
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Michael I. Ojovan | + 1022 word(s) | 1022 | 2021-04-21 10:42:41 | | | |
| 2 | Vicky Zhou | Meta information modification | 1022 | 2021-04-27 05:28:11 | | |
Video Upload Options
Glass crystalline materials (GCM) are composite solids consisting of both vitreous and crystalline phases. The major component can be the crystalline phase with a vitreous phase acting as a binding agent or alternatively the vitreous phase can be the major component, with crystalline particles dispersed in the glass matrix.
1. Introduction
GCM are of increasing interest as advanced nuclear wasteforms combining the advantages of vitreous and crystalline matrices. The GCM are versatile wasteforms envisaged for a wider use to immobilise various types of both radioactive and chemically hazardous wastes. They can be produced either via low temperature sintering using precursors composed of glass frit, oxides, and crystalline phases or through conventional melting aiming to produce first a parent glass, which is then crystallised by a controlled thermal schedule to obtain target crystalline phases within the GCM.
The spent nuclear fuel (SNF) reprocessing generates radioactive wastes, including high level radioactive waste (HLW), which is industrially immobilised in the Na-Al-P glass in Russia and A-B-Si glass elsewhere, where A stands for alkaline elements. By 2013, there were about 30,000 accumulated tonnes of vitrified HLW overall in the world [1][2]. Considering the processing rates of vitrification facilities [2][3][4][5][6], the current mass of vitrified HLW can be estimated at about 35,000–36,000 tonnes, of which almost 80% are A-B-Si, and the rest are Na-Al-P glasses. Vitrification of HLW is, however, not the optimal method of immobilisation due to the relative low radionuclide loading of glasses and their susceptibility to crystallisation, which can begin immediately after the melt pouring into canisters due to the residual heat of the melt [6][7]. Partitioning of HLW radionuclides onto groups can provide a better solution for their immobilisation by incorporation within crystalline lattice of silicates, titanates, zirconates, and phosphates. Their natural analogues are minerals zircon, britholite, pyrochlore, zirconolite, murataite, perovskite, monazite, and garnet [8][9][10][11][12][13][14][15][16]. Fission products (Cs, Sr) can be isolated in both crystalline phases such as hollandite, pollucite, perovskite, langbeinite, and glasses. Glass crystalline materials (GCM) with the same mineral-like phases are optimal for wastes of complex composition. The major component of GCM may be either the crystalline phases with the glass acting as a binding agent or alternatively the vitreous phase may be the major component, with crystalline particles dispersed in the glass matrix [17][18][19][20][21].
2. GCM with Mineral-Like Phases
2.1. Importance of Novel Matrices
Much attention is paid to the modernization of existing glass matrices, as well as the search for new types of wasteforms, for example, for HLW radionuclide fractions. Partitioning of HLW and incorporation of the most dangerous long-lived actinides and fission products in a compact and capacious matrix will improve the use of underground repository space. This will reduce the need for the construction of new storage facilities and lead to savings in finances and time for their search and construction. Potential novel matrices for HLW immobilisation are crystalline and GCM which have been studied since the 1970s, almost simultaneously with research of glasses [22][23][24][25].
2.2. Crystaline Matrices
The best known crystalline wasteforms are the Synroc polyphase ceramic and the monophase NZP matrix. In the first, artificial phases of minerals with the structure of perovskite, zirconolite, pyrochlore, and hollandite serve as carriers of radionuclides and the HLW elements are distributed between them in accordance with the radius and charge of cations. The structure of the NZP matrix of the composition NaZr2(PO4)3 as a natural analogue of the mineral kosnarite is formed by a three-dimensional network of PO43- octahedra connected by vertices to ZrO6 octahedra, and large Na+ cations occupy voids. The HLW components can enter three positions of the structure: Alkalis, alkaline earths instead of sodium; REE and actinides are in the Zr position, hexavalent Mo replaces phosphorus, etc. Usually, there is an additional phase—REE phosphate with a monazite structure. Kosnarite, KZr2(PO4)3 is a natural analogue of the NZP matrix, although unlike other phases (pyrochlore, zirconolite, brannerite, monazite, etc.), it does not contain radioactive elements such as U and Th [22]. The waste loading of Synroc and NZP ceramics is about 20 wt.%. Crystalline phases for immobilization of waste have been overviewed in many publications such as [15][16][22][26].
