
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Dmitriy Berillo | + 3212 word(s) | 3212 | 2021-04-09 04:31:09 | | | |
| 2 | Bruce Ren | -21 word(s) | 3191 | 2021-04-25 04:52:35 | | |
Video Upload Options
Bioremediation is a key process for reclaiming polluted soil and water by the use of biological agents. A commonly used approach aims to neutralise or remove harmful pollutants from contaminated areas using live microorganisms. Generally, immobilised microorganisms rather than planktonic cells have been used in bioremediation methods. Activated carbon, inorganic minerals (clays, metal oxides, zeolites), and agricultural waste products are acceptable substrates for the immobilisation of bacteria, although there are limitations with biomass loading and the issue with leaching of bacteria during the process. Various synthetic and natural polymers with different functional groups have been used successfully for the efficient immobilisation of microorganisms and cells. Promise has been shown using macroporous materials including cryogels with entrapped bacteria or cells in applications for water treatment and biotechnology. A cryogel is a macroporous polymeric gel formed at sub-zero temperatures through a process known as cryogelation. Macroporous hydrogels have been used to make scaffolds or supports for immobilising bacterial, viral, and other cells. The production of composite materials with immobilised cells possessing suitable mechanical and chemical stability, porosity, elasticity, and biocompatibility suggests that these materials are potential candidates for a range of applications within applied microbiology, biotechnology, and research.
1. Introduction
Current multistep water purification methods to remove chemical contaminants include activated sludge, hydrogenation, ion exchange, liquid–liquid extraction, adsorption by activated carbon, forward and inverse osmosis, electrolysis, sonochemistry, UV irradiation, and oxidation. Some processes require high temperatures and extreme pressures (e.g., hydrogenation, hydrodechlorination), and/or expensive catalysts such as platinum, palladium, rhodium, and gold nanoparticles on carbon or other supports [1][2][3][4][5], and are therefore not always cost-effective. Some compounds may result in the formation of more toxic or mutagenic derivatives. For example, the Fenton oxidation process, which usually involves the use of hydrogen peroxide and salts of iron (II) and generating free highly active hydroxide radicals may result in the production of polychlorinated dibenzo-p-dioxins and dibenzofurans [3][4][6]. The chemical redox process using iron nanoparticles (FeNPs) for the dechlorination process, where the dissolution of iron in water leads to the generation of radicals of hydrogen that, in turn, are involved in the interaction with organic molecules, and the other mechanism may include the interaction of iron oxide/hydroxide shell of FeNPs with chlorinated groups [5]. Several book chapters are devoted to immobilisation strategies for microorganisms (prokaryotes and eukaryotes, primarily fungi) on various organic (bark or wood chips) and inorganic supports (Fe2O3, Titanium oxide, alumosilicates Al2O3 x SiO2, cement particles) [5][7]. Bertrand et al. made a comprehensive overview of a range of efficient systems of waste water treatment at the industrial scale and accompanied each case study with a simplified yet very informative illustration of the principles of the system [5]. Advantages and drawbacks of each immobilisation approach (artificially generated biofilm, biofilm produced in nature) compared to microorganisms in a planktonic state for purifying of waste water from oil, herbicides, pesticides, xenobiotics, and heavy metals are discussed [7]. These approaches for water treatment are quite efficient but at the same time expensive and in most cases quite specific to the contaminant. Such methods require complex technological processes, which are expensive to build and operate. In comparison, biological treatment methods have fewer environmental impacts and are more energy-efficient, but their performance is limited at high concentrations of contaminants. Enzymatic treatment protocols are immobilized to be an efficient way to treat various compounds. However, they are unstable in the free state and require immobilisation on a substrate to ensure durability and efficacy. To reduce costs, whole cells can be used as the enzymatic system. The immobilisation of microorganisms onto appropriate substrates can be exploited for a number of processes and allows easy recovery and reuse of the bacteria [8]. Immobilisation can enhance the operational stability of cells and can protect them from the effects of extreme pH, toxic compounds, turbulent reaction technologies, and reduce the risk of contamination of cell cultures [4][9][10][11]. The review by Martines et al. [10] was devoted to a brief chemical description of each natural (Chitosan(CHI), Alginate(Alg), agar, collagen, agarose) and synthetic polymers (poly-acrylamide(pAAm), polyvinyl alcohol (PVA), polyethylene-glycol (PEG) and polycarbamoyl sulphonate, polypropylene, polyethylene, polyvinylchloride, poly-urethane, polyacrylonitrile) that are utilised for the preparation of inexpensive, non-toxic, and potentially with reactive