
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ichiro Imae | + 1583 word(s) | 1583 | 2021-03-30 10:41:18 | | | |
| 2 | Camila Xu | Meta information modification | 1583 | 2021-04-27 03:35:22 | | |
Video Upload Options
Graphene is a single layer of carbon atoms lined up in a hexagonal lattice, and graphite is composed of these multiple layers.
1. Introduction
Graphene is a single layer of carbon atoms lined up in a hexagonal lattice, and graphite is composed of these multiple layers. Until the 2000s, graphene was difficult to obtain, and research in this area was slow for many years. However, in 2004, Andre Geim and Konstantin Novoselov succeeded in obtaining graphene by attaching a piece of graphite to cellophane tape (Scotch tape) and peeling it off, and since then, graphene has attracted attention as a new electronic functional material due to its unique electrical properties [1]. Graphene can be obtained not only by direct exfoliation from graphite by tape (as described above) but also by the chemical vapor deposition (CVD) method. However, the former is inefficient and the latter requires large equipment, so both are not suitable for industrial use. On the other hand, the synthesis of graphene via graphene oxide, which is obtained from the oxidation of graphite, has recently attracted much interest due to its suitability for mass-scale synthesis (Figure 1). In this method, graphite oxide is first synthesized by chemical oxidation of graphite. Graphite oxide has many hydrophilic oxygen functional groups inserted between the graphite layers, which weakens the interaction between the layers, and can be easily exfoliated into a monolayer by sonication in water to produce graphene oxide (GO), which is uniformly dispersed in water. Finally, the GO can be reduced to obtain a material with electrical properties similar to graphene. Strictly speaking, however, it is difficult to obtain perfect graphene from this method, so the material obtained from this method is often called “reduced graphene oxide” (rGO).
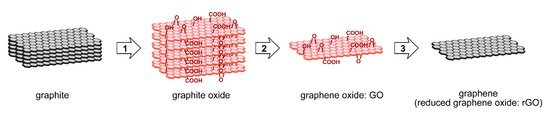
Figure 1. Synthetic route of graphene (reduced graphene oxide) through graphene oxide from graphite (1: oxidation, 2: exfoliation, 3: reduction).
There are three main methods of GO reduction: (1) chemical reduction, (2) electrochemical reduction, and (3) thermal reduction. Among them, chemical reduction is the most frequently used method to synthesize rGO by the chemical reduction of oxygen functional groups such as epoxy groups in GO using a reducing agent such as hydrazine [2]. However, due to the high toxicity of hydrazine, reduction using hydrazine is not a preferred method from an industrial viewpoint. Recently, reduction using hydrogen iodide has also been attempted [3], but this method is also industrially undesirable because hydrogen iodide is highly corrosive. In addition, a unique reduction method using food-derived substances such as vitamin C [4], glucose [5], and xylitol [6] has been reported, but their reaction efficiency is not very high. More recently, reduction methods of GO using Joule heat and microwaves have also been reported [7].
In contrast, electrochemical reduction is a method of reducing oxygen groups by electrode reaction and is therefore more environmentally friendly than chemical reduction methods in that it does not use toxic reagents. Another feature is that the electronic state of the resulting graphene can be easily manipulated by controlling the reduction level through electrolysis conditions. The thermal reduction method is not exactly a reduction reaction but rather a simple thermal decomposition which removes the oxygen functional groups (hydroxyl, epoxy, carboxyl groups, etc.) in GO. This method can synthesize rGO on a mass scale as long as there is an electric furnace that can provide heat in an inert atmosphere, making it suitable for industrial-scale production processes in addition to being environmentally friendly.
