Helv. Chim. Acta 1968, 51, 1631–1641.
- Kuhn, M.; von Wartburg, A. Mitosis-inhibiting substances. glycosidation process.II. Glycosides of 4″-demethylepipodo-phyllotoxin. Helv. Chim. Acta 1969, 52, 948–955.
- Keller-Juslen, C.; Kuhn, M.; Von Wartburg, A.; Staehelin, H. Synthesis and antimitotic activity of glycosidic lignan derivatives related to podophyllotoxin. J. Med. Chem. 1971, 14, 936–940.
- Joel, S. The clinical pharmacology of etoposide. An update. Cancer Treat. Rev. 1996, 22, 179–221.
- Stahelin, H.F.; von Wartburg, A. The Chemical and Biological Route from Podophyllotoxin Glucoside to Etoposide: Ninth Cain Memorial Award Lecture. Cancer Res. 1991, 51, 5–15.
- Ardalani, H.; Avan, A.; Ghayour-Mobarhan, M. Podophyllotoxin: A novel potential natural anticancer agent. Avicenna J. Phytomed. 2017, 7, 285–294.
- Mross, K.; Huttmann, A.; Herbst, K.; Hanauske, A.R.; Schilling, T.; Manegold, C.; Burk, K.; Hossfeld, D.K. Pharmacokinetics and pharmacodynamics of the new podophyllotoxin derivative NK 611 A study by the AIO groups PHASE-I and APOH. Cancer Chemother. Pharmacol. 1996, 38, 217–224.
- Huang, T.S.; Lee, C.C.; Chao, Y.; Shu, C.H.; Chen, L.T.; Chen, L.L.; Chen, M.H.; Yuan, C.C.; Whang-Peng, J. A novel podophyllotoxin-derived compound GL331 is more potent than its congener VP-16 in killing refractory cancer cells. Pharm. Res. 1999, 16, 997–1002.
- Lee, C.C.; Huang, T.S. A novel topoisomerase GL331 preferentially induces DNA cleavage at (C/G)T sites and can cause telomere DNA damage. Pharm. Res. 2001, 18, 846–851.
- Solary, E.; Leteurtre, F.; Paull, K.D.; Scudiero, D.; Hamel, E.; Pommier, Y. Dual inhibition of topoisomerase II and tubulin polymerization by azatoxin, a novel cytotoxic agent. Biochem. Pharm. 1993, 45, 2449–2456.
- Arora, R. Medicinal Plant Biotechnology; CAB International: Wallingford, UK, 2010.
- You, Y. Podophyllotoxin derivatives: Current synthetic approaches for new anticancer agents. J. Curr. Pharm. Des. 2005, 11, 1695–1717.
- Bromberg, L. Polyether-modified poly (acrylic acid): Synthesis and applications. Ind. Eng. Chem. Res. 1998, 37, 4267–4274.
- Von-Schreier, E. Partial synthese der 6, 7-Dimethoxy-Analogen von Podophyllotoxin, Epi-, Neo-, und Desoxy-podophyllotoxin. Helv. Chim. Acta 1964, 47, 1529–1554.
- Wang, Z.Q.; Hu, H.; Chen, H.X.; Cheng, Y.C.; Lee, K.H. New 4 beta-substituted aniline derivatives of 6,7-O,O-demethylene-4’-O-demethylpodophyllotoxin and related compounds as potent inhibitors of human DNA topoisomerase II. J. Med. Chem. 1992, 35, 871–877.
- Cho, S.J.; Kashiwada, Y.; Bastow, K.F.; Cheng, Y.C.; Lee, K.H. Podophenazine, 2″,3″-Dichloropo-dophenazine, Benzopodophenazine, and Their 4b-p-Nitroaniline Derivatives as Novel DNA Topoisomerase II Inhibitors. J. Med. Chem. 1996, 39, 1396–1402.
- Cho, S.J.; Tropsha, A.; Suffness, M.; Cheng, Y.C.; Lee, K.H. Three-Dimensional Quantitative Structure-Activity Relationship Study of 4’-O-Demethyl-epipodophyllotoxin Analogs Using the Modified CoMFA/q2 -GRS Approach. J. Med. Chem. 1996, 39, 1383–1395.
- Hartwell, J.L. α-Peltatin, a new compound isolated from podophyllum peltatum. J. Am. Chem. Soc. 1947, 69, 2918.
- Stoll, A.; von Wartburg, A.; Renz, J. The isolation of a-peltatin glucoside from the rhizomes of Podophyllum peltatum L. J. Am. Chem. Soc. 1955, 77, 1710–1711.
