
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Deepti Shrivastava | + 3438 word(s) | 3438 | 2021-04-22 04:19:23 | | | |
| 2 | Catherine Yang | -13 word(s) | 3425 | 2021-04-23 02:53:27 | | | | |
| 3 | Catherine Yang | -13 word(s) | 3425 | 2021-04-23 02:54:32 | | |
Video Upload Options
The Gingival Crevicular Fluid (GCF) consists of various host and bacterial-derived products as well as biomarkers which in turn can be evaluated for the diagnosis, prognosis as well as management of the periodontal disease.
1. Introduction
Periodontal diseases have affected almost all the fields of dentistry in one way or the other. Foremost, every individual is affected differently by the periodontal disease. Besides, due to the inability to access rear‐end segments of the oral cavity, clinical signs are sometimes inappreciable and the periodontal structures in the maxillary tuberosity region are difficult to visualize and properly examine. Additionally, clinical researches conducted in early times (approximately in the 1980s) showed that chronic periodontitis followed a slow and steady pattern of progression but later it became clear that clinical parameters are poor indicators of inactive states of disease pattern. However, Goodson in 1982 explained that it’s a natural tendency of chronic periodontitis showing regular pattern of occurrence which is interrupted by a phase of remission and acute exacerbation [1]. Day to day errors in the process of diagnosis and failure of treatment with the persistence of disease convinced investigators to devise a standard method for detection of disease. Presence and mapping the authentic treatment plan where the use of antibiotic is strictly administered in accordance to treat the symptoms.
In the light of multiple investigations performed, aiming at the detection of periodontal disease with absolute certainty.The secondary colonizing bacteria’s in the biofilm and the host‐immune response plays a significant role in the commencement and its subsequent progression of periodontal disease. Although, the cascade of damage begins with alteration in normal microflora of the oral cavity, especially polymicrobial colonization in the form of biofilms on the tooth surface and derangements in subgingival flora. Over the time, the condition worsens due to power‐play of consistent poor oral hygiene or any uncontrolled co‐morbid condition and it presents as bad breath, bleeding from gums, pain, sensitivity and on occasion mobility of teeth.Physical assessment of the aforementioned symptoms and signs are the primary diagnostic criteria. However, clinical assessment alone cannot judge the status of the disease activity of the site.
Progressive destruction of collagen fibers that were initially limited to epithelium tissue advances to deeper supportive tissues of periodontium, including bone tissue that prevail state of periodontitis. Epidemiological studies have validated that the morbidity of chronic periodontitis is 35%–50% in the adult population, with approximately 10% displaying severe disease concomitant with early tooth loss [2]. Whereas, this disease has increased the global burden by 57.3% from 1990 to 2010 [3]. It is an established fact that periodontitis has a polymicrobial etiology. It is not only the oral dysbiosis results in tissue destruction but also the host inflammatory dysregulation plays an important role in tissue destruction of periodontal supporting tissues [4]. The interesting fact is that components of host defense and pro‐inflammatory mediator elements are raised during any periodontitis infection and infused locally as well as in gingival crevicular fluid (GCF) and saliva. Hence, oral liquids play a vital role in developing an accurate picture of periodontal health for obtaining data that mimics periodontium [5]. Zhou et al. conducted a study to find the microbiome and cytokine composition in GCF in healthy and periodontitis patients and they concluded that the oral dysbiosis and immune response plays a pivotal role in the etiology of periodontitis [6]. Similarly, Pei et al. collected the GCF from healthy and periodontitis patients and concluded that oral dysbiosis and changes in the metabolic product detected within GCF can be a good predictor of diagnosis, prognosis and management of periodontal disease [7]. Like other oral fluids, GCF is a reliable tool but unlike others, it is a very delicate specimen that maintains the structural integrity of the junctional epithelium (JE), hence acting as a barrier [8]. It is a serum transudate or inflammatory exudate fluid, present in the sulcus/periodontal pocket between the tooth and marginal gingiva. Talking about oral fluids, GCF is amongst the most localized fluid that delineates the very peculiar characteristics of the site, thus making future diagnostic treatment and management of a wide variety of maladies way easier [9]. Thus, considering the pivotal role of GCF being in the close proximity with the gingival tissues, this review paper aims to highlight the various aspects of GCF and its diagnostic potential for the discrimination of periodontal health and disease condition.