2. Synthesis of Graphene Oxide
There are three main methods used for the synthesis of GO: Brodie [8], Staudenmaier [9], and Hummers [10] methods. Among these methods, the Brodie and Staudenmaier methods use potassium chlorate as the oxidizing agent, while the Hummers method uses potassium permanganate as the oxidizing agent to reduce the risk of explosion, which was a concern in the former two methods. However, in the Hummers method, only the surface of the graphite is oxidized, leaving an incompletely oxidized graphite core, which tends to produce oxidized graphite with insufficient exfoliation. Therefore, the Modified Hummers [11] and Improved Hummers [12] methods were developed to improve the synthesis efficiency of GO by pre-oxidation of graphite. Details on the characteristics of these GO synthesis methods are provided in the review by Prof. M. Mercedes Velázquez et al. [13]. In our study, the Modified Hummers method was employed and GO aqueous dispersions were synthesized by the following procedure.
The natural graphite powder (2.0 g) was put into a solution of concentrated H2SO4 (8 mL), K2S2O8 (1.0 g), and P2O5 (1.0 g), and stirred at 80 °C for 5 h. The mixture was cooled to room temperature, and 200 mL of deionized water was slowly added and left to stand overnight. The solution was filtered through a Buchner funnel, washed until the filtrate was neutral, and dried spontaneously at room temperature to obtain a pre-oxidized graphite powder. The pre-oxidized graphite was then subjected to oxidation by the Hummers method. The pre-oxidized graphite powder (2.0 g) was put into cold (0 °C) concentrated H2SO4 (50 mL). KMnO4 (7.0 g) was added gradually with stirring and cooling for 1 h. The mixture was then stirred at 35 °C for 2 h, and distilled water (110 mL) was added at 0 °C. Hydrogen peroxide solution was added until no gas was generated from the reaction solution, after which the color of the mixture changed to bright brown. Using a Buchner funnel, the mixture was filtered and washed with 9 vol % HCl solution (500 mL) in order to remove metal ions. The GO product was suspended in distilled water to give a viscous dispersion, which was subjected to dialysis using a standard regenerated cellulose dialysis membrane (Spectra/Por® 1, Repligen Corp. (formerly Spectrum Laboratories, Inc.), Rancho Dominguez, CA, USA) to completely remove metal ions and acids. This process was carried out for one week while changing the distilled water. The GO was then dispersed in water by sonication for 1 h. GO was dispersed in water by sonication for 1 h and then centrifuged to remove the few remaining impurities (this process was carried out twice). The concentration of the final GO aqueous dispersion was determined by vacuum drying 1.0 mL of the GO aqueous dispersion and measuring the weight of the resulting GO powder.
3. Electrochemical Reduction
3.1. Electrochemical Reduction of GO in Organic Solvents
The electrochemical reduction of GO is generally carried out in aqueous electrolyte solutions, but since GO is water dispersible, it is necessary to immobilize GO on the electrode by prior chemical treatment [14]. We carried out the electrochemical reduction of GO in organic solvents, taking advantage of the fact that GO does not detach from the electrode in organic solvents as compared to aqueous systems [15]. For the electrochemical reduction of GO, platinum and indium–tin oxide (ITO) could be used as working electrodes, but the reduction reaction proceeded more effectively when fluorine-doped tin oxide (FTO), which can be applied in a wide potential window, was used. Figure 2 shows the linear sweep voltammetry (LSV) of the system using propylene carbonate (PC) as the organic solvent, tetraethylammonium tetrafluoroborate (Et4NBF4) as the supporting electrolyte, and FTO coated with a drop-cast film of GO as the working electrode. For comparison, the LSV data using only FTO without GO coating (bare FTO) as the working electrode are also shown. The reduction current flowed from around −0.5 V vs. Fc/Fc+, indicating that electrochemical reduction of GO is possible. When the applied potential became more negative than −1.0 V at LSV, the color of the film changed from brown to black, suggesting that GO was changing to rGO (Figure 3). Electrochemically reduced GO is henceforth referred to as “erGO”.