functional groups carriers for entrapment of microorganisms.Bacteria have been immobilised on a range of chemically activated and/or inert supports. These include biofilm formation on activated carbon, powdered activated carbon with external surface areas of 938–978 mg/m2, inorganic metal oxides, membranes or porous polymers, and chitosan-beads [12][13]. Ionic liquids have been used in sensor-based applications as they significantly prolong the lifetime of the sensor and enable the number of immobilised cells on the electrode surface to be controlled [14]. The management of bioremediation process parameters such as oxygen concentration, pH, sulphide, and nitrite/nitrate levels is important, especially processes utilising entrapped bacterial cells. The bacterial consortium immobilised on natural coconut coir is illustrated to be the most efficient matrix to enhance the effectiveness of sewage remediation. Reactors operated in continuous mode for 15 and 16 days revealed 75.9% and 73.7% chemical oxygen demand decline and removal of nitrogen NO3 of 78.9% and NH4 of 79.7%, respectively. The organic contaminant removal rate was not significant during the first day; however, from the second day, the removal efficacy was continuously increased, due to adaptation of the bacterial community, which takes 24 h. On the eighth day, a fifty per cent decline of contaminant was registered. The maximum kinetics of remediation was exhibited on the 15th day in domestic wastewater column reactor experiments [15]. The density of bacteria on the substrate material depends on the structure, pore size, and surface area of the support, as well as the nature of the material (hydrophobicity, charge, etc.) and environmental conditions, such as ionic strength, presence of some trace essential elements, pH, and temperature. It has been discussed that the bioremediation rate can be enhanced using immobilisation methods, particularly under harsh conditions [16]. Immobilised cells are easier to separate, are more tolerant to pH and temperature changes, and have possible reutilisation potential [17][18]. The effect of cell storage at −18 °C for 18–24 months on reproductive capacity of different microorganisms (Gram-positive and Gram-negative bacteria, yeasts, and filamentous fungi) entrapped in PVA macroporous hydrogel was studied. It was observed that activity of immobilised Escherichia coli cells preserved in granules of PVA macroporous hydrogel revealed a high level of productivity of a target recombinant protein over 1.5 years [19]. Contamination of the environment with AAm is great, as it is a side product of various processes besides enormous industrial production of polyacrylamide. AAm degrades quickly at low concentrations (10 mg/L) in surface water; however, at higher concentrations, it stays stable for approximately 60 days in tap water. Such a problem for wastewater can be solved by bioremediation of AAm solution (1–5%) by Pseudomonas aeruginosa immobilised into Ca-Alg hydrogel within 2–3 days and faster than free bacteria suspension. Moreover, the presence of 200 and 400 mg/L of nickel enhanced the process [20].Cortez and co-workers presented a review of adhesive biocatalytic coatings (biocoatings), with a nanoporous microstructure of polymeric particles incorporated with a high density of viable cells [18]. Lyophilised biocoatings stabilised with carbohydrate osmo-protectants can be prepared by high-speed coating technologies, aerosol delivery, or ink-jet printers. They form multi-layered, patterned coatings on flexible nonporous or nonwoven substrates, preserving the order of 1010–1012 non-growing viable bacteria per m2 [19]. The structure of the biocoatings allows illumination of a high concentration of photo-reactive cells or algae within thin liquid films for efficient mass transfer. Biofilm formation, however, is usually a long process and represents the main cost and limitations of biotechnological processes using immobilised bacteria (Figure 1b). Biofilm develops in several steps: motile cells attach to the surface, maturate, and produce protective extracellular matrix, finally followed by biofilm dispersal [21][22]. Thermosensetive macroporous hydrogel s composed of co-polymer N-isopropylacrylamide - N-hydroxyethylacrylamide was modified with glycomonomer (mannose, glucose). These macroporous hydrogel s used for sample preparation of water after purification at the quality control step for accumulation of potentially photogenic bacteria at low concentrations via filtration of a large volume of water, that followed by elution by sugar-containing eluent [22]. This approach also can be utilised for accelerated biofilm formation for various biotechnological processes.
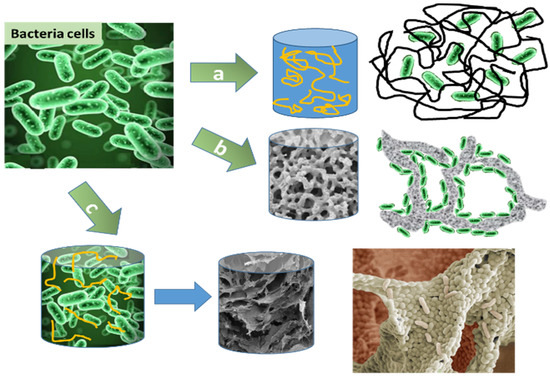
2. Application of Immobilised Bacteria
Different macroporous hydrogel configurations have been developed to immobilise bacteria, including entrapment in a polymeric matrix or attachment to a solid support. Three main methods of immobilisation are showed in Figure 1. Entrapment and encapsulation of microorganism into the polymer network with restricted diffusion of substrate to cells and corresponding metabolism products, low density of cells relatively hydrated polymer volume ratio, high viability of cells is illustrated on Figure 1a [24][25].