2. Gingival Crevicular Fluid
2.1. Formation of GCF
Early research in the field aimed to establish the nature of GCF; whether it is a transudate or exudate and its detection in the healthy gingiva. Researchers by the late 1950s and early 1960s had postulated that GCF is a result of stimulation by chemical or mechanical means which in turn results alters the permeability of the vessels beneath the junctional and sulcular epithelium [1]. Alfano and Pashley have reported that the initial fluid produced is interstitial and is produced in the crevice because of the osmotic gradient. Thus, the earlier was contemplated to be a transudate and later upon stimulation, it becomes inflammatory exudates [2].
2.2. Constituents of GCF Aliquot
The GCF is derived from serum and locally generated components such as tissue breakdown products and derivatives of subgingival biofilm. The various elements found in GCF includes inflammatory mediators, cytokines, leucocytes, enzymes, organic ions, tissue breakdown products and proteins. These components give an insight about the healing potential of periodontium. Conversely, it also reflects about the adaptive mechanism of certain bacterial survival within the gingival crevice and pocket [3]. The various biological disease markers such as interleukins (IL-Iα-IL-1β) Tumor necrosis factor alpha TNF α, enzymes such as acid phosphatase, alkaline phosphatase, matrix metalloproteinases, collagenases, elastase are widely used in periodontal research to assess the resolution of periodontal disease [4]. Along with this it also constitutes organic and inorganic ions (Figure 1).
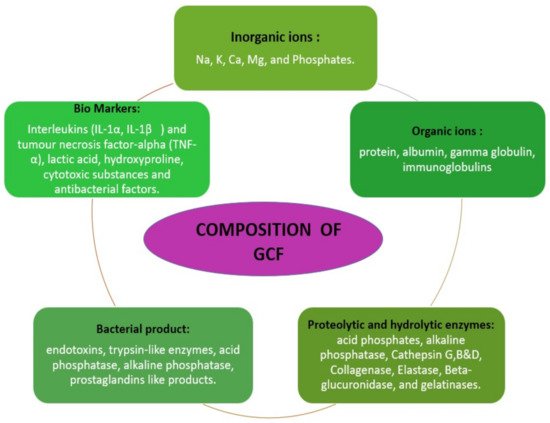
Figure 1. Description of gingival crevicular fluid composition [2].
2.3. Mechanism of Production
Under normal conditions, the sulcus contains a minimal amount of GCF. During inflammation of tissue structure investing the tooth, this fluid in the V-shaped sulcus travels from the microcapillary framework within the inflamed connective tissues consisting of biological molecular markers. Hence, GCF is considered an important tool for assessing the health and diseased condition. In a healthy state, the sulcus of each tooth is flushed by GCF to get rid of the pathogens and toxic metabolites from periodontium and cushions the tooth against any foreign insult. However, during inflammation, it changes its properties from serum transudate to an inflammatory exudate where biological biomarkers including host defense molecules and anti-inflammatory mediators are present in enormous quantity hence the nature of the fluid is converted into exudates [2] (Figure 2).
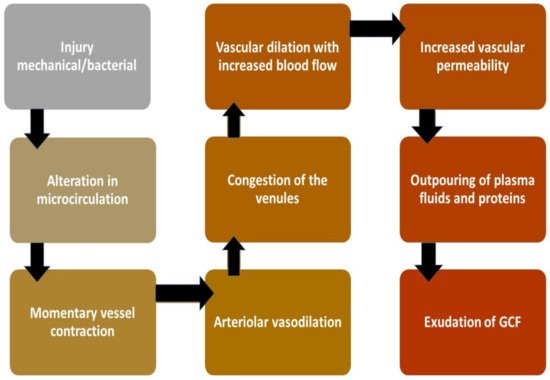
Figure 2. Mechanism of GCF production on stimulus [1][2][5].
2.4. Circadian Rhythm of GCF
Circadian periodicity of GCF is upsurged around early morning (6 a.m.) till night (10 p.m.) and down surged during the rest hours [6]. Other conditions like any changes in hormonal level, mechanical stimulations, smoking and periodontal therapy but particularly in case of a breach in the form of periodontal infection, activates host immune system to release polymorphonuclear leukocytes (PMNLs) and lymphocytes in GCF [6]. Other enzymes like proteinases are present already in the environment in its inactive proenzyme form and convert into its active form upon the stimulus of antigen by lymphocytes [7].
2.5. Collection of GCF
Collection of GCF is an easy but sensitive method. GCF collection can be achieved by employing any of the several proposed techniques [8] which can be chosen according to the aim or objective of the study. In the gingival washing method, the gingival crevice is flushed with an isotonic solution of fixed volume and then the diluted crevicular fluid is collected that contains both cells and soluble proteins [9]. This is a highly useful technique when the intent is to harvest cells from the gingival crevice region [10].