- Nagar, N.; Jat, R.K.; Saharan, R.; Verma, S.; Sharma, D.; Bansal, K. Podophyllotoxin and their Glycosidic derivatives. Pharmacophore 2011, 2, 87–97.
- Bedows, E.; Hatfield, G.M. An Investigation of the Antiviral Activity of Podophyllum peltatum. J. Nat. Prod. 1982, 45, 725–729.
- Thurston, L.S.; Irie, H.; Tani, S.; Han, F.S.; Liu, Z.C.; Cheng, Y.C.; Lee, K.H. Inhibition of human DNA topoisomerase II by podophyllotoxin and alpha-peltatin analogs. J. Med. Chem. 1986, 29, 1547–1550.
- Thurston, L.S.; Imakura, Y.; Haruna, M.; Li, D.H.; Liu, Z.C.; Liu, S.Y.; Cheng, Y.C.; Lee, K.H. Inhibition of human DNA topoisomerase II by cytotoxic ether and ester derivatives of podophyllotoxin and alpha-peltatin. J. Med. Chem. 1989, 32, 604–608.
- Beers, S.A.; Imakura, Y.; Dai, H.J.; Li, D.H.; Cheng, Y.C.; Lee, K.H. Synthetic Ring C Aromatized Podophyllotoxin Analogues as Potential Inhibitors of Human DNA Topoisomerase II. J. Nat. Prod. 1988, 51, 901–905.
- Canjanamurthy, K.; Lokanatharai, M. Synthesis of Podophyllotoxin & related analogues: Part II-Synthesis of ß-apopicrophyllin analogues with modified Hydroaromatic ring-B. Indian J. Chem. 1985, 24, 505–508.
- Canjanamurthy, K.; Lokanatharai, M. Synthesis of Podophyllotoxin & related analogues: Part III-Synthesis of ß-apopicrophyllin analogues with modified lactone ring. Indian J. Chem. 1987, 26, 131–135.
- He, Y.; Ma, W.Y.; Zhang, C.N. The effects of unsaturated factors on ring C to chemical Properties of podophyllotoxin analogues. J. Chin. Pharm. Sci. 2001, 10, 81–84.
- Kamal, A.; Reddy, S.T.; Polepalli, S.; Shalini, N.; Reddy, V.G.; Subba Rao, A.V.; Shankaraiah, N. Synthesis and biological evaluation of podophyllotoxin congeners as tubulin polymerization inhibitors. Bioorg. Med. Chem. 2014, 22, 5466–5475.
- Guo, H.Z.; Guo, D.A.; Fei, X.Y.; Cui, Y.J.; Zheng, J.H. Biotransformation of podophyllotoxin to picropodophyllin by microbes. J. Asian Nat. Prod. Res. 1998, 1, 99–102.
- Larsson, M.A. Cyclolignans as inhibitors of the insulin-like growth factor-I receptor. Cancer Res. 2007, 67, 2899.
- Vasilcanu, R.; Vasilcanu, D.; Rosengren, L.; Natalishvili, N.; Sehat, B.; Yin, S.; Girnita, A.; Axelson, M.; Girnita, L.; Larsson, O. Picropodophyllin induces down regulation of the insulin-like growth factor 1 receptor: Potential mechanistic involvement of Mdm2 and beta-arrestin. Oncogene 2008, 27, 1629–1638.
- Walter, J.; Gensler, C.D.; Murthy, M.H.T. Non-enolizable podophyllotoxin derivatives. J. Med. Chem. 1977, 20, 635–644.
- Glinski, M.B.; Freed, J.C.; Durst, T. Preparation of 2-substituted podophyllotoxin derivatives. J. Org. Chem. 1987, 52, 2749–2753.
- Van-Vliet, D.S.; Lee, K.H. A high yield preparation of 2-fluoropodophyllotoxin. Tetrahedron. Lett. 1999, 40, 2259–2262.
- Van-Vliet, D.S.; Tachibana, Y.; Bastow, K.F.; Huang, E.S.; Lee, K.H. Design, Synthesis, and Biological Testing of 4b-Anilino-2-fluoro-4’-demethylpodophyllo-toxin Analogues as Cytotoxic and Antiviral Agents. J. Med. Chem. 2001, 44, 1422–1428.
- Laatsch, H.; Ernst, B.P.; Noltemeyer, M. Synthesis of Sterically Fixed Podophyllotoxin. Liebigs Ann. 1996, 1996, 731–737.