Figure 1b shows biofilm generation on the porous surface of inorganic supports (silicates, perlite, vermiculite, Grow plant or glass aerogel) [26] or scaffold such as composite macroporous hydrogel s [27]. Microorganisms in a biofilm form are robust and usually show tolerance to toxic ions that might be present in the contaminated water due to cells incorporated in the biofilm matrix, which acts as a physical barrier. These advantages make biofilm attractive viable catalysts for organic syntheses, which may find wide technological applications [7]. In Figure 1c, macroporous hydrogel formation takes place via direct covalent linkage of bacteria cells’ membranes’ into 3D biofilm(advantages: one step, rapid biofilm formation due to cryoconcentration phenomenon leading to generation of high cells density with respect to polymer weight) [23][28][29]. Pseudomonas fluorescens(S3X), Microbacterium oxydans (EC29) and Cupriavidus spp. (1C2) were immobilised on the hydroxyapatite surface obtained from fish’s bones with a surface area of 15.5–24.4 m2/g within one hour at 30 °C at agitation. A concurrent effect of adsorbed bacteria and the material resulting in a synergistic effect of improved removal efficiencies of zinc and cadmium ions from treated water[26].
The different methods for immobilisation will affect the performance of the cells and may also impact the number of viable cells and hence the performance of the biocatalytic or bioremediation process [27]. But, the number of viable cells in these experiments was not quantitatively estimated via biochemical assays such as MTT assay, and therefore, it is difficult to obtain reproducible results. However, immobilisation strategies illustrate benefits compared to planktonic free cells, leading to significantly improved remediation rates [30][31][32]. The principal difference between cryogels and other porous materials(perlite(surfce area 1-2m2g-1), vermiculite(1 m2g-1 and grinded up to 39 m2g-1), silicate Grow plant or glass aerogel) of similar pore size is that the former exhibit tissue-like elasticity and flexibility and are able to withstand deformation without being destroyed or damaged. Preparation at low temperatures allows sufficient preservation of enzymes or cells (Figure 1c) [23][28][29].
Cryogels or also called macroporous hydrogels possessing a spongy 3D polymeric network structure are synthesised via a cryogelation or cryostructuration technique [30][31][32][33][34]; the concept of cryogel preparation based on cells is summarised in Figure 2 [29]. The available cryogelation techniques enable the production of elastic macroporous cryogels with a wide range of porosities and morphologies; for instance, Figure 3 exhibits the morphology of composite cryogels based on PVA, chitosan, and glutaraldehyde(GA) and PVA, CHI, hydroxyapatite, and GA [35]. The change of cryogelation mode gives the possibility to regulate material mechanical and physico-chemical properties (porosity, crystallinity, melting point, etc.) [5][29][31]. Cryogels can be prepared by applying several approaches, such as free-radical polymerization of a monomeric mixture or suspension with particles (metals, polymers, cells, bacteria) [25][36][37][38][39]. Other approaches include irradiation of the polymer solution or suspension with gamma-rays (electron beam, UV) [40] or modified polymers and self-assembly of supramolecular gelators [5][39][41]. The morphological characteristics of macroporous cryogels produced by cryogelation are controlled by parameters such as the rate of crystallisation of the solvent and the size of the solvent crystals, type of the solvent (water, DMSO, dioxan, N-methyl-2-pyrrolidinone (NMP), ethylene glycol) [29][31][42][43], and the phase-separation between the polymers and crystalizing solvent. For the process of hydrogel formation, covalent cross-linkers such as N,N-methylenbisacrylamide, PEG-diacrylate, glutaraldehyde(GA), oxidised dextran(Ox.Dex.), and tetramethoxypropane have been tried [28][44][45]. Noncovalent cross-linking methods include noble metal complexes, hydrophobic interactions, π-πstacking, polyelectrolyte complexes, and hydrogen bonding [33][39][44][45]. Furthermore, the temperature and the rate of freezing can impact the formation and growth of ice nuclei and hence pore size. If a solution is frozen in liquid nitrogen it brings about fast ice nuclei formation and the growth of small ice crystals, leading to scaffold with poor water permeability. If the freezing of solvent occurs at a relatively higher temperature depending on the freezing point of solvent (e.g.,−20 to 8 °C), ice nucleation is slower and the nuclei tend to grow into larger ice crystals, that results in the preparation of materials with large and randomly oriented pores after the freeze-drying process. The direction of freezing has a major effect on pore morphology; thus, by controlling the direction of freezing, the growth of ice crystal can be orientated in one direction due to a great temperature gradient across the sample with the ice crystals growing from the low to the higher temperature end [46]. Low temperatures (−10 to −25 °C) in water-based solvent systems enable the development of large solvent crystals and thus pore sizes [27]. Control of the pore size of the cryogel is achieved using a pre-freezing method, which effectively controls the freezing of large volumes of solvent and polymer solutions. The mixture is frozen to below the freezing point of the solvent with constant stirring prior to initiating gelation, leading to the freezing of up to 90% of the solvent, whilst the gel-forming compounds remain in a non-frozen liquid state. The agitation of the reaction mix improves the heat transfer and formation of ice nuclei. Cryogels formed this way have large pore volumes and near-uniform porosity with interconnected pores of diameter 20–200 µm, and exhibit twice the strength of cryogels produced by conventional methods (Young’s modulus = 12 kPa vs. 6 kPa) [43]. Covalent attachment of cells to the support is one of the most widely utilised methods that links the micro-organism with bonding reaction of reactive functionality (amino (–NH2); hydroxyl(–OH)thiol(–SH) or carboxygroups(–COOH)) at the cell’s surface. This leads to the stability of the microorganism, but the bioactivity of bacteria might be slowed down. Usually, inactive carbonyl groups presented in Alg, gelatin, and carboxymethylcellulose requires prior activation with carbodiimide for the purpose of immobilisation. Hydroxyl groups on the surface of metal oxides can be functionalised using various silane derivatives (aminopropyl -triethoxysilane, 3-(Trimethoxysilyl)propyl methacrylate, etc.) or cyanobromide(BrCN) [47] Polymers containing amino(–NH2, –NH–), hydroxyl (>C–OH), and thiol(>C–SH)can be functionalised with an active epoxy group via treatment with epichlorohydrine or di- and triepoxyderivatives [32].