However, the properties of GCF such as composition or volume from specific sites cannot be assessed correctly as the dilution factor cannot be determined precisely [8]. Nonetheless, the GCF volume can be accurately determined by capillary tubing or micropipettes which are introduced within gingival sulcus after adequate isolation and drying. Micropipettes allow precise collection of GCF from a given site in a fixed volume. This technique yields undiluted native GCF, but the collection of adequate samples may take >30 min which may pose risk of trauma to the tissues. Another drawback is the difficulty in removing the complete sample from the tubing [11]. The conclusion in accordance with a published work suggests that standard operating procedure of GCF specimen involves the use of perio-paper as best transporting source through intra-gingival absorption where the strip is kept sub-gingivally for 30 s. The components of the GCF can be analyzed by ELISA which is the most preferred method of analysis [12]. Environment before collection must be sterile and well isolated, and the perio-paper must be held at place for 30 s to avoid contamination with blood or saliva. This approach offers numerous benefits including ease of use, allowing collection from individual sites and a quick technique that avoids tissue trauma.
2.6. Conventional Diagnostic Measure Versus GCF
In the gingival sulcus, one surface is tooth while the other is the wall of the pocket, therefore during the event of phase change from transudate to exudate form, the first seen pathology is inflammation of gingival connective tissue, that is clinically signified as gingivitis [13]. Later, when the extension of deterioration involves bone, it is called periodontitis. Periodontitis being one of the most prevalent diseases known to humankind and its severe forms mark 10% of adults. Besides, it’s critical types were recently classified as the sixth most widespread ailment across the globe [14]. Diagnostic potential of the sulcular fluid was known more than six decades ago and since the 1950s this domain is under the discussion of research experts but has failed to achieve a unanimous acceptance to implement it as a clinically significant tool till to date.
Currently, research strategies are emphasizing more on advance and sensitive methods to point active phases and sites of periodontal diseases to differentiate the treatment modality and need as per the requirement [15]. The routine practice uses subjective trends that are based on three aspects. Firstly, the clinical signs and symptoms such as bleeding on probing, change in the gingival color, contour, texture, size plays a crucial role in the establishment of the gingival inflammation, Secondly, determination of periodontal probing depth (PPD) with the help of a periodontal probe. The most commonly used probe is William′s periodontal probe. The other probes used to detect PPD are Community Periodontal Index of Treatment Needs–Clinical probe (CPITN (C)) and Community Periodontal Index of Treatment Needs–Epidemiological probe (CPITN(E)) [16]. Whereas, the later CPITN (E) is commonly used in epidemiological screening. Lastly, the radiological evaluation of alveolar bone loss is essential to ascertain the grade of periodontitis.
Additionally, the present diagnostic method is evaluated based on clinical perspectives such as plaque score (PS), gingival index (GI), clinical attachment loss (CAL), bleeding on probing (BOP), periodontal probing depth (PPD) and radio-graphical analysis. These parameters can only render information about the former destruction of periodontium instead of illustrating the future state of the tissues [3][17]. Thus, it is mandatory to explore a new method of periodontal diagnosis that can provide information about future disease outcomes. The abovementioned subjective criteria is not proficient enough to diagnose the periodontal diseases with reproducibility. Therefore, core objective criteria are colossally essential at present to enable a practitioner to pre-emptively diagnose the condition/stage and intervene the pathophysiology for treatment and management.
GCF nevertheless, is a competent instrument that can influence the advancement of proteomics to the next level. The pathophysiology of periodontal diseases can be gauged by analysis of the protein composition of GCF as various cells and inflammatory biomarkers such as enzymes, cytokines, and local tissue degradation products are discharged in the GCF during the inflammatory state. Extensive use and study of GCF in different perspectives can bridge the gap in understanding many bi-directional diseases (oral-systemic and systemic-oral) and a wide range of oral conditions. In this article, the novelty of GCF is suggested in multiple maladies. Firstly, a wide variety of evidence-based studies have reported that there is no other ideal specimen that is naturally collectable, inexpensive, and biocompatible and with no post complication, which outstands GCF at both practitioner and patient level. It helps reduce the patient’s pre-test anxiety and fear along with a decrease in chair site time. Evidence showed that GCF specimen were found positive for periodontal disease [8][18], where authentic biological mediators which correspond with the pathophysiology of periodontitis in the harmonious pattern were also ascertained. Hence, it will work as a prism to identify any disease with its constituent to cater for easy management of the condition.
