
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Amani Ayyash | + 1927 word(s) | 1927 | 2021-04-15 10:33:14 | | | |
| 2 | Rita Xu | Meta information modification | 1927 | 2021-04-23 03:49:56 | | |
Video Upload Options
Cancer is considered the second leading cause of death worldwide and in 2018 it was responsible for approximately 9.6 million deaths. Globally, about one in six deaths are caused by cancer. A strong correlation was found between diabetes mellitus and carcinogenesis with the most evident correlation was with type 2 diabetes mellitus (T2DM). Research has proven that elevated blood glucose levels take part in cell proliferation and cancer cell progression.
1. Introduction
Diabetes mellitus is a class of metabolic disorders characterized by prolonged periods of hyperglycemia. According to the World Health Organization (WHO), in the year 2014, 422 million people worldwide suffered from diabetes, and 1.6 million deaths were directly related to diabetes in the year 2016. Moreover, these statistics have been rising slowly over the last few decades [1]. Diabetes mellitus (DM) is displayed as either type 1 diabetes mellitus (result from total insulin deficiency due to beta-cell death follow an autoimmune disease) or type 2 Diabetes Mellitus (predominantly resulting from insulin resistance, rendering target cells unable to effectively respond to insulin and so unable to utilize blood glucose for energy). It is well known that diabetes mellitus increases the risk of developing a variety of severe life-threatening health complications, resulting from the disruption and impairment in the function of several organs (kidneys, hearts, skin, blood vessels, or nerves), leading to both microvascular and macrovascular complications which include nephropathy, diabetic retinopathy, and neuropathy, as well as atherosclerosis, hypertension, and stroke. These diabetic vascular complications are responsible for the majority of mortality in diabetic patients [2]. In addition to these complications, elevated blood glucose levels have been shown to stimulate cancer cell proliferation and progression [3]. Studies have shown a strong correlation between diabetes mellitus and carcinogenesis and the most evident correlation is reported with type 2 diabetes mellitus (T2DM). Nevertheless, In type 1 diabetes mellitus (T1DM), the risk of carcinogenesis has also been identified but is less evident compared to that with T2DM [4]. However, in both types of diabetes mellitus cancer incidence tends to be increased [5]. Cancer is the second leading cause of death worldwide and in the year 2018, approximately 9.6 million deaths were attributed to cancer, and nearly one in six deaths around the world are caused by cancer [6]. Both diabetes and cancer have a dramatic detrimental effect on both the mortality rate and the quality of life, and the simultaneous rise in incidence rates of both diseases has encouraged the research community to search into a possible correlation in terms of pathophysiological pathways and/or the common climate [7]. Hyperglycemia causes epigenetic alterations by several mechanisms including DNA methylation and chromatin remodeling, resulting in abnormal gene expression. Moreover, in cancer, abnormal gene expression causes tumor growth by increasing the metastases, proliferation, and chemoresistance of cancer cells [8]. The proliferation of cancer cells which is induced by hyperglycemia/diabetes occurs indirectly by mediating the following processes (1) insulin and insulin-like growth factor 1 (IGF-1), (2) secretion of leptin/adiponectin, (3) inflammatory responses, (4) production of reactive oxygen species (ROS; oxidative stress) and (5) immune abnormalities (platelet activation) [9]. In addition to a direct correlation between impaired glucose tolerance/diabetes and the initiation of cancer, proliferation and invasiveness may occur due to hyperglycemia [10]. Many epidemiological reports have shown that diabetes is positively associated with several types of cancers including breast, colorectal, endometrial, liver and pancreatic cancers. On the other hand, a decreased prevalence of prostate cancer has been observed in diabetic patients [11]. Ben et al. found a significant association between DM and pancreatic with an inverse correlation between the duration of DM and the occurrence of pancreatic cancer in both sexes, thus observing the greatest risk of developing pancreatic cancer in patients with a duration of DM shorter than 1 year after diagnosis [12]. Similar findings were observed in another study [13]. Cancer risk in diabetic patients is 20% higher based on a meta-analysis of 20 studies [14]. The analysis of data obtained by Hulda Hrund Bjornsdottir et al. on 450,000 people with type 2 diabetes and more than 2 million people without diabetes in Sweden between 1998 and 2014 was unable to show a cause-and-effect relationship. Nevertheless, people with blood sugar disease were observed to have a higher risk of developing several types of cancer including a 231% higher risk of hepatocellular cancer, a 119% higher risk of pancreatic cancer, and a 78% higher risk of uterine cancer compared to those without type 2 diabetes. Additionally, people with diabetes had an elevated risk of kidney cancer (45% higher), stomach cancer (21% higher), cancer of the gallbladder and bile duct (32% higher), and penile cancer (56% higher). Moreover, the incidence of colorectal cancer and bladder cancer was also 20% higher, while the breast cancer risk was 5% higher [15]. Besides the increased risk of cancer by hyperglycemia/diabetes, several meta-analysis studies have also linked diabetes with a higher incidence of poor post-treatment prognosis in diabetic cancer subjects [16]. In addition, the effect of hyperglycemia on breast cancer chemotherapy resistance have been shown in recent studies [17]. Although epidemiological studies have shown that cancer mortality in diabetic patients is relatively increased, it is still unclear if this is a consequence of hyperglycemia and hyperinsulinemia (growth-promoting effect on cancer cells), poor health conditions attributable to diabetes comorbidity, or a combination of all these factors [18]. There is strong evidence that cancer patients with diabetes are treated less aggressively or are expected to be less likely treated with modified anti-cancer therapy than non-diabetic cancer patients [19]. Some anti-diabetic drugs, such as metformin, have been shown to reduce the incidence of cancer in diabetes patients, enhance the efficacy of anti-cancer drugs and also has shown favorable survival in cancer patients [16].