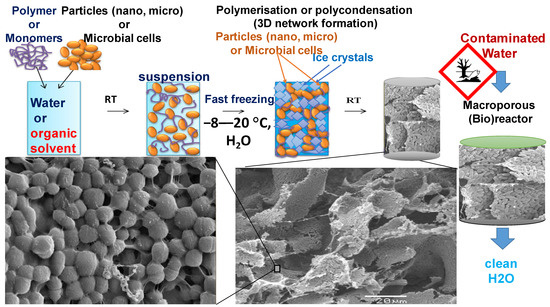
Figure 2. Scheme of preparation of cryogels from bacterial cell suspension and cross-linking polymer(left to right), mixing of components, freezing of the bacteria suspension and thawing step; illustration of the morphology of macroporous structure(SEM images at low and high magnification). The left part illustrates the principle of the water bioremediation process by macroporous bioreactor in flow-through mode.
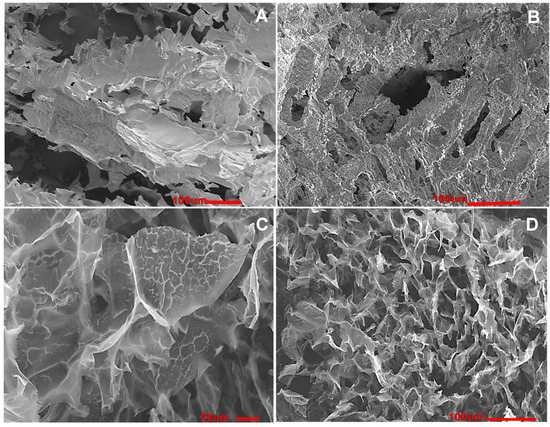
Figure 3. SEM images of composite cryogels: (A,B) composites PVA-CHI-hydroxyapatite-heparin-GA; scale bar 100; (C) polyelectrolyte complex PVA-CHI-heparin-GA scale bar 20 [35] and (D) CHI-GA; scale bar 100 [44].
The physical entrapment of bacteria in PVA cryogels was one of the first applications in microbiology [34][48] (Figure 1a), where the viability of bacteria within the cryogel and effect of temperature stress were estimated via bioluminescent activity of immobilised photobacteria [49]. A production of lactic, fumaric and succinic acids by various microbial cells (filamentous fungi Rhizopusoryzae (F-814, F-1127) and bacteria Actinobacillus succinogenes B-10111) immobilised into PVA cryogels revealed higher yields than non-immobilised cells [50]. The macroporous cryogels revealed enhanced efficacy with various cell types compared to polymeric matrices available at the time such as pAAm, hydroxymethylmethacrylate (HEMA), humic acid, polysacharides gum Arabic, and carrageenan, Alg particles cross-linked by triglycidylpropane derivatives [51][52][53][54]. Currently used supports can be classified as organic carriers, inorganic carriers, and composite one in terms of their chemical composition. Organic carriers are subdivided into natural polymeric carriers and synthetic polymers [51]. Hydrophobicity, charge of the surface, roughness, morphology and texture of carriers, water permiability, and temperature of the process are factors that affect microbial cell adsorption and therefore have to be taken into account for efficient cell immobilisation. The review contains methods of immobilisation of Chlorella spp. into Alg, carrageenan agarose, Alg and agar beads, polyurethane foam, and AAm gels for the removal of harmful heavy metals (Ni, Zn, Cd, Cu, Hg, Pb, and uranium). Moreover, those bioremediation systems illustrated 4–5 times more efficiency toward phosphate, nitrate, and ammonia removal in comparison with free planktonic cells [51]. There are quite a few publications related to pilot-scale studies. A recent report ascribes a novel design of immobilization of Pseudomonas citronellolis on a composite of PVA bamboo-biochar beads (cell-biochar beads), which is environmentally friendly scaffold that was utilised for biotrickling filters (BTFs) fabrication. On one side, cell-biochar beads provide adsorption of pollutants. On the other side, it works as a carrier for the attachment and proliferation of bacteria. Therefore, BTFs illustrated enhanced efficiency of pollutant removal (toluene removal was over 99%). The organic loading rate and elimination capacity of BTF for toluene were 46 g/m3∙h and 35 g/m3∙h at a gas flow rate of 0.015 m3/min, respectively. Cell-immobilised biochar beads were made by mixing 50 g of 9% PVA solution, 10 mL of bacterial strain, and 65 g of bamboo-bio-char powder (grain size 0.15 mm) [52]. The efficiency of cryogels produced via direct radical polymerisation of (hydroxyethylmethacrylate)HEMA with freeze-dried bacteria Trichoderma sp. for the removal of cyanide at concentration up to 20ppm at a wide pH range of 4–8 and temperature range of 20–40 °C [54]. Berillo et al. [29] tried to reproduce the experiment with direct polymerisation of different bacteria strains. Metabolic