2.7. GCF as a Biomarker
GCF has found to encompass an extensive range of bio-indicators [19], this property makes it more lucrative for professional use in the crevicular fluid and has shown encouraging co-relation between proteases, collagenase levels and pocket depth derangements among periodontal disease individuals which confirms that clinical parameters can now confidently be explained through this unique fluid [20]. Likewise, a claimable level of protein carbonyl is observed in this fluid that is linked with the initial stage and recovered stages of periodontitis patients [21]. Literature based on an extensive collection of studies through a meta-analysis has expressed realistic long-term benefits of this approach. However, it recommends the use of more than one marker as a standard. Multiple studies have suggested MMP-8 and IL-1β, various others have found that MMP-8 is the most reliable indicator present persistently in periodontitis [22][23]. Another study identified a positive correlation of increased Interleukin-1β (IL-1β) and Interleukin-6 (IL-6) in GCF with severe BOP and increased pocket depth [24].
Several other proteolytic and hydrolytic enzyme biomarkers also provide useful information to predict, diagnose, and monitor periodontal diseases. Alkaline phosphatase (ALP) is a very important indicator of bone formation and its presence in the GCF indicates inflammation and destruction of periodontal tissues. The severity of the periodontal disease is positively correlated to the level of ALP [25]. Lactate dehydrogenase (LDH) activity is elevated with increasing probing depth [26]. Aspartate aminotransferase (AST) is a non-specific marker for cell death and necrosis and its activity is associated with the severity of periodontitis [27]. Finally, cathepsin-B aids in distinguishing periodontitis from gingivitis by serving as a predictor of attachment loss [28].
GCF also contain various bone-related biomarkers to reflect the disease status of periodontal tissues [29]. Osteocalcin (OC) is the most specific biomarker of osteoblast function [30]. An increase in OC level in GCF is associated with high rates of bone turnover and seen during increased periodontal disease activity [31]. Calprotectin alters the immune response by inhibiting the immunoglobulin production and also plays a role in neutrophil recruitment and activation. Higher levels of calprotectin levels are reported in periodontitis patients [31]. Osteopontin (OPN) is mainly produced by osteoblast and macrophages and its raised levels are found associated with periodontal disease. Similarly, osteonectin is an important biomarker associated with the periodontal disease status and its increasing levels correlates with the increase in pocket depth [31][32].
Cell death and tissue breakdown products serve as reliable markers for tissue destruction. Different glycosaminoglycans are found depending on the tissue, chondroitin-4-sulfate is the most promising marker as it reflects bone degradation [4]. Elevated glycosaminoglycan concentration in GCF depicts the active destruction of periodontal tissue [33]. Fibrinogen plays a pivotal role in a variety of cellular activities and is considered an active player in inflammation [34]. It is a marker for periodontal disease status as specific fibrinogen fragments are involved in the pathogenesis of periodontitis [31].
Several other components of GCF are also shown to reflect the periodontal disease status that includes neutrophil elastase [35], periostin [36], Chitinase-3-like protein 1 (also known as YKL-40) [37], lysophosphatidic acid (LPA) [38], human beta-defensins (hBDs) [39], hypoxia-inducible factor-1α (HIF-1α) [40], osteoprotegerin [41], and anti-Hsp-70 (heat shock protein family A) [42]. In light of innumerable proactive studies, it has already been suggested that this is an ideal fluid as a strong and unmatchable latest chemical gizmo that cross matches the clinical parameters too. While negotiating the pros of GCF, there exist very few studies presenting that levels of biomarkers are not significantly different for healthy and diseased individuals [43] which need to produce more clarity in studies that suggest a scientific evolutionary rationale for these indicators and this tool.
Many well-renowned researchers in the field have put forward the role of numerous proteomes as validated biomarker for the initiation and inhibition of periodontal pathological process [23][44]. In 2016, a definite and scientifically accelerative proof presented by Barros et al. team indicated that GCF is a source of multiple biomarkers that potentially indicate disease presence with details of active and passive sites [45]. In 2017, Kaur et al. provided a comprehensive overview of the host defense mediators and their expression in the crevicular fluid around the tooth, these studies explicitly favored the use of GCF as a universal tool [46]. Various methods of manipulating GCF for evaluating gingival related diseases have been proposed such as polymerase chain reaction (PCR), enzyme-linked immunosorbent assay (ELISA) or immunofluorometric assay (IFMA). The application of liquid chromatography-mass spectrometry (LC-MS) combined with label-free quantitative proteomic approaches have led to discovery of 154 proteins of human, viral, bacterial, and fungal origin in 2010, that consequently helped scientists to expedite a detailed investigation of both microbial and host protein biochemistry that will open door for exploration of a variety of treatment modalities [47]. Thus, the emerging quantitative and qualitative proteomics will continue to provide new assessment strategies to further unravel the diagnostic and therapeutic role of GCF (Figure 3).