2. Cancer Altered Metabolism
Generally, cancer cells have the ability to proliferate from one abnormal cell to more than 109 cells (the total number of cells ~1 cm in diameter in a tumor) if suitable conditions are available [20]. Tumor cells will modify their metabolism to guarantee survival, overpower host immune attack, and maintain the proliferative capacity to induce their lethal effects and sustain survival [21]. Cancer cells undergo physiological adaptations to preserve their survival under many stressful conditions, such as hypoxia and food hunger in order to fulfill their massive growth requirements. Such metabolic modifications generate abnormal metabolic events when compared to normal cells. This reprogrammed metabolism is seen as a cancer signature, as many metabolic modifications are predominant in several other types of cancers [22]. Similar to normal cells, cancer cells also have to produce ATP to maintain both daughter cells formed through division, rely on metabolic intermediates or biosynthesis by-products and, most critically, to compete with the oxidizing effects to mitigate the impact of reactive oxygen species (ROS). These altered metabolic and bioenergetic mechanisms, significantly elevated biosynthesis and redox equilibrium, are crucial to cancer progression [23]. Therefore, proliferating cells need to obtain high amounts of lipids, nucleotides, and amino acids. Through using by-products and derivatives of the TCA (tricarboxylic acid) cycle, cells can generate this biomass [24]. It would appear that fulfilling all these requirements would require a significant boost in glucose uptake in tumors. The high glucose consumption in many, but not all, tumors have been verified by PET (positron emission tomography) imaging and glucose consumption rates beyond the levels that can be easily explained by energy or metabolite requirements [25]. Moreover, the utilization of glutamine meets a symmetrical pattern of excessive consumption [26]. It is believed that cancer metabolism could be described as upregulation of the metabolism of both glucose and glutamine for the production of energy.
2.1. Glucose
Glucose is the principal source of cellular energy and in the presence of oxygen, it is metabolized to pyruvate through glycolysis, which is transported to the mitochondria, where it is oxidatively metabolized into CO2 in the tricarboxylic acid (TCA) cycle and the oxidative phosphorylation via electron transport chain (ETC) to produce high amounts of energy (for each molecule of glucose about 32 to 34 molecules of ATP are produced) [27]. Cells may also undergo anaerobic glycolysis without oxygen, i.e., fermentation, diverting the resulting pyruvate molecules towards lactate production which is less effective in ATP production than the TCA cycle coupled with oxidative phosphorylation. Similarly, cancer cells primarily metabolize glucose, but in contrast to normal cells and in spite of the presence of oxygen, glycolysis produces lactate leading to the generation of two ATP per molecule of glucose. Consequently, cancer cells need a high glucose utilization efficiency so as to fulfill their energy and anabolic requirements, Otto Heinrich Warburg in 1920 found that even in ample oxygen, glycolysis was improved in cancer cells. However, glycolysis was commonly known to increase under anaerobic conditions [28]. Despite deficiency in oxygen, increased glycolysis in cancer cells seemed to be a new phenomenon and was referred to as aerobic glycolysis or the “Warburg effect” [29]. Consequently, the high quantity of glucose catabolism into lactate has been the most widespread metabolic phenotype detected in cancer cells, contributing to the deposition of lactate by-products within the tumor’s microenvironment [20]. Currently, it is obvious that cancer cells experience aerobic glycolysis due to oncogene stimulation, tumor suppressor genes inhibition, and activation of phosphatidylinositol 3-kinase (PI3K) pathway, and that one benefit of high glycolytic frequencies is the availability of anabolic pathway substrates [30]. The expression of glucose transporters in tumor cells is deregulated by some oncogenes (such as Myc oncogene), resulting in over-expression of these transporters, especially GLUT1 and GLUT3 [31]. The idea that cancer cells utilize glucose as a fuel is explained as follows; glucose begins to be processed in the cytosol, and is converted to glucose-6-phosphate (G6P) by Hexokinase (HK). Glucose-6-phosphate (G6P) is the splitting point from glycolysis to the pentose phosphate pathway oxidative branch (PPP) that produces the ribose group needed for nucleotide synthesis (RNA and DNA synthesis). This pathway is important in order to enable cancer cells to fulfill their anabolic demands and survive oxidative and nutritional stress [32]. In this pathway, glucose-6-phosphate (G6P) is broken up into three-carbon compounds, 3-phosphoglyceraldehyde, which is the main point for the non-essential amino acid serine synthesis. Phosphoenolpyruvate (PEP) is also formed by glycolysis and end up producing two pyruvate molecules [20]. The glycolytic enzyme pyruvate kinase enzyme (PK) is the final rate-limiting enzyme and is essential in pyruvate and ATP production. This enzyme has four isoforms (M1, M2, L, and R) which are expressed in different cell types. The PKM2 isoform is highly expressed in tumors thus shifting glucose metabolism towards anabolism through aerobic glycolysis and allowing the pyruvate product in the cytosol to be reduced to lactate by lactate dehydrogenase enzyme (LDH) [33][34][35]. In cancer cells, lactate dehydrogenase enzyme (LDH) expression is upregulated which plays a crucial role in cancer characteristics [33].To sum up, cancer cells use catabolism of glucose as a main energy-generating mechanism by glycolysis and also from glucose, many biosynthetic compounds and NADPH molecules are produced.
2.2. Glutamine
The most common circulating amino acid in humans is glutamine. Glutamine is also the second most abundant nutrient after glucose in vitro cell culture environments, and its intake surpasses protein synthesis requirements [26]. Via controlling mitochondrial reactive oxygen species (ROS) and proliferation, glutamine impacts the signaling pathways needed for cancer development, survival, and metabolism [36][37]. Glutamine is taken up by proliferating cancer cells and converted to glutamate by various deamidation and transamination reactions, especially mitochondrial amidohydrolase glutaminase [38]. Then, glutamate is transformed to alpha-ketoglutarate (α-KG) by glutamate dehydrogenase activity (or an aminotransferase) [39]. Moreover, glutamine is used by rapidly developing tumor cells as a source of carbon for energy production as well as for the regenerating intermediates of the TCA cycle such as pyruvic acid, oxaloacetate, and α-KG to compensate for the continuous depletion of citrate exported from the mitochondria for lipid synthesis [37]. (Figure 1) showing the metabolic alteration in cancer cell.
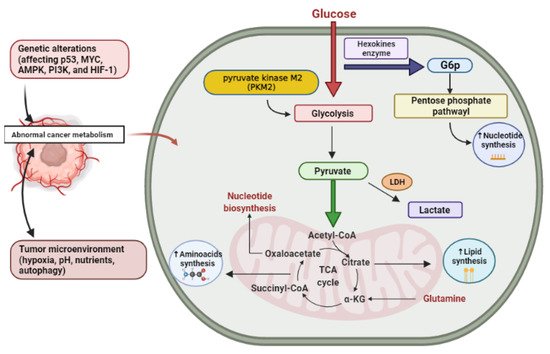
Figure 1. Cancer altered metabolism. G6P, glucose-6-phosphate; LDH, lactate dehydrogenase enzyme; α-KG, alpha-ketoglutarate. Created using Biorender software.
References
- Zimmet, P.; Alberti, K.G.; Magliano, D.J.; Bennett, P.H. Diabetes mellitus statistics on prevalence and mortality: Facts and fallacies. Nat. Rev. Endocrinol. 2016, 12, 616–622.
- Asmat, U.; Abad, K.; Ismail, K. Diabetes mellitus and oxidative stress—A concise review. Saudi Pharm. J. 2016, 24, 547–553.