activity Cell Viability MTT assays showed that cell viability was less than 5%, most probably, due to damage to the cell membrane by active radicals. Stepanov et al. [55] demonstrated the successful entrapment of Komagataei bacterxylinum B-12429 into a PVA cryogel. The bacteria produced cellulose, which passed through the pores of cryogel matrix and accumulated on the surface of the medium. The synthesis of bacterial cellulose was 1.3–1.8-fold higher than the planktonic bioreactor. Repeated utilization of the immobilised bacteria preserved 100% of their metabolic activity for 10 working cycles (60 days). Some researchers state that Bacillus pseudomycoides immobilized PVA)/GA hydrogel at mass ratio of 0.03 and acidic pH of 2 maintaining cell accessibility to external environment for bioremediation of wastewater, [56] however it won’t work for most of low pH non-resistant bacteria strains. Recent evaluation of patent applications revealed most efficient series of bioreactor design (magnetic fluidised bed reactors, fluidised bed bioreactor employing biofilm carriers, reverse fluidised loop reactor, fluidised bed reactor, packed-bed reactor, biological permeable barrier for removal groundwater contaminations, and layered packed bed bioreactor) [47].
Although cryogels have been in development since the mid-1980s, it is only within the last few years that the potential of macroporous cryogels for cell immobilisation in cell culture applications and in bioremediation approaches has been realised [19][34][35][38][56][57][58][59][60].
The inherent characteristics of macroporous cryogels, such as their high flow permeability, low flow resistance, and minimal toxicity [60], make them suitable for applications in a range of processes including aerobic and anaerobic bioreactors [28][61][62][63][64] water treatment and biomedicine.
References
- Dong, Z.; Dong, C.; Liu, Y.; Le, X.; Jin, Z.; Ma, J. Hydro dechlorination and further hydrogenation of 4-chlorophenol to cyclohexanone in water over Pd nanoparticles modified N-doped mesoporous carbon microspheres. Chem. Eng. J. 2015, 270, 215–222.
- Descorme, C. Catalytic wastewater treatment: Oxidation and reduction processes. Recent studies on chlorophenols. Catal. Today 2017, 15, 324–334.
- Oturan, M.A.; Aaron, J.-J. Advanced oxidation processes in water/wastewater treatment: Principles and applications. A review. Crit. Rev. Environ. Sci. Technol. 2014, 44, 2577–2641.
- Villegas, L.G.C.; Mashhadi, N.; Chen, M.; Mukherjee, D.; Taylor, K.E.; Biswas, N. A short review of techniques for phenol removal from wastewater. Curr. Pollut. Rep. 2016, 2, 157–167.
- Bertrand, J.C.; Doumenq, P.; Guyoneaud, R.; Marrot, B.; Martin-Laurent, F.; Matheron, R.; Moulin, P.; Soulas, G. Applied microbial ecology and bioremediation. In Environmental Microbiology: Fundamentals and Applications; Springer: Dordrecht, The Netherlands, 2015; pp. 659–753.
- Dhillon, G.S.; Kaur, S.; Pulicharla, R.; Brar, S.K.; Cledón, M.; Verma, M.; Surampalli, R.Y. Triclosan: Current status, occurrence, environmental risks and bioaccumulation potential. Int. J. Environ. Res. Public Health 2015, 12, 5657–5684.
- Dedov, A.G.; Ivanova, E.A.; Sandzhieva, D.A.; Lobakova, E.S.; Kashcheeva, P.B.; Kirpichnikov, M.P.; Buznik, V.M. New materials and ecology: Biocomposites for aquatic remediation. Theor. Found. Chem. Eng. 2017, 51, 617–630.
- Willaert, R.; Baron, G. Gel entrapment and microencapsulation: Methods, applications and engineering principles. Rev. Chem. Eng. 1996, 12, 1–205.
- Martins, S.C.S.; Martins, C.M.; Fiuza, L.M.C.G.; Santaella, S.T. Immobilization of microbial cells: A promising tool for treatment of toxic pollutants in industrial waste water. Afr. J. Biotechnol. 2013, 12, 4412–4418.