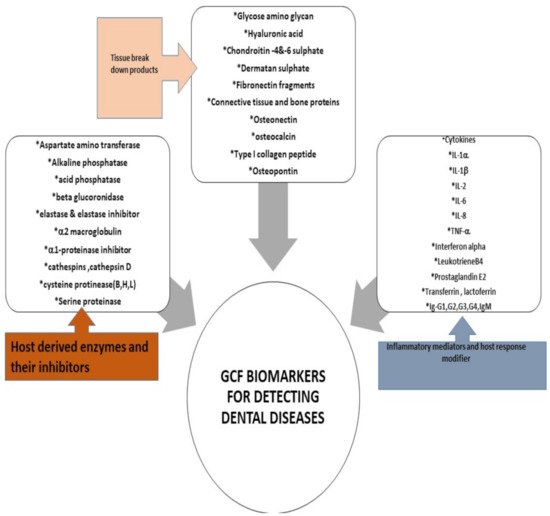
Figure 3. GCF Biomarkers for detecting dental diseases [2][15].
Furthermore, GCF is exploited to analyze the biochemical parameters to identify the periodontal disease at its early stage before the commencement of the clinical damage [2][48]. The current approaches for assessing periodontal disease include PD and BOP. However, the reliability of these tools is questioned by many studies [49]. Moreover, decrease patient compliance due to associated pain with the probing [50] and higher incidence of negative predictive value [51] highlights the need for a less invasive and efficient modality. Increase in the GCF volume is the initial sign of inflammation which is indicative of subclinical inflammation [52]. Detection of hemoglobin in the GCF has been advocated for early diagnosis as this indicator of bleeding in the periodontal pocket can be detected well in advance of BOP [53]. Matrix metalloproteinase-8 (MMP-8) levels can effectively diagnose gingivitis, which is an early inflammatory prerequisite state to periodontitis. Thus, this approach can effectively be employed to prevent periodontitis [54].
3. Conclusions
In today’s health‐ and cost‐conscious environment, it is essential to make rational and cost‐effective decisions for the prevention and treatment of periodontal disease. This prevention and treatment should be based on accurate diagnosis, reducing causative factors and risk management. A wide range of periodontal diseases exist, requiring various types of treatment. Success lies in the establishment of precise diagnoses to detect the disease type with precision which is best provided with the help of GCF–a complete bioinformatics tool. The purpose of this study was to develop a sense among dental practitioners about the use of gingival crevicular fluid as a fluid biopsy specimen.
The goal of periodontal diagnostic procedures via GCF assessment is to provide useful information to the clinician regarding the present periodontal disease location,and severity [80]. These findings serve as a basis for treatment planning and provide essential data during periodontal maintenance and disease‐monitoring phases of treatment. Therefore, via this study we want to endorse Specific, Measurable, Achievable,Realistic and Time‐bound (SMART) periodontal diagnosis to broaden the horizon of diagnostic criteria, also to shift the conventional paradigm from subjective to objective methods of assessment.
The future holds promising possibilities for GCF as a perio-diagnostic tool that offers a non‐invasive, efficient and easy to use approach to sample biomarkers of inflammation and bone resorption in the oral cavity [81] and allow to differentiate the active inflamed sites and predict future tissue destruction and to diagnose early signs of periodontitis.Furthermore, it can also allow monitoring of this condition and prompt discovery of new biomarkers that will aid in the development of new therapeutic approaches via host modulatory drugs for periodontal disease treatment leading to more individualized,targeted treatments for oral health.
The major attraction of GCF as a diagnostic marker is the site‐specific nature of the sample. This allows laboratory investigations of GCF constituents to be linked to clinical assessments at the site of sample collection which may offer the basis for patient‐specific diagnostic tests for periodontal disease. Moreover, GCF as a diagnostic marker can indicate the presence of a disease process before extensive clinical damage has occurred. Finally, the simplicity of its use along with a level of reliability and low cost favors its use over other modalities.
References
- Brill, N.; Krasse, B.O. The Passage of Tissue Fluid into the Clinically Healthy Gingival Pocket. Acta Odontol. Scand. 1958, 16, 233–245.
- Khurshid, Z.; Mali, M.; Naseem, M.; Najeeb, S.; Zafar, M.S. Human Gingival Crevicular Fluids (GCF) Proteomics: An Overview. Dent. J. 2017, 5, 12.
- Armitage, G.C. The complete periodontal examination. Periodontology 2000 2004, 34, 22–33.