- Giri, B.; Dey, S.; Das, T.; Sarkar, M.; Banerjee, J.; Dash, S.K. Chronic hyperglycemia mediated physiological alteration and metabolic distortion leads to organ dysfunction, infection, cancer progression and other pathophysiological consequences: An update on glucose toxicity. Biomed. Pharmacother. 2018, 107, 306–328.
- Wojciechowska, J.; Krajewski, W.; Bolanowski, M.; Kręcicki, T.; Zatoński, T. Diabetes and cancer: A review of current knowledge. Exp. Clin. Endocrinol. Diabetes 2016, 124, 263–275.
- Suh, S.; Kim, K.-W. Diabetes and cancer: Cancer should be screened in routine diabetes assessment. Diabetes Metab. J. 2019, 43, 733.
- De Martel, C.; Georges, D.; Bray, F.; Ferlay, J.; Clifford, G.M. Global burden of cancer attributable to infections in 2018: A worldwide incidence analysis. Lancet Glob. Health 2020, 8, e180–e190.
- Cignarelli, A.; Genchi, V.A.; Caruso, I.; Natalicchio, A.; Perrini, S.; Laviola, L.; Giorgino, F. Diabetes and cancer: Pathophysiological fundamentals of a ‘dangerous affair’. Diabetes Res. Clin. Pract. 2018, 143, 378–388.
- Lee, C.; An, D.; Park, J. Hyperglycemic memory in metabolism and cancer. Horm. Mol. Biol. Clin. Investig. 2016, 26, 77–85.
- Ferroni, P.; Riondino, S.; Buonomo, O.; Palmirotta, R.; Guadagni, F.; Roselli, M. Type 2 diabetes and breast cancer: The interplay between impaired glucose metabolism and oxidant stress. Oxid. Med. Cell. Longev. 2015, 2015, 183928.
- Srivastava, S.P.; Goodwin, J.E. Cancer biology and prevention in diabetes. Cells 2020, 9, 1380.
- Sampayo, V.; Tofthagen, C. Hyperglycemia and Cancer: An algorithm to guide oncology nurses. Clin. J. Oncol. Nurs. 2017, 21, 345–352.
- Ben, Q.; Xu, M.; Ning, X.; Liu, J.; Hong, S.; Huang, W.; Zhang, H.; Li, Z. Diabetes mellitus and risk of pancreatic cancer: A meta-analysis of cohort studies. Eur. J. Cancer 2011, 47, 1928–1937.
- Elena, J.W.; Steplowski, E.; Yu, K.; Hartge, P.; Tobias, G.S.; Brotzman, M.J.; Chanock, S.J.; Stolzenberg-Solomon, R.Z.; Arslan, A.A.; Bueno-de-Mesquita, H.B. Diabetes and risk of pancreatic cancer: A pooled analysis from the pancreatic cancer cohort consortium. Cancer Causes Control 2013, 24, 13–25.
- Villarreal-Garza, C.; Shaw-Dulin, R.; Lara-Medina, F.; Bacon, L.; Rivera, D.; Urzua, L.; Aguila, C.; Ramirez-Morales, R.; Santamaria, J.; Bargallo, E. Impact of diabetes and hyperglycemia on survival in advanced breast cancer patients. Exp. Diabetes Res. 2012, 2012, 732027.
- Bjornsdottir, H.H.; Rawshani, A.; Rawshani, A.; Franzén, S.; Svensson, A.-M.; Sattar, N.; Gudbjörnsdottir, S. A national observation study of cancer incidence and mortality risks in type 2 diabetes compared to the background population over time. Sci. Rep. 2020, 10, 1–12.
- Samuel, S.M.; Varghese, E.; Varghese, S.; Büsselberg, D. Challenges and perspectives in the treatment of diabetes associated breast cancer. Cancer Treat. Rev. 2018, 70, 98–111.
- Zeng, L.; Zielinska, H.; Arshad, A.; Shield, J.; Bahl, A.; Holly, J.; Perks, C. Hyperglycaemia-induced chemoresistance in breast cancer cells: Role of the estrogen receptor. Endocr. Relat. Cancer 2016, 23, 125–134.
- Vigneri, P.; Frasca, F.; Sciacca, L.; Pandini, G.; Vigneri, R. Diabetes and cancer. Endocr. Relat. Cancer 2009, 16, 1103–1123.
- Harding, J.L.; Andes, L.J.; Gregg, E.W.; Cheng, Y.J.; Weir, H.K.; Bullard, K.M.; Burrows, N.R.; Imperatore, G. Trends in cancer mortality among people with vs. without diabetes in the USA, 1988–2015. Diabetologia 2020, 63, 75–84.