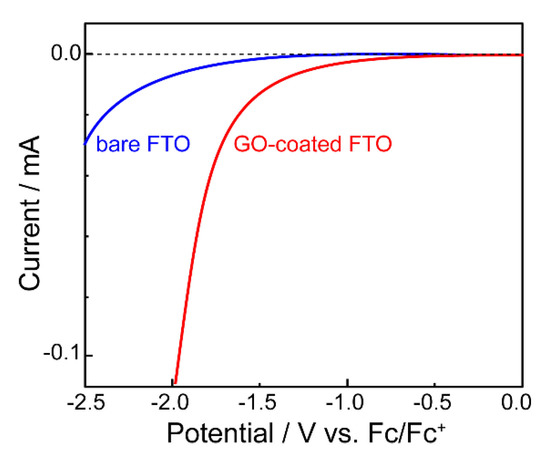
Figure 2. Linear sweep voltammogram of GO (red) (supporting electrolyte: 0.5 M Et4NBF4 in propylene carbonate). As a reference, the LSV curve of bare FTO is shown (blue).
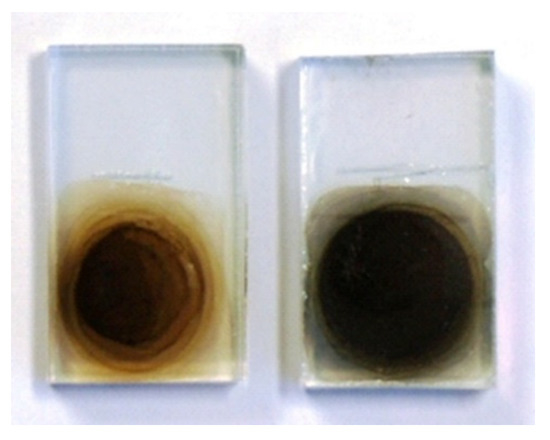
Figure 3. Photo-images of GO (left) and erGO (right).
Fourier Transform Infrared (FT-IR) and X-ray photoelectron spectroscopy (XPS) were measured to identify the chemical structure of erGO (Figure 4). Although no significant absorption bands were observed in the raw graphite, absorption bands originating from C=O stretching vibration of the carbonyl group, angular vibration of the carboxyl group, and C–OH stretching vibration of the hydroxyl group were observed in GO powder at 1740, 1370, and 1220 cm−1, respectively. However, after the electrochemical reduction of GO, the characteristic absorption bands were no longer observed in the spectrum of the films as in graphite, suggesting that most of the oxygen-containing groups were removed by the electrochemical reduction. In addition, the C1s spectra in XPS showed that the signal derived from oxidized species in GO almost completely disappeared by electrochemical reduction. In order to investigate the electrical properties of erGO, the electrical conductivity of erGO films was measured by the four-probe method and found to be about 3 S cm−1, which is comparable to that of rGO obtained by chemical reduction with hydrazine.
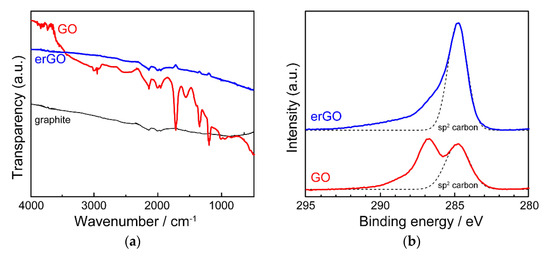
Figure 4. (a) FT-IR and (b) XPS spectra of erGO.
In addition to its high electrical conductivity, graphene has a large specific surface area of 2600 m2 g−1, which makes it promising as an electrode material for electric double-layer capacitors (EDLCs). EDLC is one of the electronic components used in a wide range of industries as a backup power source for integrated circuit (IC) and large-scale integration (LSI) memories and actuators. The sweep rate dependence of the specific capacity of EDLC prepared using erGO obtained by electrochemical reduction in propylene carbonate (PC) is shown in Figure 5. Although the rate of decrease in specific capacitance was about 20% compared to that at 10 mV s−1, it was found that the specific capacitance was maintained to some extent.