- Oswal, S.; Dwarakanath, C. Relevance of gingival crevice fluid components in assessment of periodontal disease—A critical analysis. J. Indian Soc. Periodontol. 2010, 14, 282–286.
- Griffiths, G.S. Formation, collection and significance of gingival crevice fluid. Periodontology 2000 2003, 31, 32–42.
- Günday, S.; Topcu, A.O.; Ercan, E.; Yamalik, N. Analysis of Daytime Variations in Gingival Crevicular Fluid: A Circadian Periodicity? J. Periodontol. 2014, 85, e47–e56.
- Winer, R.A.; O’Donnell, L.J.; Chauncey, H.H.; McNamara, T.F. Enzyme Activity in Periodontal Disease. J. Periodontol. 1970, 41, 449–456.
- Arias-Bujanda, N.; Regueira-Iglesias, A.; Alonso-Sampedro, M.; González-Peteiro, M.M.; Mira, A.; Balsa-Castro, C.; Tomás, I. Cytokine thresholds in gingival crevicular fluid with potential diagnosis of chronic periodontitis differentiating by smoking status. Sci. Rep. 2018, 8, 1–12.
- Skapski, H.; Lehner, T. A crevicular washing method for investigating immune components of crevicular fluid in man. J. Periodontal Res. 1976, 11, 19–24.
- Shrivastava, D.; Srivastava, K.C.; Dayakara, J.K.; Sghaireen, M.G.; Gudipaneni, R.K.; Al-Johani, K.; Baig, M.N.; Khurshid, Z. BactericidalActivity of Crevicular Polymorphonuclear Neutrophils in Chronic Periodontitis Patients and Healthy Subjects under the Influence of Areca Nut Extract: An In Vitro Study. Appl. Sci. 2020, 10, 5008.
- Ghallab, N.A. Diagnostic potential and future directions of biomarkers in gingival crevicular fluid and saliva of periodontal diseases: Review of the current evidence. Arch. Oral Biol. 2018, 87, 115–124.
- Gul, S.S.; Griffiths, G.S.; Stafford, G.P.; Al-Zubidi, M.I.; Rawlinson, A.; Douglas, C.W. Investigation of a novel predictive biomarker profile for the outcome of periodontal treatment. J. Periodontol. 2017, 88, 1135–1144.
- Nimbulkar, G.; Garacha, V.; Shetty, V.; Bhor, K.; Srivastava, K.C.; Shrivastava, D.; Sghaireen, M.G. Microbiological and clinical evaluation of Neem gel and Chlorhexidine gel on dental plaque and gingivitis in 20–30 years old adults: A randomized parallel-armed, double-blinded controlled trial. J. Pharm. Bioallied Sci. 2020, 12 (Suppl. 1), S345–S351.
- Larsson, L. Current Concepts of Epigenetics and Its Role in Periodontitis. Curr. Oral Health Rep. 2017, 4, 286–293.
- Qasim, S.S.B.; Al-Otaibi, D.; Al-Jasser, R.; Gul, S.S.; Zafar, M.S. An Evidence-Based Update on the Molecular Mechanisms Underlying Periodontal Diseases. Int. J. Mol. Sci. 2020, 21, 3829.
- Nomura, Y.; Okada, A.; Kakuta, E.; Gunji, T.; Kajiura, S.; Hanada, N. A new screening method for periodontitis: An alternative to the community periodontal index. BMC Oral Health 2016, 16, 1–7.
- Lindhe, J.; Haffajee, A.D.; Socransky, S.S. Progression of periodontal disease in adult subjects in the absence of periodontal therapy. J. Clin. Periodontol. 1983, 10, 433–442.
- Buduneli, N. Biomarkers in periodontal health and disease: Rationale, benefits, and future directions. In Biomarkers in Periodontal Health and Disease: Rationale, Benefits, and Future Directions; Springer: Cham, Switzerland, 2019; Volume 90.
- Subrahmanyam, M.V.; Sangeetha, M. Gingival crevicular fluid a marker of the periodontal disease activity. Indian J. Clin. Biochem. 2003, 18, 5–7.
- De Alencar Silva, F.G.; Gomes, S.C. Validation of an alternative absorbent paper for collecting gingival crevicular fluid/Validação de um papel absorvente para coleta de Fluido Crevicular Gengival. Periodontia 2009, 19, 85–90.
- Konopka, Ł.; Pietrzak, A.; Brzezińska-Błaszczyk, E. Effect of scaling and root planing on interleukin-1β, interleukin-8 and MMP-8 levels in gingival crevicular fluid from chronic periodontitis patients. J. Periodontal Res. 2012, 47, 681–688.