- Zhou, X.M.; Lee, K.J.H.; Cheng, J.; Wu, S.S.; Chen, H.X.; Guo, X.; Cheng, Y.C.; Lee, K.H. New gamma-lactone ring-modified arylamino etoposide analogs as inhibitors of human DNA topoisomerase II. J. Med. Chem. 1994, 37, 287–292.
- Gordaliza, M.; Faircloth, G.T.; Castro, M.A.; Miguel del Corral, J.M.; Lopez-Vazquez, M.L.; Feliciano, S.A. Immunosuppressive cyclolignans. J. Med. Chem. 1996, 39, 2865–2868.
- Subrahmanyam, D.; Renuka, B.; Laxmana-Rao, C.V.; Sagar, S.P.; Deevi, D.S.; Babu, J.M.; Vyas, K. Novel D-ring analogues of podophyllotoxin as potent anti-cancer agents. Bioorg. Med. Chem. Lett. 1998, 8, 1391–1396.
- Subrahmanyam, D.; Renuka, B.; Sunil, K.G.; Vandana, V.; Deevi, D.S. 9-Deoxopodophyllotoxin derivatives as anti-cancer agents. Bioorg. Med. Chem. Lett. 1999, 9, 2131–2134.
- Gaspar, N.; Occean, B.V.; Pacquement, H.; Bompas, E.; Bouvier, C.; Brisse, H.J.; Castex, M.P.; Cheurfa, N.; Corradini, N.; Delaye, J.; et al. Results of methotrexate-etoposide-ifosfamide based regimen (M-EI) in osteosarcoma patients included in the French OS2006/sarcome-09 study. Eur. J. Cancer 2018, 88, 57–66.
- Lau, W.; Sattely, E.S. Six enzymes from mayapple that complete the biosynthetic pathway to the etoposide aglycone. Science 2015, 349, 1224–1228.
- Jia, K.Z.; Zhan, X.; Li, H.M.; Shen, Y.; Qi, Q.; Zhang, Y.; Li, Y.Z.; Tang, Y.J. A novel podophyllotoxin derivative with higher anti-tumor activity produced via 4′-demethylepipodophyllotoxin biotransformation by Penicillium purpurogenum. Process Biochem. 2020, 96, 220–227.
- Liu, Y.Q.; Yang, L.; Tian, X. Podophyllotoxin: Current Perspectives. Curr. Bioact. Compd. 2007, 3, 37–66.
- Hernandez, A.P.; Diez, P.; Garcia, P.A.; Perez-Andres, M.; Ortega, P.; Jambriana, P.G.; Diez, D.; Castro, M.A.; Fuentes, M. A Novel Cytotoxic Conjugate Derived from the Natural Product Podophyllotoxin as a Direct-Target Protein Dual Inhibitor. Molecules 2020, 25, 4258.
- Xu, H.; Lv, M.; Tian, X. A review on hemisynthesis, biosynthesis, biological activities, mode of action, and structure-activity relationship of podophyllotoxins: 2003–2007. Curr. Med. Chem. 2009, 16, 327–349.
- Farkya, S.; Bisaria, V.S.; Srivastava, A.K. Biotechnological aspects of the production of the anticancer drug podophyllotoxin. Appl. Microbiol. Biotech. 2004, 65, 504–519.
- Brewer, C.F.; Loike, J.D.; Horwitz, S.B.; Sternlicht, H.; Gensler, W.J. Conformational analysis of podophyllotoxin and its congeners. Structure-activity relationship in microtubule assembly. J. Med. Chem. 1979, 22, 215–221.
- Zia-Ul-Haq, M. Past, Present and Future of Carotenoids Research. In Carotenoids: Structure and Function in the Human Body; Zia-Ul-Haq, M., Dewanjee, S., Riaz, M., Eds.; Springer: Cham, Switzerland, 2021; pp. 827–854.
- Desbene, S.; Giorgi-Renault, S. Drugs that inhibit tubulin polymerization: The particular case of podophyllotoxin and analogues. Curr. Med. Chem. Anti Cancer Agents 2002, 2, 71–90.
- Hu, C.; Xu, D.; Du, W.; Qian, S.; Wang, L.; Lou, J.; He, Q.; Yang, B.; Hu, Y. Novel 4 beta-anilino-podophyllotoxin derivatives: Design synthesis and biological evaluation as potent DNA-topoisomerase II poisons and anti-MDR agents. Mol. Biosyst. 2010, 6, 410–420.
- Ming-Jen, C.; Ming, D.; Hing, Y.; Sung, Y.; Jih, J.; Hsun, S.; Yueh, H.; Gwo, F.; Ih, S. A New Benzenoid Derivative from an Endophytic Fungus in Peperomia sui. Chem. Nat. Compd. 2018, 54, 625–627.