2.3. GCM as an Universal Nuclear Wasteform
GCM are thermodynamically more stable materials compared with homogeneous glasses. Indeed, the free Gibbs energy GGCM of a GCM containing the volume fraction φ of crystalline phase will be:
where the free Gibbs energy of crystalline phase GC is lower than the free Gibbs energy of glass GG. Thus, the driving force of crystallization of GCM toward a most stable fully crystalline material (for which φ = 1) will be smaller.
GCM containing both crystalline and glassy phases are optimal for radioactive waste of complex composition [8][17][19][21][22][27][28][29][30][31][32][33][34]. Compared to homogeneous glassy materials, GCMs can incorporate larger amounts of waste elements and they can be produced using lower processing temperatures than those of conventional melting. Indeed, hazardous and nuclear waste constituents can be immobilised both by direct chemical incorporation into the glass structure in a classical vitrification approach and by the physical encapsulation of the waste in a glass matrix, forming a GCM consisting of both vitreous and crystalline phases (Figure 1).
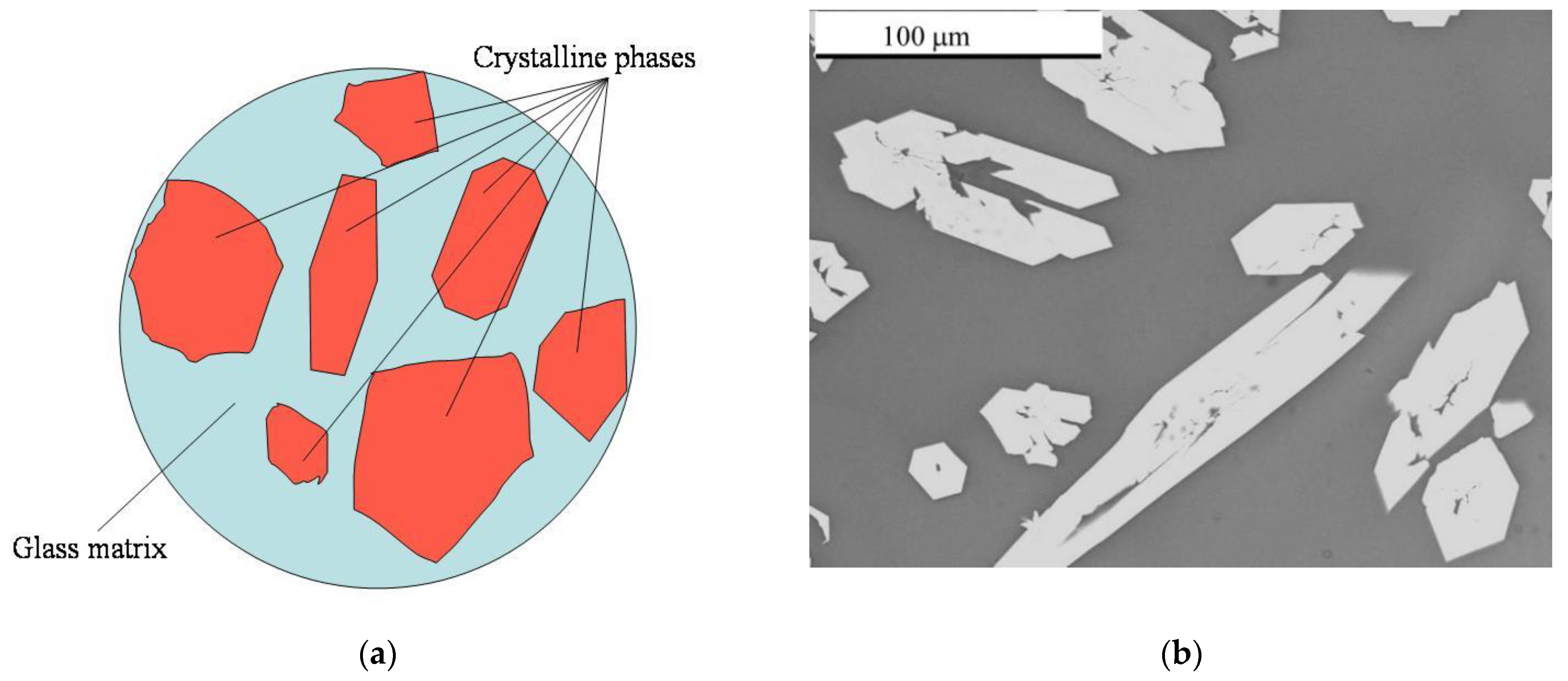
Figure 1. Schematic of a Glass crystalline material (GCM) nuclear wasteform (a) and SEM/BSE image (b) of a real specimen loaded with rare earth elements (REE)-imitator of actinides. Dark areas are composed of glass and light grains are crystals of britholite.