- Majeed, Z.N.; Philip, K.; Alabsi, A.M.; Pushparajan, S.; Swaminathan, D. Identification of Gingival Crevicular Fluid Sampling, Analytical Methods, and Oral Biomarkers for the Diagnosis and Monitoring of Periodontal Diseases: A Systematic Review. Dis. Markers 2016, 2016, 1804727.
- Graves, D. Cytokines that Promote Periodontal Tissue Destruction. J. Periodontol. 2008, 79, 1585–1591.
- Offenbacher, S.; Barros, S.P.; Singer, R.E.; Moss, K.; Williams, R.C.; Beck, J.D. Periodontal disease at the biofilm-gingival interface. J. Periodontol. 2007, 78, 1911–1925.
- Júnior, A.A.B.; Pallos, D.; Cortelli, J.R.; Saraceni, C.H.C.; Queiroz, C.S. Evaluation of organic and inorganic compounds in the saliva of patients with chronic periodontal disease. Rev. Odonto Ciência 2010, 25, 234–238.
- Wong, D.T. Salivary diagnostics powered by nanotechnologies, proteomics and genomics. J. Am. Dent. Assoc. 2006, 137, 313–321.
- Oringer, R.J.; Howell, T.H.; Nevins, M.L.; Reasner, D.S.; Davis, G.H.; Sekler, J.; Fiorellini, J.P. Relationship Between Crevicular Aspartate Aminotransferase Levels and Periodontal Disease Progression. J. Periodontol. 2001, 72, 17–24.
- Eley, B.M.; Cox, S.W. The relationship between gingival crevicular fluid cathepsin B activity and periodontal attachment loss in chronic periodontitis patients: A 2-year longitudinal study. J. Periodontal Res. 1996, 31, 381–392.
- Ahmad, P.; Arshad, A.I.; Bella, E.D.; Khurshid, Z.; Stoddart, M. Systemic Manifestations of the Periodontal Disease: A Bibliometric Review. Molecules 2020, 25, 4508.
- Fassbender, W.J.; Steinhauer, B.; Stracke, H.; Schumm-Draeger, P.-M.; Usadel, K.-H. Validation of a new automated immunoassay for measurement of intact osteocalcin. Clin. Lab. 2002, 48, 31–38.
- Khiste, S.V.; Ranganath, V.; Nichani, A.S.; Rajani, V. Critical analysis of biomarkers in the current periodontal practice. J. Indian Soc. Periodontol. 2011, 15, 104–110.
- Bowers, M.R.; Fisher, L.W.; Termine, J.D.; Somerman, M.J. Connective tissue-associated proteins in crevicular fluid: Potential markers for periodontal diseases. J. Periodontol. 1989, 60, 448–451.
- Airila-Månsson, S.; Söder, B.; Kari, K.; Meurman, J.H. Influence of Combinations of Bacteria on the Levels of Prostaglandin E2, Interleukin-1β, and Granulocyte Elastase in Gingival Crevicular Fluid and on the Severity of Periodontal Disease. J. Periodontol. 2006, 77, 1025–1031.
- Huynh, Q.N.; Wang, S.; Tafolla, E.; Gansky, S.A.; Kapila, S.; Armitage, G.C.; Kapila, Y.L. Specific Fibronectin Fragments as Markers of Periodontal Disease Status. J. Periodontol. 2002, 73, 1101–1110.
- Aral, C.A.; Ölçer, S.N.; Aral, K.; Kapila, Y. Oxidative stress, neutrophil elastase and IGFBP7 levels in patients with oropharyngeal cancer and chronic periodontitis. Oral Dis. 2020, 26, 1393–1401.
- Sophia, K.; Suresh, S.; Sudhakar, U.; Cader, S.A., Jr.; Vardhini, V.M.; Arunachalam, T.L.; Jean, S.C. Comparative Evaluation of Serum and Gingival Crevicular Fluid Periostin Levels in Periodontal Health and Disease: A Biochemical Study. Cureus 2020, 12, e7218.
- Kumar, P.A.; Kripal, K.; Chandrasekaran, K.; Bhavanam, S.R. Estimation of YKL-40 Levels in Serum and Gingival Crevicular Fluid in Chronic Periodontitis and Type 2 Diabetes Patients among South Indian Population: A Clinical Study. Contemporary Clin. Dent. 2019, 10, 304–310.