- Lunt, S.Y.; Vander Heiden, M.G. Aerobic glycolysis: Meeting the metabolic requirements of cell proliferation. Annu. Rev. Cell Dev. Biol. 2011, 27, 441–464.
- Villalba, M.; Rathore, M.G.; Lopez-Royuela, N.; Krzywinska, E.; Garaude, J.; Allende-Vega, N. From tumor cell metabolism to tumor immune escape. Int. J. Biochem. Cell Biol. 2013, 45, 106–113.
- Pavlova, N.N.; Thompson, C.B. The emerging hallmarks of cancer metabolism. Cell Metab. 2016, 23, 27–47.
- DeBerardinis, R.J.; Chandel, N.S. Fundamentals of cancer metabolism. Sci. Adv. 2016, 2, e1600200.
- Danhier, P.; Bański, P.; Payen, V.L.; Grasso, D.; Ippolito, L.; Sonveaux, P.; Porporato, P.E. Cancer metabolism in space and time: Beyond the Warburg effect. Biochim. Biophys. Acta (BBA) Bioenerg. 2017, 1858, 556–572.
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674.
- Matés, J.M.; Campos-Sandoval, J.A.; de Los Santos-Jiménez, J.; Márquez, J. Dysregulation of glutaminase and glutamine synthetase in cancer. Cancer Lett. 2019, 467, 29–39.
- Bose, S.; Le, A. Glucose Metabolism in Cancer. Adv. Exp. Med. Biol. 2018, 1063, 3–12.
- Warburg, O.; Wind, F.; Negelein, E. The metabolism of tumors in the body. J. Gen. Physiol. 1927, 8, 519–530.
- Vander Heiden, M.G.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science 2009, 324, 1029–1033.
- Patra, K.C.; Wang, Q.; Bhaskar, P.T.; Miller, L.; Wang, Z.; Wheaton, W.; Chandel, N.; Laakso, M.; Muller, W.J.; Allen, E.L. Hexokinase 2 is required for tumor initiation and maintenance and its systemic deletion is therapeutic in mouse models of cancer. Cancer Cell 2013, 24, 213–228.
- Hsieh, A.L.; Walton, Z.E.; Altman, B.J.; Stine, Z.E.; Dang, C.V. Seminars in Cell & Developmental Biology. In MYC and Metabolism on the Path to Cancer; Elsevier: Amsterdam, The Netherlands, 2015; pp. 11–21.
- Fadaka, A.; Ajiboye, B.; Ojo, O.; Adewale, O.; Olayide, I.; Emuowhochere, R. Biology of glucose metabolization in cancer cells. J. Oncol. Sci. 2017, 3, 45–51.
- Feng, Y.; Xiong, Y.; Qiao, T.; Li, X.; Jia, L.; Han, Y. Lactate dehydrogenase A: A key player in carcinogenesis and potential target in cancer therapy. Cancer Med. 2018, 7, 6124–6136.
- Palsson-McDermott, E.M.; Curtis, A.M.; Goel, G.; Lauterbach, M.A.; Sheedy, F.J.; Gleeson, L.E.; van den Bosch, M.W.; Quinn, S.R.; Domingo-Fernandez, R.; Johnston, D.G. Pyruvate kinase M2 regulates Hif-1α activity and IL-1β induction and is a critical determinant of the warburg effect in LPS-activated macrophages. Cell Metab. 2015, 21, 65–80.
- Seton-Rogers, S. Feed it forward. Nat. Rev. Cancer 2011, 11, 461.
- Daye, D.; Wellen, K.E. Seminars in Cell & Developmental Biology. In Metabolic Reprogramming in Cancer: Unraveling the Role of Glutamine in Tumorigenesis; Elsevier: Amsterdam, The Netherlands, 2012; pp. 362–369.
- Phan, L.M.; Yeung, S.-C.J.; Lee, M.-H. Cancer metabolic reprogramming: Importance, main features, and potentials for precise targeted anti-cancer therapies. Cancer Biol. Med. 2014, 11, 1.
- Hensley, C.T.; Wasti, A.T.; DeBerardinis, R.J. Glutamine and cancer: Cell biology, physiology, and clinical opportunities. J. Clin. Investig. 2013, 123, 3678–3684.
- Deshmukh, A.; Deshpande, K.; Arfuso, F.; Newsholme, P.; Dharmarajan, A. Cancer stem cell metabolism: A potential target for cancer therapy. Mol. Cancer 2016, 15, 1–10.