3. Conclusions
This entry focuses on GCM as versatile materials to immobilise nuclear waste[35]. HLW derived in the close nuclear fuel cycle is currently immobilised into Na-Al-P and A-B-Si glasses using vitrification technology. Waste immobilisation in GCMs has emerged as a versatile technology enabling reliable immobilisation of complex and varying composition waste streams, including both radioactive and hazardous residues, which are otherwise difficult to immobilise using the traditional vitrification technology. The optimisation of the GCM phase assemblages as a function of waste stream composition is important for achieving simultaneously high nuclear waste loadings and corrosion resistance. Future research may focus on practical aspects of GCM utilisation through one or another technological process using either controlled devitrification of synthesized parent glasses or sintering routes using crystalline and vitreous precursors.
References
- Gin, S.; Abdelouas, A.; Criscenti, L.J.; Ebert, W.L.; Ferrand, K.; Geisler, T.; Harrison, M.T.; Inagaki, Y.; Mitsui, S.; Mueller, K.T.; et al. An international initiative on long-term behavior of high-level nuclear waste glass. Mater. Today 2013, 16, 243–248.
- Ojovan, M.I. On Alteration Rate Renewal Stage of Nuclear Waste Glass Corrosion. MRS Adv. 2020, 5, 111–120.
- Luzhetsky, A.V.; Petrov, V.A.; Yudintsev, S.V.; Malkovsky, V.I.; Ojovan, M.I.; Nickolsky, M.S.; Shiryaev, A.A.; Danilov, S.S.; Ostashkina, E.E. Effect of gamma irradiation on structural features and dissolution of nuclear waste Na–Al–P glasses in water. Sustainability 2020, 12, 4137.
- Gin, S.; Jollivet, P.; Tribet, M.; Peuget, S.; Schuller, S. Radionuclides containment in nuclear glasses: An overview. Radiochim. Acta 2017, 105, 927–959.
- Jantzen, C.M.; Ojovan, M.I. On selection of matrix (wasteform) material for higher activity nuclear waste immobilisation (Review). Russ. J. Inorg. Chem. 2019, 64, 1611–1624.
- Kozlov, P.V.; Remizov, M.B.; Belanova, E.A.; Vlasova, N.V.; Orlova, V.A.; Martynov, K.V. Modification of the composition of aluminophosphate glasses with HLW simulators to increase their stability. 1. The effect of modifiers on the viscosity and crystallization ability of melts. Rad. Saf. Issues 2019, 1, 3–15.
- Stefanovsky, S.V.; Stefanovsky, O.I.; Remizov, M.B.; Kozlov, P.V.; Belanova, E.A.; Makarovsky, R.A.; Myasoedov, B.F. Sodium–aluminum–iron phosphate glasses as legacy high-level waste forms. Prog. Nucl. Energy 2017, 94, 229–234.
- Yudintsev, S.V. Isolation of fractionated waste of nuclear industry. Radiochemistry 2021, in press.
- Ewing, R.C. The Design and Evaluation of Nuclear-Waste Forms: Clues from Mineralogy. Canad. Miner. 2001, 39, 697–715.
- Omel’yanenko, B.I.; Livshits, T.S.; Yudintsev, S.V.; Nikonov, B.S. Natural and artificial minerals as matrices for immobilization of actinides. Geol. Ore Depos. 2007, 49, 173–193.
- Vance, E.R. Ceramic Waste Forms. In Comprehensive Nuclear Materials; Konings, R., Allen, T., Stoller, R., Yamanaka, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2012; Volume 5, Chapter 5.19; pp. 485–503.
- Stefanovsky, S.V.; Yudintsev, S.V. Titanates, zirconates, aluminates and ferrites as waste forms for actinide immobilization. Russ. Chem. Rev. 2016, 85, 962–994.
- Orlova, A.I.; Koryttseva, A.K.; Loginova, E.E. A family of phosphates of langbeinite structure. Crystal-chemical aspect of radioactive waste immobilization. Radiochemistry 2011, 53, 51–62.
- Orlova, A.I.; Lizin, A.A.; Tomilin, S.V.; Lukinykh, A.N. On the possibility of realization of monazite and langbeinite structural types in complex americium and plutonium phosphates. Synthesis and X-ray diffraction studies. Radiochemistry 2011, 53, 63–68.