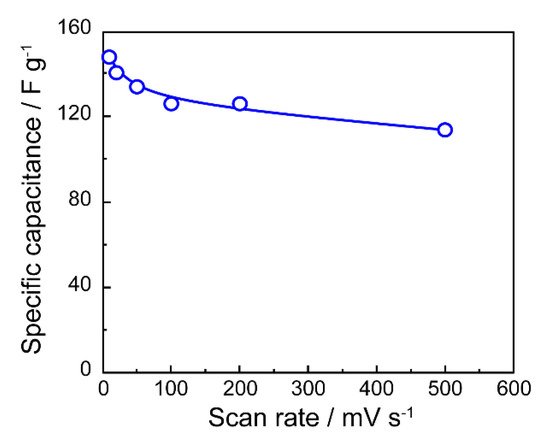
Figure 5. Specific capacitances of erGO/FTO in Et4NBF4 (0.5 M) in propylene carbonate plotted against the scan rate.
References
- Rashid bin Mohd Yusoff, A. Graphene-Based Energy Devices; Wiley-VCH: Weinheim, Germany, 2015.
- Stankovich, S.; Dikin, D.A.; Piner, R.D.; Kohlhaas, K.A.; Kleinhammes, A.; Jia, Y.; Wu, Y.; Nguyen, S.B.T.; Ruoff, R.S. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 2007, 45, 1558–1565.
- Pei, S.; Zhao, J.; Du, Z.; Ren, W.; Cheng, H.-N. Direct reduction of graphene oxide films into highlyconductive and flexible graphene films by hydrohalic acids. Carbon 2010, 48, 4466–4474.
- Chen, W.; Yan, L. In situ self-assembly of mild chemical reduction graphene for three-dimensional architectures. Nanoscale 2011, 3, 3132–3137.
- Xu, Y.-L.; Qi, J.-M.; Sun, F.-F.; Ma, N. Carbocatalysis: Reduced graphene oxide-catalyzed Boc protection of hydroxyls and graphite oxide-catalyzed deprotection. Tetrahedron Lett. 2015, 56, 2744–2748.
- Liu, Y.; Feng, J. An attempt towards fabricating reduced graphene oxide composites with traditional polymer processing techniques by adding chemical reduction agents. Comp. Sci. Technol. 2017, 140, 16–22.
- Dong, L.; Yang, J.; Chhowalla, M.; Loh, K.P. Synthesis and reduction of large sized graphene oxide sheets. Chem. Soc. Rev. 2017, 46, 7306–7316.
- Brodie, B.C. Researches on the atomic weight of graphite. Quart. J. Chem. Soc. Lond. 1860, 12, 261–268.
- Staudenmaier, L. Verfahren zur Darstellung der Graphitsäure (Method for the preparation of graphitic acid). Ber. Dtsch. Chem. Ges. 1898, 31, 1481–1487.
- Hummers, W.S., Jr.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339.
- Kovtyukhova, N.I.; Ollivier, P.J.; Martin, B.R.; Mallouk, T.E.; Chizhik, S.A.; Buzaneva, E.V.; Gorchinskiy, A.D. Layer-by-layer assembly of ultrathin composite films from micron-sized graphite oxide sheets and polycations. Chem. Mater. 1999, 11, 771–778.
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved synthesis of graphene oxide. ACS Nano 2010, 4, 4806–4814.
- López-Diaz, D.; Merchán, M.D.; Velázquez, M.M. The behavior of graphene oxide trapped at the air water interface. Adv. Colloid Int. Surf. 2020, 286, 102312.
- Shao, Y.; Wang, J.; Engelhard, M.; Wang, C.; Lin, Y. Facile and controllable electrochemical reduction of graphene oxide and its applications. J. Mater. Chem. 2010, 20, 743–748.
- Harima, Y.; Setodoi, S.; Imae, I.; Komaguchi, K.; Ooyama, Y.; Ohshita, J.; Mizota, H.; Yano, J. Electrochemical reduction of graphene oxide in organic solvents. Electrochim. Acta 2011, 56, 5363–5368.