- Anku, W.W.; Mamo, M.A.; Govender, P.P. Phenolic compounds in water: Sources, reactivity, toxicity and treatment methods. Phenolic Compd. Nat. Sources Importance Appl. 2017, 420–443.
- Yazdi, M.K.; Vatanpour, V.; Taghizadeh, A.; Taghizadeh, M.; Ganjali, M.R.; Munir, M.T.; Habibzadeh, S.; Saeb, M.R.; Ghaedi, M. Hydrogel membranes: A review. Mater. Sci. Eng. C 2020, 114, 111023.
- Lim, J.W.; Zaid, H.F.M.; Isa, M.H.; Oh, W.D.; Adnan, R.; Bashir, M.J.; Wang, D.K. Shielding immobilized biomass cryogel beads with powdered activated carbon for the simultaneous adsorption and biodegradation of 4-chlorophenol. J. Clean. Prod. 2018, 205, 828–835.
- Lee, S.H.; Lee, S.; Lee, K.; Nahm, C.H.; Jo, S.J.; Lee, J.; Park, P.K. Enhancing the Physical Properties and Lifespan of Bacterial Quorum Quenching Media through Combination of Ionic Cross-Linking and Dehydration. J. Microbiol. Biotechnol. 2017, 27, 552–560.
- Memon, H.; Lanjewar, K.; Dafale, N.; Kapley, A. Immobilization of Microbial Consortia on Natural Matrix for Bioremediation of Wastewaters. Int. J. Environ. Res. 2020, 14, 403–413.
- Shukla, S.K.; Mangwani, N.; Rao, T.S. Bioremediation Approaches for Persistent Organic Pollutants Using Microbial Biofilms. In Microbial Biofilms in Bioremediation and Wastewater Treatment; CRC Press: Boca Raton, FL, USA, 2019; pp. 179–206.
- Hailei, W.; Ping, L.; Yu, Q.; Hui, Y. Removal of phenol in phenolic resin wastewater by a novel biomaterial: The Phanerochaete chrysosporium pellet containing chlamydospore-like cells. Appl. Microbiol. Biotechnol. 2016, 100, 5153–5164.
- Partovinia, A.; Behnam, R. Review of the immobilized microbial cell systems for bioremediation of petroleum hydrocarbons polluted environments. Crit. Rev. Environ. Sci. Technol. 2018, 48, 1–38.
- Cortez, S.; Nicolau, A.; Flickinger, M.C.; Mota, M. Biocoatings: A new challenge for environmental biotechnology. Biochem. Eng. J. 2017, 121, 25–37.
- Martynenko, N.N.; Gracheva, I.M.; Sarishvili, N.G.; Zubov, A.L.; El’Registan, G.I.; Lozinsky, V.I. Immobilization of champagne yeasts by inclusion into cryogels of polyvinyl alcohol: Means of preventing cell release from the carrier matrix. Appl. Biochem. Microbiol. 2004, 40, 158–164.
- Efremenko, E.N.; Tatarinova, N.Y. The effect of long-term preservation of bacterial cells immobilized in poly(vinyl alcohol) cryogel on their viability and biosynthesis of target metabolites. Microbiology 2007, 76, 336–341.
- Yordanova, G.; Ivanova, D.; Godjevargova, T.; Krastanov, A. Biodegradation of phenol by immobilized Aspergillus awamori NRRL 3112 on modified polyacrylonitrile membrane. Biodegradation 2009, 20, 717–726.
- Von der Ehe, C.; Buś, T.; Weber, C.; Stumpf, S.; Bellstedt, P.; Hartlieb, M.; Schubert, U.S.; Gottschaldt, M. Glycopolymer-functionalized cryogels as catch and release devices for the pre-enrichment of pathogens. Acs Macro Lett. 2016, 5, 326–331.
- Al-Jwaid, A.K.; Berillo, D.; Savina, I.N.; Cundy, A.B.; Caplin, J.L. One-step formation of three-dimensional macroporous bacterial sponges as a novel approach for the preparation of bioreactors for bioremediation and green treatment of water. RSC Adv. 2018, 8, 30813–30824.
- Philip, J.C.; Balmand, S.; Hajto, E.; Bailey, M.J.; Wiles, S.; Whiteley, A.S.; Lilley, A.K.; Hajto, J.; Dunbar, S.A. Whole cell immobilised biosensors for toxicity assessment of a wastewater treatment plant treating phenolics-containing waste. Anal. Chim. Acta 2003, 487, 61–74.
- Zhang, W.; Yang, Y.; Guan, T.; Guan, J.; Zheng, S.; Chen, B.; Yun, J. Formation dynamics of cell-loading alginate droplets in the micro-tube dripping and cryo-crosslinking process for cell-entrapped cryogel beads as the biocatalysts towards phenyl lactic acid biosynthesis. Ind. Eng. Chem. Res. 2018, 57, 7291–7300.