- Hashimura, S.; Kido, J.; Matsuda, R.; Yokota, M.; Matsui, H.; Inoue-Fujiwara, M.; Inagaki, Y.; Hidaka, M.; Tanaka, T.; Tsutsumi, T.; et al. A low level of lysophosphatidic acid in human gingival crevicular fluid from patients with periodontitis due to high soluble lysophospholipase activity: Its potential protective role on alveolar bone loss by periodontitis. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2020, 1865, 158698.
- Pereira, A.G.; Costa, L.C.M.; Soldati, K.R.; De Abreu, M.H.N.G.; Costa, F.O.; Zandim-Barcelos, D.L.; Cota, L.O.M. Gingival Crevicular Fluid Levels of Human Beta-defensin 2 and 3 in Healthy and Diseased Sites of Individuals with and without Periodontitis. J. Int. Acad. Periodontol. 2020, 22, 90–99.
- Zorina, O.A.; Amkhadova, M.A.; Abaev, Z.M.; Khamukova, A.A.; Demidova, A.A. Hypoxia-dependent transcriptional control of activity of destructive inflammatory and malignant periodontium changes. Stomatologiia 2020, 99, 32–36.
- Kinney, J.S.; Morelli, T.; Oh, M.; Braun, T.M.; Ramseier, C.A.; Sugai, J.V.; Giannobile, W.V. Crevicular fluid biomarkers and periodontal disease progression. J. Clin. Periodontol. 2014, 41, 113–120.
- Takai, H.; Furuse, N.; Ogata, Y. Anti-heat shock protein 70 levels in gingival crevicular fluid of Japanese patients with chronic periodontitis. J. Oral Sci. 2020, 62, 281–284.
- Najeeb, S.; Zafar, M.S.; Khurshid, Z.; Zohaib, S.; Almas, K. The Role of Nutrition in Periodontal Health: An Update. Nutrients 2016, 8, 530.
- Garlet, G.P. Critical reviews in oral biology & medicine: Destructive and protective roles of cytokines in periodontitis: A re-appraisal from host defense and tissue destruction viewpoints. J. Dent. Res. 2010, 89, 1349–1363.
- Barros, S.P.; Williams, R.C.; Offenbacher, S.; Morelli, T. Gingival crevicular fluid as a source of biomarkers for periodontitis. Periodontology 2000 2016, 70, 53–64.
- Kaur, G.; Mohindra, K.; Singla, S. Autoimmunity—Basics and link with periodontal disease. Autoimmun. Rev. 2017, 16, 64–71.
- Bostanci, N.; Bao, K.; Greenwood, D.; Silbereisen, A.; Belibasakis, G.N. Periodontal disease: From the lenses of light microscopy to the specs of proteomics and next-generation sequencing. In Advances in Clinical Chemistry; Elsevier: London, UK, 2019; pp. 263–290.
- Khurshid, Z.; Warsi, I.; Moin, S.F.; Slowey, P.D.; Latif, M.; Zohaib, S.; Zafar, M.S. Biochemical analysis of oral fluids for disease detection. In Advances in Clinical Chemistry; Elsevier: London, UK, 2021; pp. 205–253.
- Armitage, G.C.; Svanberc, G.K.; Loe, H. Microscopic evaluation of clinical measurements of connective tissue attachment levels. J. Clin. Periodontol. 1977, 4, 173–190.
- Heft, M.W.; Perelmuter, S.H.; Cooper, B.Y.; Magnusson, I.; Clark, W.B. Relationship between gingival inflammation and painfulness of periodontal probing. J. Clin. Periodontol. 1991, 18, 213–215.
- Lang, N.P.; Adler, R.; Joss, A.; Nyman, S. Absence of bleeding on probing an indicator of periodontal stability. J. Clin. Periodontol. 1990, 17, 714–721.
- Rüdin, H.J.; Overdiek, H.F.; Rateitschak, K.H. Correlation between sulcus fluid rate and clinical and histological inflammation of the marginal gingiva. Helv. Odontol. Acta 1970, 14, 21–26.
- Ito, H.; Numabe, Y.; Hashimoto, S.; Sekino, S.; Murakashi, E.; Ishiguro, H.; Sasaki, D.; Yaegashi, T.; Takai, H.; Mezawa, M.; et al. Correlation between Gingival Crevicular Fluid Hemoglobin Content and Periodontal Clinical Parameters. J. Periodontol. 2016, 87, 1314–1319.
- Hong, I.; Pae, H.-C.; Song, Y.W.; Cha, J.-K.; Lee, J.-S.; Paik, J.-W.; Choi, S.-H. Oral Fluid Biomarkers for Diagnosing Gingivitis in Human: A Cross-Sectional Study. J. Clin. Med. 2020, 9, 1720.