- Orlova, A.I.; Ojovan, M.I. Ceramic Mineral Waste-Forms for Nuclear Waste Immobilization. Materials 2019, 12, 2638.
- Burakov, B.E.; Ojovan, M.I.; Lee, W.E. Crystalline Materials for Actinide Immobilization; Imperial College Press: London, UK, 2011; 197p.
- Ojovan, M.I.; Lee, W.E.; Kalmykov, S.N. An Introduction to Nuclear Waste Immobilisation, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2019; 497p.
- Ojovan, M.I.; Juoi, J.M.; Boccaccini, A.R.; Lee, W.E. Glass Composite Materials for Nuclear and Hazardous Waste Immobilisation. Mater. Res. Soc. Symp. Proc. 2008, 1107, 245–252.
- Caurant, D.; Loiseau, P.; Majérus, O.; Aubin-Cheval-Donnet, V.; Bardez, I.; Quintas, A. Glasses, Glass-Ceramics and Ceramics for Immobilization of Highly Radioactive Nuclear Wastes; Nova Science Publishers: New York, NY, USA, 2009; 445p.
- Ojovan, M.I.; Lee, W.E. New Developments in Glassy Nuclear Wasteforms; Nova Science Publishers: New York, NY, USA, 2007; 131p.
- Donald, I.W. Waste Immobilization in Glass and Ceramic-Based Hosts; Wiley: Chichester, UK, 2010; 507p.
- Lutze, W.; Ewing, R.C. Radioactive Waste Forms for the Future; Elsevier: New York, NY, USA, 1988; 778p.
- Ojovan, M.I. Handbook of Advanced Radioactive Waste Conditioning Technologies; Woodhead: Cambridge, UK, 2011; 512p.
- Hayward, P.J. Glass-ceramics. In Radioactive Waste Forms for the Future; Lutze, W., Ewing, R.C., Eds.; Elsevier: New York, NY, USA, 1988; pp. 427–494.
- National Research Council. Waste Forms Technology and Performance: Final Report; National Academies Press: Washington, DC, USA, 2011; 308p.
- Weber, W.J.; Ewing, R.C.; Catlow, C.R.A.; Diaz de la Rubia, T.; Hobbs, L.W.; Kinoshita, C.; Matzke Hj Motta, A.T.; Nastasi, M.; Salje, E.K.H.; Vance, E.R.; et al. Radiation effects in crystalline ceramics for the immobilization of high-level nuclear waste and plutonium. J. Mater. Res. 1998, 13, 1434–1484.
- Vashman, A.A.; Demin, A.V.; Krylova, N.V.; Kushnikov, V.V.; Matyunin, Y.I.; Poluektov, P.P.; Polyakov, A.S.; Teterin, E.G. Phosphate Glasses with Radioactive Waste; CNIIatominform: Moscow, Russia, 1997; p. 172.
- McCloy, J.S.; Goel, A. Glass-ceramics for nuclear-waste immobilization. Mater. Res. Soc. Bull. 2017, 42, 233–238.
- Donald, I.W. Immobilisation of radioactive and non-radioactive wastes in glass-based systems: An overview. Glass Technol. 2007, 48, 155–163.
- Rawling, R.D.; Wu, J.P.; Boccaccini, A.R. Glass-ceramics: Their production from wastes—A Review. J. Mater. Sci. 2006, 41, 733–761.
- Colombo, P.; Brusatin, G.; Bernardo, E.; Scarinci, G. Inertization and reuse of waste materials by vitrification and fabrication of glass-based products. Curr. Opin. Solid State Mater. Sci. 2003, 7, 225–239.
- Lee, W.E.; Ojovan, M.I.; Stennett, M.C.; Hyatt, N.C. Immobilisation of radioactive waste in glasses, glass composite materials and ceramics. Adv. Appl. Ceram. 2006, 105, 3–12.
- Lewis, M.A.; Fischer, D.F. Properties of glass-bonded zeolite monoliths. Ceram. Trans. 1994, 45, 277–286.
- Juoi, J.M.; Ojovan, M.I.; Lee, W.E. Microstructure and leaching durability of glass composite wasteforms for spent clinoptilolite immobilisation. J. Nucl. Mater. 2008, 372, 358–366.
- Ojovan, M.I.; Petrov, V.A.; Yudintsev, S.V. Glass Crystalline Materials as Advanced Nuclear Wasteforms. Sustainability 2021, 13, 4117. https://doi.org/10.3390/su13084117.