- Piccirillo, C.; Pereira, S.I.A.; Marques, A.P.; Pullar, R.C.; Tobaldi, D.M.; Pintado, M.E.; Castro, P.M. Bacteria immobilisation on hydroxyapatite surface for heavy metals removal. J. Environ. Manag. 2013, 121, 87–95.
- Plieva, F.M.; Galaev, I.Y.; Mattiasson, B. Macroporous gels prepared at subzero temperatures as novel materials for chromatography of particulate-containing fluids and cell culture applications. J. Sep. Sci. 2007, 30, 1657–1671.
- Zaushitsyna, O.; Berillo, D.; Kirsebom, H.; Mattiasson, B. Cryostructured and crosslinked viable cells forming monoliths suitable for bioreactor applications. Top. Catal. 2014, 57, 339–348.
- Berillo, D.A.; Caplin, J.L.; Cundy, A.B.; Savina, I.N. A cryogel-based bioreactor for water treatment applications. Water Res. 2019, 153, 324–334.
- Lozinsky, V.I.; Galaev, I.Y.; Plieva, F.M.; Savina, I.N.; Jungvid, H.; Mattiasson, B. Polymeric cryogels as promising materials of biotechnological interest. Trends Biotechnol. 2003, 21, 445–451.
- Lozinsky, V.; Kolosova, O.; Michurov, D.; Dubovik, A.; Vasil’ev, V.; Grinberg, V. Cryostructuring of Polymeric Systems. 49. Unexpected “Kosmotropic-Like” Impact of Organic Chaotropes on Freeze–Thaw-Induced Gelation of PVA in DMSO. Gels 2018, 4, 81.
- Baimenov, A.; Berillo, D.A.; Poulopoulos, S.G.; Inglezakis, V.J. A review of cryogels synthesis, characterization and applications on the removal of heavy metals from aqueous solutions. Adv. Colloid Interface Sci. 2020, 276, 102088.
- Gun’ko, V.M.; Savina, I.N.; Mikhalovsky, S.V. Cryogels: Morphological, structural and adsorption characterisation. Adv. Colloid Interface Sci. 2013, 187–188, 1–46.
- Lozinsky, V.I.; Plieva, F.M. Poly(vinyl alcohol) cryogels employed as matrices for cell immobilization. 3. Overview of recent research and developments. Enzym. Microb. Technol. 1998, 23, 227–242.
- Sultankulov, B.; Berillo, D.; Kauanova, S.; Mikhalovsky, S.; Mikhalovska, L.; Saparov, A. Composite Cryogel with Polyelectrolyte Complexes for Growth Factor Delivery. Pharmaceutics 2019, 11, 650.
- Galaev, I.; Dainiak, M.B.; Plieva, F.; Mattiasson, B. Effect of matrix elasticity on affinity binding and release of bioparticles. Elution of bound cells by temperature induced shrinkage of the smart macroporous hydrogel. Langmuir 2007, 23, 35–40.
- Saylan, Y.; Tamahkar, E.; Denizli, A. Recognition of lysozyme using surface imprinted bacterial cellulose nanofibers. J. Biomater. Sci. Polym. Ed. 2017, 28, 1950–1965.
- Dainiak, M.B.; Plieva, F.M.; Galaev, I.Y.; Hatti-Kaul, R.; Mattiasson, B. Cell chromatography: Separation of different microbial cells using IMAC supermacroporous monolithic columns. Biotechnol. Prog. 2005, 21, 644–649.
- Berillo, D.; Mattiasson, B.; Galaev, I.Y.; Kirsebom, H. Formation of macroporous self-assembled hydrogels through cryogelation of Fmoc–Phe–Phe. J. Colloid Interface Sci. 2012, 368, 226–230.
- Reichelt, S.; Abe, C.; Hainich, S.; Knolle, W.; Decker, U.; Prager, A.; Konieczny, R. Electron-beam derived polymeric cryogels. Soft Matter 2013, 9, 2484–2492.
- Topuz, F.; Uyar, T. Poly-cyclodextrincryogels with aligned porous structure for removal of polycyclic aromatic hydrocarbons (PAHs) from water. J. Hazard. Mater. 2017, 335, 108–116.
- Farías, T.; Hajizadeh, S.; Ye, L. Cryogels with high cisplatin adsorption capacity: Towards removal of cytotoxic drugs from wastewater. Sep. Purif. Technol. 2020, 235, 116203.
- Savina, I.N.; Ingavle, G.C.; Cundy, A.B.; Mikhalovsky, S.V. A simple method for the production of large volume 3D macroporous hydrogels for advanced biotechnological, medical and environmental applications. Sci. Rep. 2016, 6, 21154.
- Berillo, D. Gold nanoparticles incorporated into cryogel walls for decomposition of 4-nitrophenol. J. Clean Prod. 2019, 247, 119089.
- Berillo, D.; Volkova, N. Preparation and physicochemical characteristics of cryogel based on gelatin and oxidised dextran. J. Mater. Sci. 2014, 49, 4855–4868.