- Markkanen, T.; Makinen, M.L.; Nikoskelainen, J.; Ruohonen, J.; Nieminen, K.; Jokinen, P.O.; Rannio, R.; Hirvonen, T. Biological activities of lignans. Drugs Exp. Clin. Res. 1981, 7, 691.
- Fife, K.H. New treatments for genital warts less than ideal: Abstract and commentary. JAMA 1998, 279, 2003–2004.
- Sudo, K.; Konno, K.; Shigeta, S.; Yokota, T. Inhibitory Effects of Podophyllotoxin Derivatives on Herpes Simplex Virus Replication. Antivir. Chem. Chemother. 1998, 9, 263–267.
- MacRae, W.D.; Hudson, J.B.; Towers, G.H. The antiviral action of lignans. Planta Med. 1989, 55, 531–535.
- Syed, T.A.; Cheema, K.M.; Khayyami, M.; Ahmad, S.A.; Ahmad, S.H.; Ahmad, S. Human leukocyte interferon-alpha versus podophyllotoxin in cream for the treatment of genital warts in males. A placebo-controlled, double-blind, comparative study. Dermatology 1995, 191, 129.
- Beautner, K.R. Podophyllotoxin in the treatment of genital warts. In EP Eischman (edn) Sexually Transmitted Diseases. Adv. Treat. 1996, 24, 122–232.
- Zhao, W.; Cong, Y.; Li, H.M.; Li, S.; Shen, Y.; Qi, Q.; Zhang, Y.; Li, Y.Z.; Tang, Y.J. Challenges and potential for improving the druggability of podophyllotoxin-derived drugs in cancer chemotherapy. Nat. Prod. Rep. 2021, 38, 470–488.
- Zhang, J.; Liu, Y.Q.; Yang, L.; Feng, G. Podophyllotoxin Derivatives Show Activity against Brontispa Longissima Larvae. Nat. Prod. Commun. 2010, 5, 1247–1250.
- Leander, K.; Rosen, B. Medicinal Uses for Podophyllotoxin. U.S. Patent 4,788,216, 29 November 1988. Available online: (accessed on 15 February 2021).
- Lerndal, T.; Svensson, B. A clinical study of CPH 82 vs. methotrexate in early rheumatoid arthritis. Rheumatology 2000, 39, 316.
- Gordaliza, M.; Castro, M.A.; Miguel del Corra, J.M.; Feliciano, S.A. Antitumor Properties of Podophyllotoxin and Related Compounds. Curr. Pharm. Des. 2000, 6, 1811–1839.
- Hensel, A.; Bauer, R.; Heinrich, M.; Spiegler, V.; Kayser, O.; Hempel, G.; Kraft, K. Challenges at the Time of COVID-19: Opportunities and innovations in the Antivirals from Nature. Planta Med. 2020, 86, 659–664.
- Thanh, V.T.T.; Pham, V.C.; Mai, H.D.T.; Litaudon, M.; Gueritte, F.; Retailleau, P.; Nguyen, V.H.; Chau, V.M. Cytotoxic Lignans from Fruits of Cleistanthus indochinensis: Synthesis of Cleistantoxin Derivatives. J. Nat. Prod. 2012, 75, 1578–1583.
- Nanjundaswamy, N.; Satishi, S.; Lokanatha Rai, K.M.; Shashikanth, S.; Raveesha, K.A. Antibacterial Activity of Synthetic Precursors of Podophyllotoxin. Int. J. Biomed. Sci. 2007, 3, 112–115.
- Dombernowsky, P.; Nissen, N.I. Schedule dependency of the antileukemic activity of the podophyllotoxin-derivative VP 16-213 (NSC-141540) in L1210 leukemia. Acta Physiol. Microbiol. Scand. Sec. A Path. 1973, 81, 715–724.
- Liu, Y.Q.; Feng, G.; Yang, L.; Zhang, J.; Li, H.Y. Podophyllotoxin-derived insecticidal agents: Part XIII—Evaluation of insecticidal activity of podophyllotoxin derivatives against Brontispa longissima. Nat. Prod. Res. 2010, 25, 1570–1576.
- Wang, J.; Long, L.; Chen, Y. Design, synthesis and antineoplastic activity of novel hybrids of podophyllotoxin and indirubin against human leukemia cancer cells as multifunctional anti-MDR agents. Bioorg. Med. Chem. Lett. 2018, 28, 1817–1824.