- Suner, S.S.; Sahiner, N. Humic acid particle embedded super porous gum Arabic cryogel network for versatile use. Polym. Adv. Technol. 2018, 29, 151–159.
- Zhou, L.; Li, G.; An, T.; Fu, J.; Guoying, S. Recent patents on immobilized microorganism technology and its engineering application in wastewater treatment. Recent Patent Eng. 2008, 2, 28–35.
- Choi, M.; Cho, K.; Lee, S.; Chung, Y.C.; Park, J.; Bae, H. Effective seeding strategy using flat type poly (vinyl alcohol) cryogel for anammox enrichment. Chemosphere 2018, 205, 88–97.
- Aleskerova, L.E.; Alenina, K.A.; Efremenko, E.N.; Ismailov, A.D. The factor stabilizing the bioluminescence of PVA-immobilized photobacteria. Microbiology 2017, 86, 218–224.
- Maslova, O.; Stepanov, N.; Senko, O.; Efremenko, E. Production of various organic acids from different renewable sources by immobilized cells in the regimes of separate hydrolysis and fermentation (SHF) and simultaneous saccharification and fermentation (SFF). Bioresour. Technol. 2019, 272, 1–9.
- Bouabidi, Z.B.; El-Naas, M.H.; Zhang, Z. Immobilization of microbial cells for the biotreatment of wastewater: A review. Environ. Chem. Lett. 2019, 17, 241–257.
- Liu, S.H.; Lin, H.H.; Lai, C.Y.; Lin, C.W.; Chang, S.H.; Yau, J.T. Microbial community in a pilot-scale biotrickling filter with cell-immobilized biochar beads and its performance in treating toluene-contaminated waste gases. Int. Biodeterior. Biodegrad. 2019, 144, 104743.
- du Toit, J.P.; Pott, R.W. Transparent polyvinyl-alcohol cryogel as immobilisation matrix for continuous biohydrogen production by phototrophic bacteria. Biotechnology for Biofuels. Biotechnol. Biofuels 2020, 13, 1–16.
- Aslıyüce, S.; Denizli, A. Design of PHEMA Cryogel as Bioreactor Matrices for Biological Cyanide Degradation from Wastewater. Hacet. J. Biol. Chem. 2017, 45, 639–645.
- Stepanov, N.; Efremenko, E. “Deceived” Concentrated Immobilized Cells as Biocatalyst for Intensive Bacterial Cellulose Production from Various Sources. Catalysts 2018, 8, 33.
- Mehrotra, T.; Zaman, M.N.; Prasad, B.B.; Shukla, A.; Aggarwal, S.; Singh, R. Rapid immobilization of viable Bacillus pseudomycoides in polyvinyl alcohol/glutaraldehyde hydrogel for biological treatment of municipal wastewater. Environ. Sci. Pollut. Res. 2020, 27, 9167–9180.
- Milakin, K.A.; Capáková, Z.; Acharya, U.; Vajďák, J.; Morávková, Z.; Hodan, J.; Humpolíček, P.; Bober, P. Biocompatible and antibacterial gelatin-based polypyrrole cryogels. Polymer 2020, 197, 122491.
- Wasi, S.; Tabrez, S.; Ahmad, M. Use of Pseudomonas sp. for the bioremediation of environmental pollutants: A review. Environ. Monit. Assess. 2013, 185, 8147–8155.
- Dainiak, M.B.; Kumar, A.; Galaev, I.Y.; Mattiasson, B. Methods in cell separations. In Cell Separation; Mattiasson, B., Ed.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 1–18.
- Bansal, V.; Roychoudhury, P.K.; Mattiasson, B.; Kumar, A. Recovery of urokinase from integrated mammalian cell culture cryogel bioreactor and purification of the enzyme using p-aminobenzamidine affinity chromatography. J. Mol. Recognit. 2006, 19, 332–339.
- Jain, E.; Kumar, A. Disposable polymeric cryogel bioreactor matrix for therapeutic protein production. Nat. Protoc. 2013, 8, 821–835.
- Börner, R.A.; Zaushitsyna, O.; Berillo, D.; Scaccia, N.; Mattiasson, B.; Kirebom, H. Immobilization of Clostridium acetobutylicum DSM 792 as macroporous aggregates through cryogelation for butanol production. Process Biochem. 2014, 49, 10–18.
- Zaushitsyna, O.; Dishisha, T.; Hatti-Kaul, R.; Mattiasson, B. Crosslinked, cryostructured Lactobacillus reuteri monoliths for production of 3-hydroxypropionaldehyde, 3-hydroxypropionic acid and 1,3-propanediol from glycerol. J. Biotechnol. 2017, 241, 22–32.
- Sam, S.P.; Adnan, R.; Ng, S.L. Statistical optimization of immobilization of activated sludge in PVA/alginate cryogel beads using response surface methodology for p-nitrophenol biodegradation. J. Water Process Eng. 2021, 39, 101725.




